* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download atoms. - Unicam
Nuclear transmutation wikipedia , lookup
Nuclear binding energy wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Electron configuration wikipedia , lookup
Chemical bond wikipedia , lookup
Atomic nucleus wikipedia , lookup
Periodic table wikipedia , lookup
Abundance of the chemical elements wikipedia , lookup
Isotopic labeling wikipedia , lookup
History of chemistry wikipedia , lookup
Chemical element wikipedia , lookup
Stoichiometry wikipedia , lookup
Extended periodic table wikipedia , lookup
Gas chromatography–mass spectrometry wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
History of molecular theory wikipedia , lookup
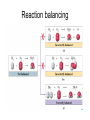
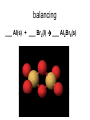
http://biologybiotechnology.unicam.it/intranet/courses/2015-2016/First%20year/First%20semester/ Basilar chemistry laws Dr.ssa Rossana Galassi 320 4381420 [email protected] Atom structure: protons number (in the nucleus) equal to the electrons number (around the nucleus). In the nucleus are located also the neutrons. These latters are neutral Electricity: Two faced gold foils connected with a metal pome. When electrized ebane is put close the gold foils disclose to each other Radioactivity: alfa, beta particles and gamma radiation Atom contains subparticles Electron discovery and determination of its charge/mass. • : Cathod rays Experiments to Determine the Properties of Cathode Rays OBSERVATION CONCLUSION 1. Ray bends in magnetic field. 2. Ray bends towards positive plate in electric field. consists of charged particles 3. Ray is identical for any cathode. consists of negative particles Determination of the electron charge (Millikan) Millikan used his findings to also calculate the mass of an electron. mass of electron = mass determined by J.J. Thomson and others X charge charge = (-5.686x10-12 kg/C) (-1.602x10-19C) = 9.109x10-31kg = 9.109x10-28g Scoperta del protone (esperienza di Goldstein): raggi canale Rutherford’s a-scattering experiment and discovery of the atomic nucleus. General features of the atom today. •The atom is an electrically neutral, spherical entity composed of a positively charged central nucleus surrounded by one or more negatively charge electrons. •The atomic nucleus consists of protons and neutrons. Properties of the Three Key Subatomic Particles Charge Name(Symbol) Mass Location Absolute(C)* Relative(amu)† Absolute(g) in the Atom Proton (p+) +1.60218x10-19 1.00727 Neutron (n0) 0 1.00866 Electron (e-) -1.60218x10-19 0.00054858 1.67262x1024 1.67493x10-24 Nucleus 9.10939x10-28 * The coulomb (C) is the SI unit of charge. † Nucleus The atomic mass unit (amu) equals 1.66054x10-24 g. Outside Nucleus Atomic Symbols, Isotopes, Numbers A Z X X = Atomic symbol of the element A = mass number; A = Z + N Z = atomic number (the number of protons in the nucleus) N = number of neutrons in the nucleus Isotope = atoms of an element with the same number of protons, but a different number of neutrons Determining the Number of Subatomic Particles in the Isotopes of an Element PROBLEM: Silicon(Si) is essential to the computer industry as a major component of semiconductor chips. It has three naturally occurring isoltopes: 28Si, 29Si, and 30Si. Determine the number of protons, neutrons, and electrons in each silicon isotope. PLAN: We have to use the atomic number and atomic masses. SOLUTION: The atomic number of silicon is 14. Therefore 28Si has 14p+, 14e- and 14n0 (28-14) 29Si has 14p+, 14e- and 15n0 (29-14) 30Si has 14p+, 14e- and 16n0 (30-14) Calculating the Atomic Mass of an Element PROBLEM: Silver(Ag: Z = 47) has 46 known isotopes, but only two occur naturally, 107Ag and 109Ag. Given the following mass spectrometric data, calculate the atomic mass of Ag: PLAN: Isotope Mass(amu) Abundance(%) 107Ag 106.90509 51.84 109Ag 108.90476 48.16 We have to find the weighted average of the isotopic masses, so we multiply each isotopic mass by its fractional abundance and then sum those isotopic portions. SOLUTION: mass(g) multiply of each by fractional portion of atomic mass atomic mass add isotopic portions isotope abundance of each from each isotope isotope mass portion from 107Ag = 106.90509amu x 0.5184 = 55.42amu mass portion from 109Ag = 108.90476amu x 0.4816 = 52.45amu atomic mass of Ag = 55.42amu + 52.45amu = 107.87amu The periodic table Elements are ordered in increasing number of atomic number Z -there vertical groups and horizontal periods -due formalisms for groups: 1-8 A main groups o B groups or groups from 1 to 18 Three areas: metals, not metals and metalloids The periodic table This table was built, first by Mendeleev, by following some rules: • Chemical elements are arranged in the table accordingly to the increasing Atomic Number • Elements are arranged in the table by considering some chemical properties. Dimitri Mendeleev table (18341907) Mendeleev developed the periodic table based on atomic weights because the concept of atomic numbers was not known till the development of the structure of the atom in the early 20th century. Dimitri Mendeleev table (1834-1907) • • • • • • • • The world’s first view of Mendeleev’s Periodic Table – an extract • from Zeitschrift fϋr Chemie, 1869. • Concerning the relation between the properties and atomic weights of elements. By D. Mendeleev. Arranging the elements in vertical columns with increasing atomic weights, so that the horizontal rows contain similar elements, again in increasing weight order, the following table is obtained from which general predictions can be drawn Elements show a periodicity of properties if listed in order of size of atomic weights. Elements with similar properties either have atomic weights that are about the same (Pt, Ir, Os) or increase regularly (K, Rb, Cs). The arrangement of the elements corresponds to their valency, and somewhat according to their chemical properties (eg Li, Be, B, C, N, O, F). The commonest elements have small atomic weights. It continues .... The value of the atomic weight determines the character of the element. There are unknown elements to discover eg elements similar to Al and Si with atomic weights in range 65-75. The atomic weights of some elements may be changed from knowing the properties of neighbouring elements. Thus the atomic weight of Te must be in range 123-126. It cannot be 128. Some typical properties of an element can be predicted from its atomic weight. Dimitri Mendeleev table (1834-1907) The greatness of Mendeleev was that not only did he leave spaces for elements that were not yet discovered but he predicted properties of five of these elements and their compounds. How foolish he would have seemed if these predictions had been incorrect but fortunately for him three of these missing elements were discovered by others within 15 years (ie within his lifetime). The first of these Mendeleev had called eka-aluminium because it was the one after aluminium (eka = 1 in Sanskrit) and was identified in Paris (1875) by Paul Emile Lecoq de Boisbaudran who named it gallium after the Latin name for France. Mendeleev was ecstatic when he heard of its properties which nearly matched his eka-aluminium. However de Boisbaudran's value for gallium's density (4.9 g/cm3) differed from Mendeleev's prediction. Mendeleev told the Frenchman, who re-measured the density to find Mendeleev was right! It is interesting to speculate whether de Boisbaudran was pleased or irritated by this. The table compares Mendeleev's predictions with de Boisbaudran's discovery. Eka-aluminium (Ea) Atomic weight Density of solid Melting point Valency Method of discovery Oxide About 68 3 6.0 g/cm low 3 Probably from its spectrum Formula Ea2O3, density 3 5.5 g/cm . Soluble in both acids and alkalis. Gallium (Ga) 69.72 3 5.9 g/cm o 29.78 C 3 Spectroscopically Formula Ga2O3, density 5.88 3 g/cm . Soluble in both acids and alkalis. Dimitri Mendeleev table (1834-1907) The photograph shows a giant wall Periodic Table erected in St Petersburg, Russia, in 1934. The periodic table at Milan EXPO 2015 pavilion The modern periodic table. hydrogen H from Hydrogenum Phosphoros P from Phosphorum Gold Au from Aurum Sodium Na from Natrium 10 elements are the most abundant: O(47%), Si(26%), Al, Fe, Ca, Mg, Na, K, Ti, H About 99,5% w/w in hydrosphere, atmosphere and outer shell of lithosphere http://www.tavolaperiodica.unicam.it/ http://www.rsc.org/periodic-table http://www.ptable.com/?lang=it http://www.chemicalelements.com/ Metals, not metals and metalloids Metals, metalloids, and nonmetals. Metals They are solids (except for mercury), high heat and electrical conducibility, ductility, malleability, and alloys forming. Not metals Solids, liquids and gas, generally do not conduct electricity (except graphite, C) Metalloids B, Si, Ge, As, Sb, Te, are elements that can behave as metals or not metals Alkali metals Earth alkali metals 13° or 3 A group elements allotropes Silicon compounds Gases in the periodic table: diatomic molecules Sulfur: S8 molecule http://www.tavolaperiodica.unicam.it/ Sulfur: allotropes Noble gases http://www.tavolaperiodica.unicam.it/ Transition metals Stretching from 2A to 3 A is a series of elements called the transition metals, called also B-groups. These elements fill the 1 B to 8B in the fourth till the seventh periods in the center of the periodic table. Most occur naturally combined with other elements, but Cu, Ag, Au and Pt are less reactive and they can be found in nature as pure elements. They are very important for their commercial use, in the technological applications (materials, paints, coins, batteries, catalytic converters ….), biological involvments (hemoglobin, vitamins, enzymes…) Rare earths The rows at the bottom of the table accomodate the lanthanides (the series of elements between lanthanium Z=57, and hafnium Z=72), and the actinides (the series of elements between actinium Z=89, and They have important applications in the field of nuclear reactors (U), in the color television picture tubes (La, Gd) or as contrast agent in diagnostic imaging. Molecules, compounds and formulas A molecule is the smallest identifiable unit by which a pure compound such as sugar or water can be divided still retaining the composition and the chemical properties of the substance. Such substances are composed of identical molecules consisting of atoms of two or more elements firmly bound together Molecules and atoms The modern atomic theory is based on ancient postulates They give the basis of the understanding of chemical reactions and compound formations Dalton (1766-1844 d. C.) defined that elements are constitued by atoms putting the basis of the modern theory. However, Dalton postulated the wrong assumption that atoms were not possible to divide Joseph Proust French chemist Joseph Proust proposed the law of definite composition or proportions based on his experiments conducted between 1798 and 1804 on the elemental composition of water and copper carbonate. In 1806, Proust summarized his observations in what is now called Proust's Law. It stated that chemical compounds are formed of constant and defined ratios of elements, as determined by mass. For example, carbon dioxide is composed of one carbon atom and two oxygen atoms. Therefore, by mass, carbon dioxide can be described by the fixed ratio of 12 (mass of carbon):32 (mass of oxygen), or simplified as 3:8. Dalton’s Atomic Theory The Postulates 1. All matter consists of atoms. 2. Atoms of one element cannot be converted into atoms of another element. 3. Atoms of an element are identical in mass and other properties and are different from atoms of any other element. 4. Compounds result from the chemical combination of a specific ratio of atoms of different elements. 1. All matter is composed of atoms. The atom is the smallest body that retains the unique identity of the element. 2. Atoms of one element cannot be converted into atoms of another element in a chemical reaction. Elements can only be converted into other elements in nuclear reactions. 3. All atoms of an element have the same number of protons and electrons, which determines the chemical behavior of the element. Isotopes of an element differ in the number of neutrons, and thus in mass number. A sample of the element is treated as though its atoms have an average mass. 4. Compounds are formed by the chemical combination of two or more elements in specific ratios. • When this theory was postulated the subatomic particles were not known. • However, this theory introduces the mass ratio and the mass conservation concepts. These latters are still valid and recognised by modern theories. Dalton’s Atomic Theory explains the mass laws Mass conservation Atoms cannot be created or destroyed postulate 1 or converted into other types of atoms. postulate 2 𝐴 = 𝜋𝑟 2 Since every atom has a fixed mass, postulate 3 during a chemical reaction atoms are combined differently and therefore there is no mass change overall. 2H2 + O2 => 2H2O Dalton’s Atomic Theory explains the mass laws Definite composition Atoms are combined in compounds in postulate 3 specific ratios and each atom has a specific mass. postulate 4 So each element has a fixed fraction of the total mass in a compound. Dalton’s Atomic Theory explains the mass laws Multiple proportions Atoms of an element have the same mass postulate 3 and atoms are indivisible. postulate 1 So when different numbers of atoms of elements combine, they must do so in ratios of small, whole numbers. Mass conservation law (Lavoisier 1743-1794) The total mass of the substance involved in a trasformation remains unchanged By performing the following reaction above a balance, weighting before and after the reaction BaCl2 + CuSO4 => BaSO4 + CuCl2 The law of mass conservation: mass remains constant during a chemical reaction. Law of Mass Conservation: The total mass of substances does not change during a chemical reaction. reactant 1 + reactant 2 total mass = calcium oxide + carbon dioxide CaO + CO2 56.08 + product 44.00 total mass calcium carbonate CaCO3 100.08 Law of Definite (or Constant) Composition: No matter the source, a particular compound is composed of the same elements in the same parts (fractions) by mass. Calcium carbonate Analysis by Mass (grams/20.0g) 8.0 g calcium 2.4 g carbon 9.6 g oxygen 20.0 g Mass Fraction (parts/1.00 part) Percent by Mass (parts/100 parts) 0.40 calcium 0.12 carbon 0.48 oxygen 40% calcium 12% carbon 48% oxygen 1.00 part by mass 100% by mass Calculating the Mass of an Element in a Compound PROBLEM: Pitchblende is the most commercially important compound of uranium. Analysis shows that 84.2 g of pitchblende contains 71.4 g of uranium, with oxygen as the only other element. How many grams of uranium can be obtained from 102 kg of pitchblende? PLAN: The mass ratio of uranium/pitchblende is the same no matter the source. We can use the ratio to find the answer. SOLUTION: mass(kg) of pitchblende mass(kg) of uranium mass (kg) of uranium = mass(kg) pitchblende x mass(kg) uranium in pitchblende mass(kg) pitchblende 71.4kg uranium mass(g) of uranium = 86.5 kg = 102 kg pitchblende x 84.2kg pitchblende uranium 86.5 kg uranium x 1000g kg = 8.65 x 104g uranium Law of Multiple Proportions: If elements A and B react to form two compounds, the different masses of B that combine with a fixed mass of A can be expressed as a ratio of small whole numbers. Example: Carbon Oxides A & B Carbon Oxide I : 57.1% oxygen and 42.9% carbon Carbon Oxide II : 72.7% oxygen and 27.3% carbon Assume that you have 100g of each compound. In 100 g of each compound: g O = 57.1 g for oxide I & 72.7 g for oxide II g C = 42.9 g for oxide I & 27.3 g for oxide II gO gC 57.1 = 42.9 gO 72.7 gC 27.3 = 1.33 = 2.66 2.66 g O/g C in II 1.33 g O/g C in I = 2 1 Law of Multiple Proportions: If elements A and B react to form two compounds, the different masses of B that combine with a fixed mass of A can be expressed as a ratio of small whole numbers. Given the following data for two compounds, SnO2 and SnO, what is the whole number ratio of the oxygen of SnO to the oxygen of SnO2? Does this follow the law of multiple proportions? •1:1. Yes, it follows the law of multiple proportions. •2:1. Yes, it follows the law of multiple proportions. •1:2. Yes, it follows the law of multiple proportions. Law of Multiple Proportions: If elements A and B react to form two compounds, the different masses of B that combine with a fixed mass of A can be expressed as a ratio of small whole numbers. Which of the following compounds CANNOT demonstrate the law of multiple proportions? 1. NO, NO2 2. CO, CO2 3. H2O, H2O2 4. Na2S, NaF Chemical reactions Matter cannot be created neither destroyed In a reaction, matter undergoes a transformation without mass loss. C (solid) + O2 (gaseous) => CO2 (gas) + heat (energy) 12 amu + 32 amu => 44 amu (micro) 12 g + 32 g => 44 g (macro) Reaction balancing balancing ___ Al(s) + ___ Br2(l) ___ Al2Br6(s) 2 Al + 3 Br2 =>1Al2Br6 6Cl2(g) + P4(s) 4PCl3 (l) Equation intial (mol) initial (g) variation 6Cl2 (g) 6 mol 425 g -6 mol (-425 g) + + + + P4(s) 1 mol 124 g -1 mol (-124 g) Complete reaction 0 mol + 0 mol 4PCl3(l) 0 mol 0 g +4 mol 4 mol (425+124=549g) Cl2 (g) + P4 (s) PCl3 (l) The rapresentation of a chemical reaction is named chemical equation • Reactants are written on the left of the arrow whilst products are on the right Cl2(g) + P4(s) reactants PCl3 (l) products letters (s), (g), and (l) represent the physical state of the reagents and the products 6Cl2(g) + 1 P4(s) 4PCl3 (l) • The bold numbers in the chemical equation are the stoichiometric coefficients Conservation of the mass 6x2=12 atomi di Cl 6Cl2(g) + P4(s) 4 atomi di P 4x3=12 atomi di Cl 4PCl3 (l) 4 atomi di P The relationship between the reagents and the products amount is called stoichiometry and the coefficients in the chemical equation are called stoichiometric coefficients 6Cl2(g) + P4(s) 4PCl3 (l) The mole Definition of mole(mol) - the amount of a substance that contains the same number of entities as there are atoms in exactly 12 g of carbon-12. This amount is 6.022x1023. The number is called Avogadro’s number and is abbreviated as N. One mole (1 mol) contains 6.022x1023 entities (to four significant figures) 602200000000000000000000 a huge number! The mole concept of mole(mol) – being atoms and molecules very very very small to have an amount which is macroscopically measurable the number of them should be huge, as the Avogadro number is. As usually the term a couple of glasses, a dozen of eggs, a pair of socks delimits a certain amoun of daily life objects, in chemistry is used the mole of a substance to quantify the amount we refer. The Molar Mass (MM) is the mass in grams of a mole of that substance. Measure unit: g/mol The mole 1. How many molecules are present in 5 moles of C? n atoms = 𝐴𝑣𝑜𝑔𝑎𝑑𝑟𝑜 ′ 𝑠 𝑁𝑢𝑚𝑏𝑒𝑟 Ɲ 𝑥 5 = 3.011 𝑥 10 24 2. How many mole are present in 9.011 𝑥 10 24 atoms of C? n mole = 9.011 𝑥 10 24 𝐴𝑣𝑜𝑔𝑎𝑑𝑟𝑜 ′ 𝑠 𝑁𝑢𝑚𝑏𝑒𝑟 Ɲ = 15 3. How many molecules are present in 10 𝑚𝑜𝑙𝑒𝑠 𝑜𝑓 𝐻2𝑂? n molecules = 𝐴𝑣𝑜𝑔𝑎𝑑𝑟𝑜 ′ 𝑠 𝑁𝑢𝑚𝑏𝑒𝑟 Ɲ 𝑥 10 = 6.022 𝑥 10 24 The mole 12 g of C (AW = relative atomic weigh= 12 amu) contains Ɲ of atoms, and it consists of 1 mole 18 g of water (MW= relative molecular weigh = 18 amu) contains Ɲ of molecules, and it consists of 1 mole Molar Mass is the mass in g of a mole of substance. Number of mole n = 𝑀𝑀 𝑚𝑎𝑠𝑠 𝑔 𝑚𝑜𝑙𝑎𝑟 𝑚𝑎𝑠𝑠 How many moles for 180 g of water? Number of mole n = 𝑀𝑀 𝑚𝑎𝑠𝑠 𝑔 𝑚𝑜𝑙𝑎𝑟 𝑚𝑎𝑠𝑠 = 180 g /18 g /mole = 10 moles The mole C (solid) + O2 (gas) => CO2 (gas) + cal (energy) 12 amu + 32 amu => 44 amu (micro) 12 g + 32 g => 44 g (macro) The proportionality constant between the mcroscopic units (amu) and macroscopic units (grams) is the Avogadro number Ɲ One mole of common substances. CaCO3 100.09 g Oxygen 32.00 g Water 18.02 g Copper 63.55 g Information Contained in the Chemical Formula of Glucose C6H12O6 ( M = 180.16 g/mol) Carbon (C) Hydrogen (H) Oxygen (O) Atoms/molecule of compound 6 atoms 12 atoms 6 atoms Moles of atoms/ mole of compound 6 moles of atoms 12 moles of atoms 6 moles of atoms Atoms/mole of compound 6(6.022 x 1023) atoms 12(6.022 x 1023) atoms 6(6.022 x 1023) atoms Mass/molecule of compound 6(12.01 amu) =72.06 amu 12(1.008 amu) =12.10 amu 6(16.00 amu) =96.00 amu 12.10 g 96.00 g Mass/mole of compound 72.06 g Summary of Mass Terminology Term Definition Unit Isotopic mass Mass of an isotope of an element amu Atomic mass Average of the masses of the naturally occurring isotopes of an element weighted according to their abundance amu Sum of the atomic masses of the atoms (or ions) in a molecule (or formula unit) amu (also called atomic weight) Molecular (or formula) mass (also called molecular weight) Molar mass (M) Mass of 1 mole of chemical entities (also called (atoms, ions, molecules, formula units) gram-molecular weight) g/mol Interconverting Moles, Mass, and Number of Chemical Entities no. of grams Mass (g) = no. of moles x g 1 mol No. of moles = mass (g) x 1 mol no. of grams No. of entities = no. of moles x 6.022x1023 entities 1 mol 1 mol No. of moles = no. of entities x 6.022x1023 entities M MASS(g) of element Summary of the mass-molenumber relationships for elements. M (g/mol) AMOUNT(mol) of element Avogadro’s number (atoms/mol) ATOMS of element Calculating the Mass and the Number of Atoms in a Given Number of Moles of an Element PROBLEM: (a) Silver (Ag) is used in jewelry and tableware but no longer in U.S. coins. How many grams of Ag are in 0.0342mol of Ag? (b) Iron (Fe), the main component of steel, is the most important metal in industrial society. How many Fe atoms are in 95.8g of Fe? PLAN: (a) To convert mol of Ag to g we have to use the #g Ag/mol Ag, the molar mass M. SOLUTION: 0.0342mol Ag x 107.9 g Ag = 3.69g Ag mol Ag PLAN: (b) To convert g of Fe to atoms we first have to find the #mols of Fe and then convert mols to atoms. SOLUTION: 95.8g Fe x mol Fe = 1.72mol Fe 55.85g Fe 6.022x1023atoms Fe = 1.04x1024 atoms 1.72mol Fe x mol Fe Fe amount(mol) of Ag multiply by M of Ag (107.9g/mol) mass(g) of Ag mass(g) of Fe divide by M of Fe (55.85g/mol) amount(mol) of Fe multiply by 6.022x1023 atoms/mol atoms of Fe MASS(g) of compound Summary of the mass-molenumber relationships for compounds. M (g/mol) AMOUNT(mol) of compound chemical formula Avogadro’s number (molecules/mol) MOLECULES (or formula units) of compound AMOUNT(mol) of elements in compound Calculating the Moles and Number of Formula Units in a Given Mass of a Compound PROBLEM: Ammonium carbonate is white solid that decomposes with warming. Among its many uses, it is a component of baking powder, first extinguishers, and smelling salts. How many formula unit are in 41.6 g of ammonium carbonate? After writing the formula for the compound, we find its M by adding the masses of the elements. Convert the given mass, 41.6 g to mols using M and then the mols to formula units with Avogadro’s number. SOLUTION: The formula is (NH4)2CO3. mass(g) of (NH4)2CO3 divide by M amount(mol) of (NH4)2CO3 multiply by 6.022x1023 formula units/mol number of (NH4)2CO3 formula units M = (2 x 14.01 g/mol N)+(8 x 1.008 g/mol H) +(12.01 g/mol C)+(3 x 16.00 g/mol O) mol (NH4)2CO3 41.6 g (NH4)2CO3 x 96.09 g (NH4)2CO3 6.022x1023 formula units (NH4)2CO3 mol (NH4)2CO3 2.61x1023 formula units (NH4)2CO3 Mass percent from the chemical formula Mass % of element X = atoms of X in formula x atomic mass of X (amu) x 100 molecular (or formula) mass of compound(amu) Mass % of element X = moles of X in formula x molar mass of X (amu) molecular (or formula) mass of compound (amu) x 100 Calculating the Mass Percents and Masses of Elements in a Sample of Compound PROBLEM: Glucose (C6H12O6) is the most important nutrient in the living cell for generating chemical potential energy. (a) What is the mass percent of each element in glucose? (b) How many grams of carbon are in 16.55g of glucose? PLAN: We have to find the total mass of glucose and the masses of the constituent elements in order to relate them. SOLUTION: (a) Per mole glucose there are 6 moles of C 12 moles H 6 moles O amount(mol) of element X in 1mol compound multiply by M(g/mol) of X mass(g) of X in 1mol of compound divide by mass(g) of 1mol of compound mass fraction of X multiply by 100 mass % X in compound continued 6 mol C x 12.01 g C = 72.06 g C 12 mol H x 16.00 g O = 96.00 g O = 12.096 g H mol H mol C 6 mol O x 1.008 g H M = 180.16 g/mol mol O (b) mass percent of C = 72.06 g C 180.16 g glucose = 0.3999 x 100 = 39.99 mass %C 12.096 g H mass percent of H = mass percent of O = 180.16 g glucose 96.00 g O 180.16 g glucose = 0.06714 x 100 = 6.714 mass %H = 0.5329 x 100 = 53.29 mass %O The Molar Mass (MM) is the mass in grams of a mole of that substance. Measure unit: g/mol