* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download ELECTRON CLOUD MODEL
Survey
Document related concepts
Minimal Supersymmetric Standard Model wikipedia , lookup
ALICE experiment wikipedia , lookup
Weakly-interacting massive particles wikipedia , lookup
Double-slit experiment wikipedia , lookup
ATLAS experiment wikipedia , lookup
Electric charge wikipedia , lookup
Introduction to quantum mechanics wikipedia , lookup
Grand Unified Theory wikipedia , lookup
Mathematical formulation of the Standard Model wikipedia , lookup
Nuclear force wikipedia , lookup
Compact Muon Solenoid wikipedia , lookup
Electron scattering wikipedia , lookup
Nuclear structure wikipedia , lookup
Standard Model wikipedia , lookup
Transcript
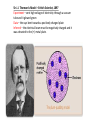
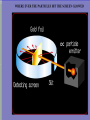
VOCABULARY OF ATOMS: 1. ATOMS – THE BUILDING BLOCKS OF THE UNIVERSE, MOST BASIC UNIT OF MATTER 2. ELEMENTS – ATOMS WITH UNIQUE PROPERTIES, CANNOT BE BROKEN DOWN 3. ELECTRONS – NEGATIVELY CHARGED SUBATOMIC PARTICLE 4. PROTONS – POSITIVELY CHARGED SUBATOMIC PARTICLE FOUND IN THE NUCLEUS OF THE ATOM 5. NEUTRON – NEUTRAL SUBATOMIC PARTICLE FOUND IN THE NUCLEUS OF THE ATOM, ABOUT THE SIZE MASS AS (+) BUT NO CHARGE, TO FIND SUBTRACT MASS FROM ATOMIC # 6. NUCLEUS – CENTER OF THE ATOM, CONTAINS MOST OF THE 7. ATOMIC NUMBER - # OF (-), # OF (+), SAME NUMBER 8. ISOTOPE – ATOM OF THE SAME ELEMENT WITH A DIFFERENT # OF NEUTRONS THEN THE ATOMIC # 9. MASS NUMBER – TOTAL NUMBER OF PROTONS AND NEUTRONS 10.ATOMIC MASS UNIT – DESCRIBES THE ATOM’S MASS, PUT A 1 OVER 12 FOR AN ISOTOPE, i.e. CARBON-12 HAS AN AMU OF 1/12 What Is the Difference Between Atomic Mass and Mass Number? MASS NUMBER IS THE COUNT OF PROTONS AND NEUTRONS ATOMIC MASS UNIT IS THE TOTAL NUMBER OF PROTONS, NEUTRONS AND ELECTRONS IN THE ATOM, OR THE AVERAGE MASS IN A GROUP OF ATOMS, ALSO KNOWN AS ATOMIC WEIGHT (SINCE WEIGHT AND MASS ARE THE SAME ON EARTH) Sir J.J. Thomson’s Model – British Scientist, 1897 Experiment – sent high voltage of electricity through a vacuum tube and it glowed green Data – the rays bent towards a positively charged plate Inferred – the electrical beam must be negatively charged and it was attracted to the (+) metal plate. Ernest Rutherford’s Atomic Model – Thomas’s student, 1909 Planetary model – Peach Pit Model Experiment –aim a (+) charged beam at a gold foil surrounded by a detection screen Data- most particles passed through, some particles reflected off Analyzing Data – some particles must be larger then a single proton from Thomson’s model Conclusion – the atom must have a nucleus and it has a (+) charge Neil Bohr’s Atomic Model – Planetary Model WHILE STUDYING RUTHFORD’S PLANETARY MODEL, HE THEORIZED: 1. ELECTRON ORBIT AROUND NUCLEUS 2. ELECTRONS CAN JUMP IN AND OUT OF LAYERS 3. WHEN THEY JUMP THEY GIVE OFF ENERGY Erwin Schrodinger and Werner Heisenberg 1926 Scientists described the electron region of the atom as a cloud around the nucleus. This cloud area shows that electrons do not orbit the nucleus in definite paths, but are likely to be in a given region at any particular time. ‘Modern Electron ELECTRON CLOUD MODEL James Chadwick •1932 •Worked with E. Rutherford in the discovery of a third subatomic particle. •He concluded this particle to be free of electrical charge and he called it the ‘neutron.’ •Concluded it was located in the nucleus along with the protons. Today’s newest memebers: Quarks and Leptons Quarks combine to form protons and neutrons, the components of atomic nuclei. Leptons: The other type of matter particles are the leptons. There are six leptons, three of which have electrical charge and three of which do not. Hydrons – the 3 sets of quarks, up and down form the world around us. IONS = charged atoms SAME