* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Document
Chemical potential wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Elementary particle wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Metastable inner-shell molecular state wikipedia , lookup
Electronegativity wikipedia , lookup
Biochemistry wikipedia , lookup
Atomic orbital wikipedia , lookup
Molecular orbital diagram wikipedia , lookup
Chemical element wikipedia , lookup
Metallic bonding wikipedia , lookup
Molecular Hamiltonian wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Resonance (chemistry) wikipedia , lookup
Size-exclusion chromatography wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Nuclear binding energy wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Computational chemistry wikipedia , lookup
Extended periodic table wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Implicit solvation wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Stoichiometry wikipedia , lookup
Gas chromatography–mass spectrometry wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
History of chemistry wikipedia , lookup
Electron configuration wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
Chemical bond wikipedia , lookup
Molecular dynamics wikipedia , lookup
Atomic nucleus wikipedia , lookup
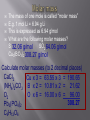
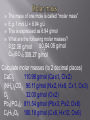
Mr. Chimenti Room 209 The number of digits in a measurement that are definitely known plus one estimate Ex. 13.5cm 2 known + 1 estimated=3 sig. figs. 1. 2. 3. 4. Zeros between nonzero numbers are significant ex. 50.33 sig. figs. Zeros in front of nonzero digits are insignificant ex. .oo8233 sig. figs. Zeros that are at the end of a number and also to the right are significant ex. 2,000.006 sig. figs. Zeros to the left of a deimal at the end of a number are significant only if they are measured to the smallest zero (insignificant zeros may be denoted by placing a bar over the numbers) + or – Takes place in columns, round the final answer to the first column from the left with a sig. fig. for both numbers. Ex. On board X or / Final answer has the same # of sig. figs. As the measurement with the least number of sig. figs. Quantities can only be added or subtracted if they have the same dimensions (units) Both sides of an algebraic equation or formula must have the same final units or it is invalid Formulas whose dimensions do not agree are invalid Abercrombie analyzed my dimensions and decide to pay me to not wear their clothes! Def.-study of the properties of chemicals and the changes they can undergo Chemical-def-any substance with a “definite composition” (it’s always made of all the same stuff) Chemical reaction-anytime a substance or substances change into one or more new substance(s) Solid-fixed volume and shape, particles are held in a rigid structure, particles vibrate Liquid-fixed volume but no fixed shape, take shape of container, move about slowly Gas-no fixed shape or volume, fill any container that they occupy Plasma-behaves like a gas whose particles have broken apart and are charged Changes to a substance where it’s identity remains the same The arrangement, location or speed of the particles may change Ex. Dissolving sugar, breaking a rock, ice melting, Chuck Norris bumping into a boulder Changes to a substance where it’s identity changes Reactants-what is consumed by a chemical reaction Products-what is created during a chemical reaction Evidence of a chemical change Evolution of a gas Formation of a precipitate Release or absorbtion of energy Color change Matter-anything that has mass and volume Volume-amount of space an object occupies Mass-amount of matter an object has Weight-force produced by gravity on an object Characteristic of a substance that can be determined without changing the nature of a substance Density-the amount of matter per unit volume Density=mass/volume or d=m/v Atoms Smallest particles that exist of each element Each atom of an element has the same number of protons in its nucleus Pure substances All of the molecules are the same 2 types: elements and compunds Pure substance whose atoms are all the same type Molecule-two or more atoms that are chemically bonded together in a definite ratio Diatomic element-an element that exists in nature as two atoms bonded together Allotrope-one of many different forms of an element Ex. Oxygen and ozone; graphite, coal and diamond Pure substances whose molecules are composed of atoms of different elements in fixed ratios Are represented by formulas Molecular formula-symbols and subscripts are used to represent elements and their ratios in each molecule of a compound Structural formula-shows how the atoms are connected in a molecule using symbols to represent atoms and lines to represent chemical bonds Ball and stick model-shows 3-D representation of molecules using different colored balls for atoms and sticks for bonds Space filling model-uses balls of different size to demonstrate the different sizes of atoms in the molecules Def.-sample of matter containing 2 or more types of pure substances Can be seperated by physical means 2 main types: homogeneous and heterogeneous Homogeneous-same composition throughout Heterogeneous-different composition throughout energy=the ability to do work (bring about a change) Physical change-altering physical properties of a substance Chemical change-creating a new substance During a physical or chemical change energy can not be created or desroyed Endothermic process-when energy is absorbed from the environment to bring about a change (feels cold) Exothermic process-when energy is released to the environment to bring about a change (feels hot) Energy can exist in many forms (heat, electricty, chemical bonds, motion, sound and light are some examples) Def-energy transferred between objects of different temperatures Temperature=measure of the average kinetic energy of a substance (how fast molecules are moving) Kinetic energy=energy possessed in the form of motion Heat and temperature are different 1 kelvin is the same size as 1 degree celsius The scale is simply moved 273 degrees up to give us a better way of expressing the temperature of a substance Degrees Celsius+273= degrees Kelvin Degrees Celsius=degrees Kelvin-273 Heat transfer can occur in 3 ways Conduction-by molecules touching Convection-by the movement of heated molecules in a fluid (liquid, gas or plasma) Radiation-movement of electromagnetic radiation Atomic theory Atoms are the building blocks of all matter Atoms are made of 3 main subatomic particles Protons-positive charge, mass=1amu Neutrons-no charge, mass=1amu Electrons-negative charge, mass=approx. 0 (barely large enough to be detected) 2 samples of the same compound have the same elements in exactly the same proportions Ex. All water molecules have 2 hydrogen and 1 oxygen, all sulfur dioxide molecules have 1 sulfur atom for every 2 oxygen atoms Matter is not created or destroyed in a chemical reaction Mass of reactants=mass of products 1. 2. 3. 4. 5. All matter is composed of atoms which can not be created, subdivided or destroyed Atoms of an element are identical in their physical and chemical properties Atoms of different elements have different chemical and physical properties Atoms of different elements combine in whole number ratios to form compounds Chemical reactions combine, separate or rearrange atoms. ATOMS ARE NEVER DESTROYED Discovered using cathode rays (TV tubes) by JJ Thompson Cathode rays are made of electrons (which are negatively charged) Thompson deflected them with a magnet Electrons have virtually no mass and negative charge Earnest Rutherford fired positively charged alpha particles at a piece of gold foil, occasionally one would be deflected back at him He theorized that the deflection occurred because of a dense positively charged nucleus It is now known that the nucleus is composed of protons and neutrons Neutrons keep the positive charge of the protons from repelling one another Def.-the number of protons located in the nucleus of an atom of a certain element Is also the number of electrons in a neutrally charged atom of a certain element Hydrogen atomic number =1 Helium atomic number=2 Def-the total average number of protons and neutrons in the nucleus of an atom Mass number-atomic number=number of neutrons Def.-atoms of the same element with different numbers of neutrons May have slightly different physical and chemical properies Chemical Chemical reaction Solid Liquid Gas Molecules Heat Specific heat Isotope Plasma Physical change Chemical change Matter Volume Homogeneous mixture Conduction protons Mass Weight Meter Liter Gram Heterogeneous mixture Convection Neutron Second Density Physical property Chemical property Atom Energy Radiation electron Element Protons Neutrons Electrons Atomic mass Atomic number He 2 2 2 4 2 N 7 8 7 15 7 V 23 29 23 52 23 Zn 30 34 30 64 30 Tc 43 56 43 99 43 Lu 71 105 71 176 71 Fr 87 137 87 224 87 Md 101 157 101 258 101 Gd 64 91 64 155 64 No 102 157 102 259 102 Sm 62 88 62 150 62 Def.-how electrons are arranged around the nucleus of an atom Electrons orbit the nucleus in a cloud Niels Bohr created a model of the atom that confines electrons to energy levels Each energy level is composed of one or more orbital (these behave like clouds of electrons) Electrons will remain as close to the nucleus of an atom as possible unless they become “excited” (this means that they have gained energy and moved away from the nucleus) When electrons become “excited” they move to a higher energy level away from the nucleus The electrons will return to their ground state closer to the nucleus when the energy is emitted as light Ground state-when an electron that is in the lowest possible energy level Excited state-when an electron has gained energy and moved away from the nucleus Q: how long would it take to spend a mole of $1 coins if they were being spent at a rate of 1 billion per second? Look at the “atomic masses” on the periodic table. What do these represent? E.g. the atomic mass of C is 12 (atomic # is 6) We know there are 6 protons and 6 neutrons Protons and neutrons have roughly the same mass. So, C weighs 12 u (atomic mass units). What is the actual mass of a C atom? Answer: approx. 2 x 10-23 grams (protons and neutrons each weigh about 1.7 x10-24 grams) Two problems 1. Atomic masses do not convert easily to grams 2. They can’t be weighed (they are too small) With these problems, why use atomic mass at all? 1. Masses give information about # of p+, n0, e– 2. It is useful to know relative mass E.g. Q - What ratio is needed to make H2O? A - 2:1 by atoms, but 2:16 by mass It is useful to associate atomic mass with a mass in grams. It has been found that 1 g H, 12 g C, or 23 g Na have 6.02 x 1023 atoms 6.02 x 1023 is a “mole” or “Avogadro’s number” “mol” is used in equations, “mole” is used in writing; one gram = 1 g, one mole = 1 mol. Q: how long would it take to spend a mole of $1 coins if they were being spent at a rate of 1 billion per second? A: $ 6.02 x 1023 / $1 000 000 000 = 6.02 x 1014 payments = 6.02 x 1014 seconds 6.02 x 1014 seconds / 60 = 1.003 x 1013 minutes 1.003 x 1013 minutes / 60 = 1.672 x 1011 hours 1.672 x 1011 hours / 24 = 6.968 x 109 days 6.968 x 109 days / 365.25 = 1.908 x 107 years A: It would take 19 million years Same 1 gram each 1 mol each No, they have dif. No, molecules volume? densities. have dif. sizes. Yes, that’s what No, molecules mass? grams are. have dif. masses No, they have dif. Yes. # of moles? molar masses No, they have dif. Yes (6.02x1023 # of in each) molecules? molar masses No, sugar has # of atoms? No more (45:3 ratio) The mass of one mole is called “molar mass” E.g. 1 mol Li = 6.94 g Li This is expressed as 6.94 g/mol What are the following molar masses? S 32.06 g/mol SO2 64.06 g/mol Cu3(BO3)2 308.27 g/mol Calculate molar masses (to 2 decimal places) CaCl2 Cu x 3 = 63.55 x 3 = 190.65 (NH4)2CO3 B x 2 = 10.81 x 2 = 21.62 O2 O x 6 = 16.00 x 6 = 96.00 308.27 Pb3(PO4)2 C6H12O6 The mass of one mole is called “molar mass” E.g. 1 mol Li = 6.94 g Li This is expressed as 6.94 g/mol What are the following molar masses? S 32.06 g/mol SO264.06 g/mol Cu3(BO3)2 308.27 g/mol Calculate molar masses (to 2 decimal places) CaCl2 110.98 g/mol (Cax1, Clx2) (NH4)2CO3 96.11 g/mol (Nx2, Hx8, Cx1, Ox3) O2 32.00 g/mol (Ox2) Pb3(PO4)2 811.54 g/mol (Pbx3, Px2, Ox8) C6H12O6 180.18 g/mol (Cx6, Hx12, Ox6) If we are given the # of grams of a compound we can determine the # of moles, & vise-versa In order to convert from one to the other you must first calculate molar mass g g = mol x g/mol mol g/mol mol = g g/mol This can be represented in an “equation triangle” Formula HCl H2SO4 NaCl Cu g/mol 36.46 98.08 58.44 63.55 g mol (n) 9.1 0.25 53.15 0.5419 207 3.55 1.27 0.0200 Equation g= g/mol x mol mol= g g/mol g= g/mol x mol mol= g g/mol Consider NaCl (ionic) vs. H2O2 (covalent) Na Cl Na Cl Cl Na Cl Na • Chemical formulas are either “simplest” (a.k.a. “empirical”) or “molecular”. Ionic compounds are always expressed as simplest formulas. • Covalent compounds can either be molecular formulas (I.e. H2O2) or simplest (e.g. HO) Q - Write simplest formulas for propene (C3H6), C2H2, glucose (C6H12O6), octane (C8H14) Q - Identify these as simplest formula, molecular formula, or both H2O, C4H10, CH, NaCl For more lessons, visit www.chalkbored.com Q - Write simplest formulas for propene (C3H6), C2H2, glucose (C6H12O6), octane (C8H14) Q - Identify these as simplest formula, molecular formula, or both H2O, C4H10, CH, NaCl A - CH2 CH CH2O C 4H 7 A - H2O is both simplest and molecular C4H10 is molecular (C2H5 would be simplest) CH is simplest (not molecular since CH can’t form a molecule - recall Lewis diagrams) NaCl is simplest (it’s ionic, thus it doesn’t form molecules; it has no molecular formula)