* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Lab 6
Electrical substation wikipedia , lookup
Control system wikipedia , lookup
Scattering parameters wikipedia , lookup
Current source wikipedia , lookup
Negative feedback wikipedia , lookup
Variable-frequency drive wikipedia , lookup
Flip-flop (electronics) wikipedia , lookup
Stray voltage wikipedia , lookup
Power inverter wikipedia , lookup
Pulse-width modulation wikipedia , lookup
Alternating current wikipedia , lookup
Voltage optimisation wikipedia , lookup
Zobel network wikipedia , lookup
Wien bridge oscillator wikipedia , lookup
Two-port network wikipedia , lookup
Voltage regulator wikipedia , lookup
Integrating ADC wikipedia , lookup
Analog-to-digital converter wikipedia , lookup
Mains electricity wikipedia , lookup
Power electronics wikipedia , lookup
Buck converter wikipedia , lookup
Oscilloscope history wikipedia , lookup
Regenerative circuit wikipedia , lookup
Resistive opto-isolator wikipedia , lookup
Switched-mode power supply wikipedia , lookup
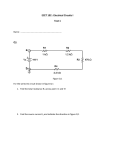
Laboratory Exercise 6 – Op. Amps 2 – Useful Op Amp Circuits You’ve learned enough about analog electronics at this point to make some fairly realistic chemical measurement circuits. We will start with one of the most common chemical measurements, the pH of an aqueous solution. pH Meter Amplifier The potential (voltage) of a glass electrode (measured versus a reference electrode) follows the equation E = C - 2.303 (RT/F) log aH+ = C + 0.0592 V * pH (at 298 K) where C is a constant, aH+ is the activity of the hydronium ion (often taken to be equal to the molar concentration [H+]), and the calibration sensitivity (slope of voltage vs. pH) is often quoted as 59.2 (or 60) mV/pH unit. Both the constant and the slope of the response are expected to depend on temperature. Clearly, pH measurement requires calibration, as do most instrumental measurements. There are good descriptions of the glass electrode and the associated reference electrodes in most analytical texts. Note that the sensitivity of the glass electrode (and all other potentiometric measurements) is low: 59 mV per unit pH change (a factor of 10 change in concentration). This is an example of a signal that would benefit from amplification, if we can maintain reasonable signal to noise and not distort the response. An amplifier for this purpose must have a very high input impedance, optimally greater than or equal to 1010 (10 G) because the glass electrode has an output impedance of about 108 . It would be nice if the amplifier gain was adjustable so that each unit of pH change (59.2 mV) was indicated as one unit on the readout, for example, one volt. An offset control for calibration of the zero point would also help to make the output indicate the pH of the solution. Concept Question 1 – If the voltage coming from a glass electrode was monitored using an oscilloscope with a 1 M input impedance, how much smaller would the observed signal be? (Treat it as a voltage divider where the voltage to be measured is applied across the 100 M of the pH electrode and the scope resistance is after that.) Circuit Exercise 1 – Breadboard the circuit below using the high impedance 3140 op amp for the input stage (from the pH meter) and a 741 for additional gain and offset. The output, when calibrated, will be one volt per pH unit. In this circuit potentiometer Pl adjusts the offset and P2 sets the slope. It is hard to get this circuit to work well, because the high output impedance of the glass electrode and the high gain tends to “pick up” external fields. It may help to use some kind of shielding around the connections and to stand back from the circuit for a few minutes while the output stabilizes for each solution. Concept Question 2 – Referring to the schematic, what type of amplifier (from last lab) is the 3140 used in? And what type of amplifier is the 741 used for? Before applying power, connect the electrode(s) to the circuit, immerse in a pH = 7 buffer, and set the DMM for the 20 V range. Activate the power supply and adjust Pl for a reading of 7.0 ±0.1 V on the DMM (P2 should be at about mid-range). Rinse the electrodes in water and immerse them in a buffer of pH = 4, adjust P2 for a reading of 4.0 ±0.1 V. Repeat the calibration at least once. Measure the pH of the pH 4, 7, and 10 buffers and record below. Comment on the reliability of the three measurements. Measure the pH of tap water and record below. If you wanted to create an auto-titrator, you would probably read the voltage with a PMD and feed the output directly into a graphical display. Would this work? (Explain your answer.) What else would you need to complete the auto-titrator? (Qualitatively – you don’t have to know how to build one yet.) Active Band Pass Filter/ Photometer When we worked with RC filters earlier, we noted that it would be possible to make better filters using active elements. The next circuit uses an op amp to produce a filter with characteristics that are superior to those made with only passive elements. The combination of RC filters (more than one) and an op amp shown below has high gain over a narrow frequency range, with small gain elsewhere. It is called a band pass filter and can be used to focus on signals with a particular frequency (often one that we control). We’ll use it with a photo cell to build a photodetector that selectively measures one light source, while ignoring any other light sources nearby. If we are successful at that, we’ll build a rudimentary absorption spectrometer and see how well it works. Circuit Exercise 2 – Set up the band pass filter circuit shown below and drive it with the function generator to characterize it. We’ll hook up the photocell and source after we’re confident that this piece works. Record the input and output voltages (peak to peak) for sine waves over the following frequency range. Input amplitude should be about 1 Vpp. Also note the phase shift in the usual way, although you may want to characterize the shift as a lead or lag (i.e., is the output ahead of or behind the input) instead of as a full 360 shift. Frequency 400 Hz 600 Hz 800 Hz 1 kHz 1.1 kHz 1.2 kHz 1.3 kHz 1.4 kHz 1.8 kHz 3 kHz lnput/volts Output/volts Lead/Lag Phase Locate the frequency (f0) that gives the maximum output voltage. Estimate the Quality factor Q for the filter. Q = f0/f, where f0 is the center frequency and f is the frequency difference between the –3 db points on either side (approximately the FWHM.) Take a guess at the changes you would have to make to get a filter with a pass band centered at 100 Hz. (If you’ve got time, you might try it out.) Power one of the monolithic LEDs (any color) with the function generator. Try a 100 Ω current limiting resistor in series with the LED. If that isn’t bright enough, try a smaller resistor. That will be the source of your photometer. Put a photocell in series with a resistor of about the same value (make a light-dependent ½ voltage divider) and power it with the 5 VDC power supply. This is the detector. Point the photocell at the LED, leaving enough space between for a 1 cm cuvette. Try to monitor the output of the detector directly with the scope (you should monitor the output of the FG that’s driving the LED with the other probe and use this to trigger the scope). Describe what you see. Connect the output of the detector circuit to the bandpass filter and monitor the output of the bandpass circuit with the scope. Assuming that you can see the modulated signal, verify that there is a dependence of the output signal magnitude on the modulation frequency. Maximize the signal. If you can detect something that you think is proportional to light before the bandpass filter, measure that too. Describe the waveform you see after the bandpass filter. Obtain a cuvette (plastic is fine) and a solution that you think will absorb at the wavelength of your LED. Acid-base indicators or food colors both work, since they come in a variety of colors and absorb strongly. Keep in mind the complementarity of color and absorption. Construct a calibration curve of absorbance vs. concentration. This system is probably going to be very alignment sensitive, so the best way to do this might be to use double sided tape to hold the cuvette rigidly in place, and then use a Pasteur pipette to fill and empty liquid from the cuvette. Don’t forget to measure a blank (water only) and the signal with no light (if there is one). What part of the waveform are you measuring? What is proportional to light intensity at the detector? Show your calibration curve below. Don’t forget to include the slope, intercept, and correlation coefficient. If you measured the unfiltered signal, show the curve for that too. Comment on the values of the slope, intercept, and correlation coefficient (linearity!) for the graph(s). Why doesn’t this “spectrometer” require a monochromator? (These instruments are sometimes called photometers.) Why doesn’t this photometer require a light-tight box like most of them have? (Assuming it worked without one.) Bridging the Analog/Digital Divide - The Comparator Now that we have started to think about the “land of digital”, we’ll also introduce you to a circuit that derives a simple digital response from an analog signal, the comparator. A comparator gives a yes or no response to the question “is an input voltage smaller than a provided reference voltage?” Specialized comparators are better than the op amp analogs that we will build, but their principle of operation is the same. The 311 (which we’ll see in the next exercise) is a popular comparator chip that has a high impedance op amp front end (input) and an open collector bipolar transistor back end (output). This allows the input to trigger any output voltage that you want, consistent with a standard bipolar transistor switch circuit. In a comparator, the voltage input is connected directly to the inverting input of an op amp, while the reference voltage is connected to the non-inverting input. No feedback loop is provided. Assume that the input voltage is bigger than the reference voltage. From the golden rules you know that the output will do everything in its power to try to make the voltages at the two inputs equal, usually by trying to supply negative voltage to the inverting input. In this case, it is unable to make any changes to the inputs because of the lack of feedback. Frustrated, the output swings to the negative rail (and hopefully stays there) and the -15 V is the answer to our question, no. Now assume that the voltage at the inverting input drops below that at the non-inverting input, at which time the output swings to the other rail: + 15 V = yes. That’s how digital works – only two voltages allowed, one is called “yes” or 1 and the other is called “no” or 0. The problem with using the comparator is, “what if the two voltages are very close to one another and are both pretty noisy?” The op amp’s output suffers from indecision and tends to go out of control, oscillating rapidly from one rail to the other. This is a bad thing, so the comparator is one of the few cases you will see where we will use positive feedback (a feedback loop to the non-inverting input). What you end up with is the op amp deciding that it needs to respond and in responding it will tend to aggravate the situation, moving the “low” input even lower (or vice versa) accelerating the circuit towards the correct decision. Circuit Exercise 4 – Breadboard the simple comparator below and drive it with the function generator using a sine wave that crosses ground (the reference voltage). This circuit doesn’t benefit from positive feedback, but it usually works just fine. We could call this a zero-crossing detector. Rationalize the output pattern as I did above. (What is the output “trying” to do.) Do you see any “funny” behavior at the edges of the square wave? (Try changing the frequency a bit to see if you can induce oscillations). Usually you can’t. The reason we still use 741s in this lab is that they are really stable, perhaps bordering on sluggish. It’s hard to get them to go fast, but it’s equally hard to get them to “freak out” on fast changes. If you want to see the “funny” behavior, you can try a faster op amp, like the 3140 or the 355, we have those in the lab. If you do, describe what you got and what you did to get it. Now add the following resistors to the circuit to provide positive feedback. Are the transitions sharper? Are they at the same voltages as before? Again walk through the analysis of this circuit, describing what the output is “trying” to do in response to different values of the input. Think about what determines the reference value. (Hint: it is easiest to start with a presumed value of the input and follow that result through the circuit, and then let that value change). This is a tricky one – ask for help if you’re struggling. This circuit makes use a concept called hysteresis, which we will see again when we talk about the Schmitt trigger in the digital section. In this context, hysteresis just means that where the thing switches in one signal direction (inverting input coming down with respect to noninverting) is different from where it switches in the other direction. This helps make the system more immune to noise. Real World Example This lab has been all about real world examples, so we don’t need to use our imagination to come up with one. Which circuits were DC measurements and which were inherently time dependent? What other characteristics can you identify about the signal sources and the nature of the output for the various circuits? Often the first step to optimizing an instrument is thinking carefully about the characteristics of the signal itself. Revised 6/12/13 DBA