* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Lecture Notes Part A
Survey
Document related concepts
Chemistry: A Volatile History wikipedia , lookup
Electronegativity wikipedia , lookup
Molecular orbital diagram wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Resonance (chemistry) wikipedia , lookup
Atomic nucleus wikipedia , lookup
Metallic bonding wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Electron configuration wikipedia , lookup
History of molecular theory wikipedia , lookup
Transcript
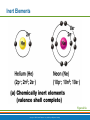
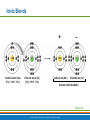
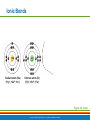
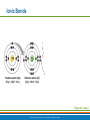
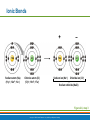
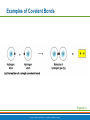
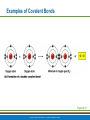
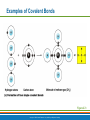
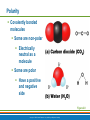
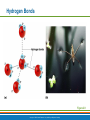
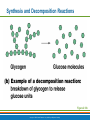
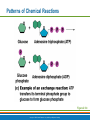
PowerPoint® Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College Basic Chemistry 2 PART A Copyright © 2009 Pearson Education, Inc., publishing as Benjamin Cummings Matter and Energy Matter—anything that occupies space and has mass (weight) Energy—the ability to do work Chemical Electrical Mechanical Radiant Copyright © 2009 Pearson Education, Inc., publishing as Benjamin Cummings Composition of Matter Elements—fundamental units of matter 96% of the body is made from four elements Carbon (C) Oxygen (O) Hydrogen (H) Nitrogen (N) Atoms—building blocks of elements Copyright © 2009 Pearson Education, Inc., publishing as Benjamin Cummings Atomic Structure Nucleus Protons (p+) Neutrons (n0) Outside of nucleus Electrons (e-) Figure 2.1 Copyright © 2009 Pearson Education, Inc., publishing as Benjamin Cummings Atomic Structure of Smallest Atoms Figure 2.2 Copyright © 2009 Pearson Education, Inc., publishing as Benjamin Cummings Identifying Elements Atomic number—equal to the number of protons that the atom contains Atomic mass number—sum of the protons and neutrons Copyright © 2009 Pearson Education, Inc., publishing as Benjamin Cummings Isotopes and Atomic Weight Isotopes Have the same number of protons Vary in number of neutrons Figure 2.3 Copyright © 2009 Pearson Education, Inc., publishing as Benjamin Cummings Isotopes and Atomic Weight Atomic weight Close to mass number of most abundant isotope Atomic weight reflects natural isotope variation Copyright © 2009 Pearson Education, Inc., publishing as Benjamin Cummings Radioactivity Radioisotope Heavy isotope Tends to be unstable Decomposes to more stable isotope Radioactivity—process of spontaneous atomic decay Copyright © 2009 Pearson Education, Inc., publishing as Benjamin Cummings Molecules and Compounds Molecule—two or more like atoms combined chemically Compound—two or more different atoms combined chemically Figure 2.4 Copyright © 2009 Pearson Education, Inc., publishing as Benjamin Cummings Chemical Reactions Atoms are united by chemical bonds Atoms dissociate from other atoms when chemical bonds are broken Copyright © 2009 Pearson Education, Inc., publishing as Benjamin Cummings Electrons and Bonding Electrons occupy energy levels called electron shells Electrons closest to the nucleus are most strongly attracted Each shell has distinct properties The number of electrons has an upper limit Shells closest to the nucleus fill first Copyright © 2009 Pearson Education, Inc., publishing as Benjamin Cummings Electrons and Bonding Bonding involves interactions between electrons in the outer shell (valence shell) Full valence shells do not form bonds Copyright © 2009 Pearson Education, Inc., publishing as Benjamin Cummings Inert Elements Atoms are stable (inert) when the outermost shell is complete How to fill the atom’s shells Shell 1 can hold a maximum of 2 electrons Shell 2 can hold a maximum of 8 electrons Shell 3 can hold a maximum of 18 electrons Copyright © 2009 Pearson Education, Inc., publishing as Benjamin Cummings Inert Elements Atoms will gain, lose, or share electrons to complete their outermost orbitals and reach a stable state Rule of eights Atoms are considered stable when their outermost orbital has 8 electrons The exception to this rule of eights is Shell 1, which can only hold 2 electrons Copyright © 2009 Pearson Education, Inc., publishing as Benjamin Cummings Inert Elements Figure 2.5a Copyright © 2009 Pearson Education, Inc., publishing as Benjamin Cummings Reactive Elements Valence shells are not full and are unstable Tend to gain, lose, or share electrons Allow for bond formation, which produces stable valence Figure 2.5b Copyright © 2009 Pearson Education, Inc., publishing as Benjamin Cummings Chemical Bonds Ionic bonds Form when electrons are completely transferred from one atom to another Ions Charged particles Anions are negative Cations are positive Either donate or accept electrons Copyright © 2009 Pearson Education, Inc., publishing as Benjamin Cummings Ionic Bonds Na Cl Sodium atom (Na) (11p+; 12n0; 11e–) Chlorine atom (Cl) (17p+; 18n0; 17e–) + – Na Cl Sodium ion (Na+) Chloride ion (Cl–) Sodium chloride (NaCl) Figure 2.6 Copyright © 2009 Pearson Education, Inc., publishing as Benjamin Cummings Ionic Bonds Na Cl Sodium atom (Na) (11p+; 12n0; 11e–) Chlorine atom (Cl) (17p+; 18n0; 17e–) Figure 2.6, step 1 Copyright © 2009 Pearson Education, Inc., publishing as Benjamin Cummings Ionic Bonds Na Cl Sodium atom (Na) (11p+; 12n0; 11e–) Chlorine atom (Cl) (17p+; 18n0; 17e–) Figure 2.6, step 2 Copyright © 2009 Pearson Education, Inc., publishing as Benjamin Cummings Ionic Bonds Na Cl Sodium atom (Na) (11p+; 12n0; 11e–) Chlorine atom (Cl) (17p+; 18n0; 17e–) + – Na Cl Sodium ion (Na+) Chloride ion (Cl–) Sodium chloride (NaCl) Figure 2.6, step 3 Copyright © 2009 Pearson Education, Inc., publishing as Benjamin Cummings Chemical Bonds Covalent bonds Atoms become stable through shared electrons Single covalent bonds share one pair of electrons Double covalent bonds share two pairs of electrons Copyright © 2009 Pearson Education, Inc., publishing as Benjamin Cummings Examples of Covalent Bonds Figure 2.7a Copyright © 2009 Pearson Education, Inc., publishing as Benjamin Cummings Examples of Covalent Bonds Figure 2.7b Copyright © 2009 Pearson Education, Inc., publishing as Benjamin Cummings Examples of Covalent Bonds Figure 2.7c Copyright © 2009 Pearson Education, Inc., publishing as Benjamin Cummings Cation: Anion: Copyright © 2009 Pearson Education, Inc., publishing as Benjamin Cummings Polarity Covalently bonded molecules Some are non-polar Electrically neutral as a molecule Some are polar Have a positive and negative side Figure 2.8 Copyright © 2009 Pearson Education, Inc., publishing as Benjamin Cummings Chemical Bonds Nonpolar Covalent: electrons shared equally between these elements Polar Covalent: electrons not shared equally Hydrogen bonds Weak chemical bonds Hydrogen is attracted to the negative portion of polar molecule Provides attraction between molecules Copyright © 2009 Pearson Education, Inc., publishing as Benjamin Cummings Hydrogen Bonds Figure 2.9 Copyright © 2009 Pearson Education, Inc., publishing as Benjamin Cummings Patterns of Chemical Reactions Synthesis reaction (A + BAB) Atoms or molecules combine Energy is absorbed for bond formation Decomposition reaction (ABA + B) Molecule is broken down Chemical energy is released Copyright © 2009 Pearson Education, Inc., publishing as Benjamin Cummings Synthesis and Decomposition Reactions Figure 2.10a Copyright © 2009 Pearson Education, Inc., publishing as Benjamin Cummings Synthesis and Decomposition Reactions Figure 2.10b Copyright © 2009 Pearson Education, Inc., publishing as Benjamin Cummings Patterns of Chemical Reactions Exchange reaction (AB + CAC + B) Involves both synthesis and decomposition reactions Switch is made between molecule parts and different molecules are made Copyright © 2009 Pearson Education, Inc., publishing as Benjamin Cummings Patterns of Chemical Reactions Figure 2.10c Copyright © 2009 Pearson Education, Inc., publishing as Benjamin Cummings