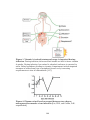
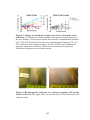
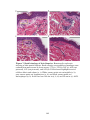
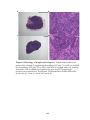
* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download The Innate and Adaptive Immune Response to
Survey
Document related concepts
Common cold wikipedia , lookup
Adaptive immune system wikipedia , lookup
Hygiene hypothesis wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Neonatal infection wikipedia , lookup
Psychoneuroimmunology wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
DNA vaccination wikipedia , lookup
Globalization and disease wikipedia , lookup
Adoptive cell transfer wikipedia , lookup
Childhood immunizations in the United States wikipedia , lookup
Molecular mimicry wikipedia , lookup
Innate immune system wikipedia , lookup
Human cytomegalovirus wikipedia , lookup
West Nile fever wikipedia , lookup
Marburg virus disease wikipedia , lookup
Transcript
The Innate and Adaptive Immune Response to Measles Virus By: Nicole Putnam A thesis submitted to Johns Hopkins University in conformity with the requirements for the degree of Master of Science. Baltimore, Maryland April 2014 © Nicole Putnam All Rights Reserved i Abstract Measles is one of the most important causes of childhood morbidity and mortality worldwide. Although a vaccine is available, the high transmission rate of measles virus requires population of 95% to interrupt its transmission. The World Health Organization and the United Nations Children’s Fund recommend that children that develop measles receive vitamin A supplementation, as a safe, cheap, and efficacious way to reduce the burden of disease. Due to differences between strains and confounding data of measles stocks contaminated with defective interfering RNA particles, the immune response to measles virus infection has not been well defined. Furthermore, the mechanism by which vitamin A protects against severe measles-induced disease is unknown. In this thesis, I investigate the innate and adaptive immune response to measles virus infection. Measles virus strains were purified of defective interfering RNA particles and used for in vitro infections of monocyte derived dendritic cells. Gene expression changes of interferon-stimulated genes and viral stress-induced genes, IFIT1 and Mx1, were upregulated in response to infection with the Edmonston measles virus vaccine strain, as well as the wild-type strains of Bilthoven, IC-B, and C- and V-protein knockout strains, as compared to mock infected cells. Unexpectedly, there were no differences between transcript levels of these genes between C and V protein knockout strains and the respective wild-type infection. Additionally, the absence of ii type I interferon production supports the theory that measles virus induces the transcription of these genes through the viral stress-induced pathway, and not the interferon-stimulated pathway. While a previous study had detected measles virus-specific IL-17producing T cells in measles virus-infected rhesus macaques, the Th17 response to measles virus has not been characterized. Th17 cell differentiation was inhibited early after measles virus infection in vitro. There was a significant decrease in IL-23A transcript ts and a significant increase in IL-27 transcripts, both of which affect Th17 cell differentiation negatively. However, in a rhesus macaque model of infection, a biphasic Th17 response was observed with peaks at days 18 and 56. The effects of vitamin A supplementation following measles virus infection on the immune response was explored in a rhesus macaque model using supplemented and non-supplemented groups. While some data has yet to be explored, major differences were not observed between the two groups up to three months following infection, in regards to clearance of infectious virus, immune cell composition, or immune cell function. Archived data will elucidate the role of vitamin A in measles virus RNA persistence, and Th1 and T follicular helper cell responses. Data will continue to be analyzed out to six months post infection. A larger cohort will be necessary to elucidate the role of vitamin A in protection against severe disease and death due to measles. iii Acknowledgements First and foremost, I would like thank my advisor, Dr. Diane E. Griffin, for allowing me to do my master’s research in her laboratory. Her guidance and support was invaluable throughout my time here. I would like to thank her for the opportunity to get involved in the dynamic, challenging, and rewarding research that she had entrusted with me. Furthermore, a huge thank you to Dr. Rupak Shivakoti for passing down his knowledge of the basics of how to work with measles virus and acquainting me with Dr. Griffin’s lab in general. Additional thanks go out to Rupak to teaching me many, many techniques. Although he was available to ask questions while he was here, it was helpful that he encouraged me to ―jump right in‖ and conduct my experiments independently early on. I would like to also thank Rupak for being responsive to questions much after he had graduated from the laboratory, which was especially helpful. I would like to thank Dr. Wendy Lin, for providing me with her knowledge of the logistics of working with measles virus in rhesus macaques. Her ability to pass down her understandings and techniques was invaluable. Furthermore, I would like to thank Wendy for taking time from her career at Columbia University to come down to Baltimore to meet with us, as well as making herself available to talk about techniques or data analysis. Importantly, this project would not have run as smoothly as it did without the help of Ashley Nelson, the PhD student with whom I shared iv responsibility in this project. With Ashley’s flexibility to work around my schedule, we were able to make sure the assays for the monkey study could be completed and analyzed in a timely manner so I could complete my thesis work. I would also like to thank Ashley for her support and friendship throughout my time here! The vitamin A/monkey study was largely a success due to the time and effort of Dr. Bob Adams and Dr. Tori Baxter. I would like to thank them immensely for their time and expertise in handling the monkeys, obtaining samples, and for being flexible with their schedules around the holidays, while also granting this project many of their early mornings. I would like to give a huge thanks to my roommate, Dr. Cailin Deal, who was able to provide me her knowledge and skills in so many areas of virology and immunology as a whole. Her expertise in writing in science was crucial to the process of editing my thesis, as well as her general knowledge of techniques and data analysis. Furthermore, I would like to thank Debbie Hauer for her assistance in teaching me techniques and processing samples that were essential for my projects and this thesis. I would like to extend my warmest thanks to the rest of Dr. Griffin’s laboratory for being so welcoming to me as a master’s student, for passing down their expertise and insight when I needed assistance, and for their general support and friendship. Dr. Kim Shulz, Dr. Tori Baxter, Dr. Kirsten Kulscar, Stephen Goldstein and Siva Manivannan, your help was v instrumental towards my experience as a student in this laboratory. Additionally, I would like to acknowledge Gui Nilaratanakul, Rachy Abraham, and Nina Martin for their presence and livelihood in the lab. Finally, I would like to thank my mother, father, and brother Ryan, as well as my extended family and friends for providing their unwavering support. As a little girl, my parents told me I could do whatever I put my mind to, and when I decided to pursue a science and research their enthusiasm was there to match my own. This thesis is the product of the hard work and support of many people, and I would again like to extend a tremendous thanks to all of the people by my side! vi Table of Contents Abstract………………………………………………………………………………. ii Acknowledgements……………………………………………………………….. iv List of Tables……………………………………………………………………...... ix List of Figures………………………………………………………………………. x Chapter 1: Introduction to measles virus…………………………………… 1 Public health implications…………………………………………………... 2 Measles virus pathogenesis…………………………………………………. 2 Prevention of measles virus infection……………………………………... 3 Measles virus virology……………………………………………………….. 6 Measles virus infection………………………………………………………. 7 Defective replication of measles virus genome…………………………… 8 Innate immune response to viral infection……………………………….. 9 TLRs…………………………………………………………………….. 9 Cytoplasmic PRRs…………………………………………………… 10 Type I interferon…………………………………………………….. 11 Interferon-inducible antiviral proteins…………………………... 12 Innate immune response to measles virus infection…………………… 12 Role of dendritic cells……………………………………………….. 14 Adaptive immune response to measles virus infection………………... 14 Antibody response…………………………………………………… 15 T lymphocyte response……………………………………………… 16 Effector CD4+ T lymphocytes……………………………………… 16 Immunosuppression following measles virus infection……………….. 18 Figures………………………………………………………………………… 20 Chapter 2: Comparision of in vitro immune responses to wild-type measles virus with C and V protein-knock out strains; wild-type and vaccine strains of measles virus……………………………………………… 24 Introduction…………………….……………………………………………. 25 Measles virus immune evasion……………………………………………. 25 Block of type I interferon production…………………………………….. 25 Block of type I interferon signaling………………………………………. 27 Interferon-stimulated genes (ISGs) and virus stress-induced genes (VSIGs)……………………………………………………..…………. 28 Role of dendritic cells in measles virus infection……………………….. 29 Defective interfering (DI) particles……………………………………….. 29 Th17 response to viral infection…………………………………………... 30 Th17 response to measles virus infection……………………………….. 30 Materials and methods……………………………………………………... 31 Results………………………………………………………………………… 38 Discussion…………………………………………………………………….. 46 Tables…………………………………………………………………………. 53 Figures………………………………………………………………………... 54 vii Chapter 3: Effects of vitamin A supplementation on the immune response and the Th17 response to measles virus infection in rhesus macaques……………………………………………………………………………. 63 Introduction………………………………………………………………….. 64 Vitamin A and measles infection…………………………………………. 64 Role of vitamin A in CD4+ T cell differentiation……………………….. 66 Vitamin A supplementation……………………………………………….. 67 Vitamin A: Potential roles in improving measles outcome………..….. 69 Repair of lung epithelium………………………………………….. 69 Effect on lymphopenia and T cell-mediated viral clearance….. 69 Inhibition of viral replication……………………………………… 70 Enhanced antibody response……………………….……………… 71 Th17 response to measles infection………………………………………. 71 Materials and methods……………………………………………………... 72 Results………………………………………………………………………… 81 Discussion…………………………………………………………………….. 91 Tables…………………………………………………………………………. 98 Figures………………………………………………………………………. 100 Chapter 4: Discussion of the immune responses to measles virus infection in vitro and in vivo………………………………………………… 120 The innate immune response to measles virus infection……………. 121 Type I interferon…………………………………………………… 121 The role of measles virus and its C and V proteins on interferonstimulated genes (ISGs) and virus stress-induced genes (VSIGs)………………………………………………………………. 122 The early adaptive immune response to measles virus infection…... 122 Th17 regulatory cytokine expression to measles virus infection…… 123 The Th17 response to measles virus infection………………………… 124 The measles virus antibody response…………………………………… 124 The role of vitamin A on the immune response to measles virus infection……………………………………………………………………… 125 References……………………………………………………………………….... 127 Curriculum vitae……………………………………………………………....... 144 viii List of Tables Chapter 2: Table 1: PCR primers, targets, and cycling conditions for P gene sequencing and detection of measles virus standard and defective genomes……………. 53 Chapter 3: Table 1: PCR primers, targets, and cycling conditions for detection of the measles virus N gene.…………….…………….…………….…………….………. 98 Table 2: Measles virus shedding in respiratory secretions…….…………….. 98 Table 3: IL-17A ELISAs…….……….…….……….…….……….…….………… 99 ix List of Figures Chapter 1: Figure 1: Measles virus infection and pathogenesis…………..……………… 20 Figure 2: 2012 immunization coverage rates with measles-containing vaccines in infants……….………………………………………………………….. 21 Figure 3: Measles virus structure, RNA genome, and replication………..… 21 Figure 4: Defective interfering particle formation from the measles virus genome……………………………………………………………………………...… 22 Figure 5: Biopsies of the measles virus rash show CD4+ and CD8+ lymphocyte infiltration……………………………………………………………... 22 Figure 6: Measles virus RNA over the course of infection………………..…. 23 Figure 7: Potential mechanisms leading to measles virus-induced immune suppression…………………………………………………………………………... 23 Chapter 2: Figure 1: Signaling pathways leading to virus stress-inducible gene (VSIG) induction…………………………………………………………………………….... 54 Figure 2: Generation of wild-type measles virus defective for the C or V protein……………………………………………………………………………….... 55 Figure 3: Primer binding sites for sequencing……………………………….... 55 Figure 4: Sequencing confirms correct viral sequences. …………………..… 56 Figure 5: Gel of measles virus stocks. ………………………………………..… 57 Figure 6: Measles virus standard and DI genome PCR products of measles virus stocks used in in vitro experiments. ……………………………………… 58 Figure 7: mRNAs for interferon-stimulated genes, IFIT1 and Mx1, are upregulated in moDCs by the Edmonston measles virus vaccine strain and Bilt Wt strain at MOIs of 0.4 and 4.0…………………………………………..… 59 Figure 8: mRNAs for interferon-stimulated genes, IFIT1 and Mx1, are comparably upregulated moDCs in response to Wt measles virus, and its respective C- and V-protein KO strains at MOIs of 0.01 and 0.1………….… 59 Figure 9: Positive regulators of Th17 cell differentiation, IL-1β, IL-23A and IL-6, mRNA expression levels from moDCs in response to Edmonston and Bilthoven measles virus infection at MOIs of 0.4 and 4.0………………….…. 60 Figure 10: Positive regulators of Th17 cell differentiation, IL-1β and IL-6, mRNA expression levels from moDCs in response to wild-type measles virus and its respective C- and V-protein knock out strains at MOIs of 0.01 and 0.1…………………………………………………………………………………….... 61 Figure 11: Negative regulators of Th17 cell differentiation, IL-27 and IL-10, mRNA expression levels from moDCs in response to Edmonston and Bilthoven measles virus infection at MOIs of 0.4 and 4.0..…………………… 61 x Figure 12: Negative regulators of Th17 cell differentiation, IL-27 and IL-10, mRNA expression levels from moDCs in response to wild-type measles virus and its respective C- and V-protein knock out strains at MOIs of 0.01 and 0.1…………………………………………………………………………………….... 62 Chapter 3: Figure 1: Vitamin A (retinol) status and usage is impaired during infection……………………………………………………………………………… 100 Figure 2: Plasma retinol levels averaged between two rhesus macaques after measles infection.………………………………………………………………..… 100 Figure 3: Time course of measles virus clearance…………………………… 101 Figure 4: Viremia is present by day 7, and is cleared in all animals by day 18. ………………………………………………………………………………….... 101 Figure 5: Change in total body weight over course of measles virus infection.……………………………………………………………………….……. 102 Figure 6: Maculopapular rash was very robust on monkey 50Y on day 10 post-infection……………………………………………………………………….. 102 Figure 7: Rash histology of skin biopsies……………………………………... 103 Figure 8: Histology of lymph node biopsies…………………………………… 104 Figure 9: Vitamin A levels begin to drop at day 21 in the non-supplemented group of monkeys (17Y, 31Y, 46Y) but remain stable in vitamin Asupplemented monkeys (14Y, 24Y, 50Y). ……………………………………… 105 Figure 10: Comprehensive blood counts and differential leukocyte counts following rash.…………………………………………………………………….... 106 Figure 11: Frequency of CD4+ and CD8+ cells within the CD14-CD20- live cell population, and CD4:CD8 cell ratio……………………………………...… 107 Figure 12: Measles virus H, N, and F protein-specific IFN-γ secreting T cells peak at 21 days post-infection………………………………………………….... 108 Figure 13: Intracellular staining for IL-17A……..…………………………... 109 Figure 14: Intracellular staining for IL-21…………………………………… 110 Figure 15: Frequency of IL-17+ cells as a percentage of total CD4+ cells peaked at day 18…………………………………………………………………... 111 Figure 16: Frequency of IL-21+ cells as a percentage of total CD4+ T cells showed peaks at day 18 and day 39 post-infection…………………………… 112 Figure 17: RORγt expression was upregulated in CD4+ T cells by day 18 post-infection, and is higher in IL-17+ cells than IL-17- cells………………. 113 Figure 18: IL-21 production begins to increase by day 18, and is much greater by day 56 post-infection in IL-17+ cells than IL-17- cells…..……… 114 Figure 19: IL-17A-secreting T cells are present in a biphasic response, with an early peak at day 14 and a late peak at day 52.…………………….…..… 115 Figure 20: Measles virus-specific IgG as detected by ELISA………….…... 116 Figure 21: Total antibody- and measles virus-specific antibody-secreting cells in PBMCs as detected by ASC assays………………………..…….….…. 117 xi Figure 22: Total antibody- and measles virus-specific antibody-secreting cells in BM as detected by ASC assays…………………………………………. 118 Figure 23: Neutralizing antibody response as detected by PRNTs……….. 119 xii Chapter One: Introduction to Measles Virus 1 Public health implications Measles virus is one of the most important causes of childhood morbidity and mortality throughout the world (1, 2, 3, 4). Mortality can result from complications due to young age (9), viral dose as a result of overcrowding (10) and immunosuppression (11). Additionally, mortality due to measles can be further increased by malnutrition (12) and hyporetinolemia (13). Death due to acute measles virus infection is most common in young children, and is most often attributed to viral and bacterial secondary infections that are acquired during a stage of measles-induced immune suppression (1). Measles virus pathogenesis Measles virus infects cells in the respiratory mucosa, such as epithelial cells, dendritic cells and macrophages (61, 62, 63). Infected immune cells then traffic to local lymph nodes, where measles virus can establish a viremia and disseminate into the blood stream through measles virus-infected cells (62). From the blood stream, measles virus is spread systemically to various tissues that are subsequently infected (Figure 1a). The incubation period of measles lasts for approximately ten days. This is followed by a prodromal phase characterized by generalized fever, cough, coryza, conjunctivitis (7). Subsequent development of a maculopapular rash on the trunk and limbs of the body can be used to clinically diagnose measles (7). Clinical symptoms begin to develop after the systemic spread of 2 measles virus, and correspond to the development of adaptive immune responses to the virus (Figure 1b, 1c). During the prodrome and rash, measles virus can spread from the infected host to susceptible individuals through direct transmission (7). This allows measles virus to be spread in a susceptible population before measures are taken to prevent transmission. Measles virus is a human virus that is not found in any reservoir populations and is maintained in the population by an unbroken chain of acute infections, as latent or persistent measles virus infections do not have the ability to be spread to new hosts (6). Before the development of a measles vaccine, measles was estimated to claim between 5-8 millions deaths annually (6,7). Recently, measles deaths globally have decreased to 158,000 in 2011, down 71% from 2000 (8). A safe, efficacious measles virus vaccine is available, but it has proven difficult to reach a high proportion of children in developing countries. In 2004 alone, almost half of the estimated measles deaths were in sub-Saharan Africa (7). Measles virus is one of the most highly contagious infectious agents, with outbreaks occurring even in populations where only 10% of individuals are susceptible (6). Prevention of measles virus infection Prevention of measles virus infection is important, because of the significant case fatality rates. In different locations in Africa, case fatality rates due to measles can range between 5-10% (3, 14). Vaccination against 3 measles virus infection as introduced in the 1960s, in the forms of attenuated and killed vaccines (6). The killed virus vaccine was associated with complications and withdrawn. Currently, safe and efficacious live attenuated vaccines are available either as a measles-only vaccine or coupled with other vaccine viruses, such as mumps and rubella (MMR) (1, 7). Most measles vaccines currently in use have been derived from the Edmonston strain of measles virus that was isolated in 1954 by Enders and Peebles (7,19). Despite variations in attenuation of vaccines and sequence differences among wild-type measles virus strains, measles virus is an antigenically stable virus with only one serotype (60). Though vaccination is an important method of prevention, interruption of measles transmission in a population requires that approximately 95% of the population is immune (7,15). Notably, that doesn’t guarantee protection. Once measles virus transmission has been halted in a geographical area, introduction of measles virus and outbreaks can still be imported via an infected individual (7). In 2007 in Quebec, Canada, population immunity was estimated to be at 95% when an outbreak leading to 94 measles cases occurred, when several groups of unvaccinated people became exposed to measles virus (16). Clusters of unvaccinated individuals can create pockets of susceptible individuals, allowing for sustained transmission of measles virus. Recently, measles vaccination rates have dropped in some developed nations 4 due to complacency, public concerns of safety, and philosophical objections (17,18). Unfortunately, immunization rates upwards of 90% are difficult to establish in many nations (Figure 2). Current vaccination strategies rely on subcutaneous inoculation and are difficult to sustain in developing countries for financial and logistical reasons (1). Attenuated measles vaccines are inactivated by heat and light, which means that immunization requires a cold chain (7). Furthermore, once the vaccines do reach these areas, trained health care workers, sterile needles and syringes are needed for proper and safe vaccination. In developed nations, where there are few measles virus infections, the measles vaccine is usually given in the form of the MMR vaccine at 12-15 months of age. However, in countries where measles is endemic, measles vaccines are typically administered at 9 months of age (7). It is thought that this is a reasonable time to vaccinate infants, because maternal antibodies to measles virus begin to wane around 6 months, and the development of protective antibody responses to measles vaccination is inhibited by maternal antibody (7,20). Not all vaccinated individuals will develop protective immunity, so a single dose of measles vaccine will not achieve levels of population immunity necessary for the elimination of measles. To reach levels of population immunity greater than 90%, it is necessary to provide a second measles virus vaccination (7). 5 In regions of the world where measles remains endemic, the burden of disease can be lessened through secondary prevention mechanisms. The World Health Organization (WHO) and United Nations Children’s Fund (UNICEF) have recommended two large doses of vitamin A to be given at the time of measles diagnosis in children under 5 years of age (21, 22). Vitamin A decreases morbidity and mortality due to measles infection, but the mechanism is unclear (23-29). Measles virus virology Measles virus is a member of the family Paramyxoviridae, and is in the morbillivirus genus. Measles virus has a 16 kb negative-sense RNA genome that is non-segmented, encapsidated, and found within a lipid envelope (1). The genome encodes six structural and two non-structural proteins (Figure 3b). The structural proteins are associated with the viral RNA and the envelope (Figure 3a). The hemagluttinin (H) and fusion (F) glycoproteins, are embedded in the lipid envelope and interact with host cells for attachment, fusion and entry (7). The interior of the lipid bilayer is lined with the matrix (M) protein. Inside the enveloped virus, the genomic RNA is maintained in a helical nucleocapsid by interaction with the nucleocapsid (N) protein, which also associates with the phosphoprotein (P) and large polymerase (L) proteins. The gene encoding the P protein also encodes the two nonstructural proteins. The C protein is translated from a separate start codon downstream in the gene, whereas the V protein is a product of RNA editing. 6 The C and V proteins regulate the cellular response to measles virus infection (7). Measles virus infection Measles virus H protein is responsible for virus attachment to cellular receptors, which determines the cell type specificity for infection through receptor protein interactions. Three host cell receptors have been identified. CD46 is ubiquitously expressed on all nucleated cells and can be utilized by vaccine strains of measles virus for attachment (30). Signaling lymphocyte activation molecule (SLAM) or CD150 is found on activated immune cells and can interact with the H protein from wild-type measles virus and vaccine strains; it is normally used as a co-stimulatory molecule (31). The association between the H protein and nectin-4 has recently been discovered (32, 33). Nectin-4 is an adherens junction protein expressed on epithelial cells. It is possible that other host receptors play a role in measles virus infection, but these are not yet identified. In persistent infections, glial cells and neurons in the central nervous system are targeted, supporting the notion that other receptors may be important in the infection process (1). Fusion with the cellular membrane and entry into the cell requires interaction between the F and H proteins, along with the cellular receptor (1). Following fusion with the viral envelope, host cells express viral glycoproteins at the cellular membrane (Figure 3c). This allows for subsequent fusion with surrounding, uninfected host cells, forming giant cells. Giant cells are one 7 hallmark of measles virus infection, and although they do not occur in all cell types, these multinucleated cells can be found in the lung, skin, and lymphatic tissue (1). Release of the measles virus genome into the cytoplasm occurs following fusion of the viral and cellular membranes. In the cytoplasm, transcription of the measles virus genome occurs first, producing mRNAs for viral proteins. Once translation of the mRNA transcripts forms sufficient nucleocapsid proteins to encapsidate other genomes, the viral RNA polymerase begins to read through the intergenic regions to replicate the genome. The negative strand RNA genome that enters the cell is used to form a positive, antigenome strand template for replication of negative strand RNA genomes. The new genomes are encapsidated by the N protein, packaged with other structural proteins and released by budding from the cellular membrane (Figure 3c). Defective replication of measles virus genome Replication of the measles virus genome can result in RNA forms other than the full length antigenome and genomes. Defective replication results in an incomplete form known as defective interfering (DI) particles or virus (55). These forms may be produced as a method of attenuation, to allow persistence in host cells (55). One theory of DI generation of negative-strand viruses such as measles virus, proposes a 5’ copy-back model (55) (Figure 4). In this model, the polymerase copying the template RNA begins to form the 8 negative sense measles virus genome, but the polymerase detaches from the template strand and resumes replication on the nascent chain. This partially synthesized chain is then copied back and a stem-loop structure is formed as the final RNA product (55). DI particle formation in vitro is partially dependent on the cell type used to grow measles virus (56). The frequent presence of DI RNA in measles virus stocks has confounded data from several in vitro studies looking at the innate immune response (1). Innate immune response to viral infections Measles virus infection introduces foreign RNA and proteins into the cell. Typically, during viral infections the host immune system will respond to these structures, pathogen-associated molecular patterns (PAMPs), that are recognized by host pathogen recognition receptors (PRRs) (34). The activation of PRRs results in cell signaling to produce various cytokines, which affect the immune environment in the host (35). In response to many viral infections, these cytokines are often pro-inflammatory and include interferons (34). Cytokines that are released early in response to a viral infection are important for inducing an antiviral state to protect uninfected cells and to modulate the induction of the adaptive immune response (34). TLRs Virus antigens and RNA at the cell surface or in the endosome, may be detected by Toll-like receptors (TLRs). In response to measles virus, several TLRs may be activated. TLR2 is found on the cell surface and interacts with 9 viral glycoproteins (39). TLR3 can sense double-stranded RNAs in the endosome, though its role in the antiviral response remains unclear (39, 157). TLR7, which is also expressed on the endosome, has been implicated in the innate antiviral response by detecting single-stranded RNA (158). TLRs dimerize after binding their ligands, causing conformational changes and allowing for the recruitment of adaptor molecules. Differential recruitment of adaptor molecules to these TLRs leads to the activation of distinct signaling pathways (39). MyD88 is the adaptor protein responsible for the production of proinflammatory cytokines, whereas TIR-domaincontaining adaptor protein-inducing IFN-β (TRIF) leads to production of type I interferons (39). TLR2 stimulation results in an inflammatory response, but not an antiviral response, because TLR2 interacts with MyD88 but not TRIF. Conversely, MyD88 does not interact with TLR3, but TRIF does. In fact, most virus-infected cells use a TLR3-triggered mechanism to produce type I interferon through its TRIF-dependent pathway (39). TLR7 and TLR8 can also interact with MyD88 to generate proinflammatory cytokines, and TLR7 can also interact with TRIF to induce production of type I interferon as well (39). Cytoplasmic PRRs Once the virus has reached the cytoplasm, it will no longer be detected by the TLRs (39). Instead, RNA helicases are the predominant PRRs involved in virus recognition. These helicases are retinoic acid inducible gene I protein 10 (RIG-I) and melanoma differentiation antigen 5 (MDA-5) (35). Inside the virus-infected cell, RIG-I can recognize single-stranded RNAs with 5’triphosphate or short double-stranded RNAs and MDA-5 can detect long dsRNAs (36, 37, 38). Studies using RIG-I-deficient cells have revealed that RIG-I is essential for induction of type I IFN responses following many RNA virus infections (40). Following activation, these RNA helicases associate with the adaptor protein IPS-1 via their CARD domains (39). IPS-1 then functions to activate specific kinases that phosphorylate Interferon-regulatory factors, IRF-3 and IRF-7 (6). IRF-3 is constitutively expressed, whereas IRF-7 must be transcriptionally activated along with other interferon-induced genes. The phosphorylated IRFs then translocate to the nucleus and function as transcription factors leading to the expression of type I interferons (39, 42). Type I interferon Type I interferons are a family of cytokines that include 12 subtypes of IFNα, IFNβ, IFNε, IFNκ, and IFNω (43). Primarily referring to IFNα and IFNβ, type I interferons are produced in direct response to many viral infections by the mechanisms outlined above (34). In response to TLR stimulation, type I interferons are produced by macrophages, conventional dendritic cells (cDCs), plasmacytoid dendritic cells (pDCs), and epithelial cells (43). However, once the virus is cytoplasmic, multiple cell types may 11 produce interferon through interaction with RNA helicases and other intracellular PRRs (43). After production and release, type I IFNs bind to the two-chain IFNα/β receptor IFNAR1/IFNAR2. Receptor binding induces dimerization and phosphorylation, followed by the phosphorylation of receptor-associated Janus kinases (Jaks), Jak1 and Tyk2 (34). Jaks then phosphorylate signal transducer and activator of transcription proteins (STATs), STAT1 and STAT2 (34). Phosphorylated STAT1 and STAT2 heterodimerize and interact with IRF-9 to form the transcription factor interferon-stimulated gene factor 3 (ISGF3). ISGF3 binds the interferon stimulated response element (ISRE) to activate the transcription of interferon stimulated genes (ISGs) (34). Interferon-inducible antiviral proteins ISGs encode proteins that have varying abilities to inhibit viral growth and spread through distinct mechanisms (41). Antiviral proteins that have well-established roles in measles virus infection are protein kinase R (PKR), 2’,5’-oligoadenylate synthetase, and Mx1 (41). PKR becomes activated by binding dsRNA and plays a role in inhibiting virus translation (44), OAS is involved in cleaving viral RNA, and Mx1 is involved in sequestering viral N proteins to prevent encapsidation of the viral genome (42, 45). Innate immune response to measles virus infection Many innate immune responses have been implicated in response to viral infection. Measles virus is unique in the immune responses that are 12 activated upon infection. The innate immune response to measles virus infection has not been well defined, due to differences observed in vitro and in vivo to different strains of measles virus (1). Measles virus replication is sensitive to the inhibitory effects of interferon in vitro (51), and some studies have reported the production of type I interferon in vitro in response to measles virus infection (46, 47, 48, 53). These studies could be confounded by the presence of DI RNA in measles virus stocks, which are potent inducers of interferon (1, 57). However, generally little or no type I interferon is produced in vivo during the acute response to measles virus infection (49, 50). Measles virus efficiently inhibits the induction of interferon and interferon signaling in infected cells (1). Nonstructural proteins V and C encoded by measles virus interact with cellular signaling pathways to interfere with the host interferon response. PRRs involved in the recognition of measles virus infection include RIG-I and MDA-5 (35). However, the V protein encoded by paramyxoviruses can bind MDA-5, interfering with downstream activation of the interferon β promoter (39). RIG-I plays an important role in the recognition of measles virus, most likely through detection of leader RNA sequences (35). It remains unclear as to why little or no interferon production is observed after activation of RIG-I and interference with MDA-5. 13 TLR2 detects the wild-type measles virus hemagglutinin protein (39, 52). Activation of TLR2 by the wild-type H protein, but not the measles vaccine H protein, leads to the production of TLR-responsive genes IL-1α/β, IL-6, and IL-12 p40 in monocytes, and also increases expression of measles virus receptor SLAM (CD150) (52). These signaling events contribute to enhanced immune activation and measles virus spread. Cytokines IL-1 and IL-8 have been detected in plasma of patients with measles (1). Role of dendritic cells Dendritic cells play a multifaceted role in the immune response to measles virus. They are able to contribute to both the innate and adaptive immune responses and bridge these processes through the production of proinflammatory cytokines as well as their ability to function as efficient antigen-presenting cells (APCs) (77). Dendritic cells contribute to the innate immune response by producing pro-inflammatory cytokines. Dendritic cells are present at mucosal surfaces (77), such as the lung, and can become infected with pathogens such as measles virus through direct infection or by phagocytizing infected cells in the airways. Infection of dendritic cells promotes their maturation, leading to increased expression of MHC class II molecules (78). Adaptive immune response to measles virus infection Mature dendritic cells play a role in activating the adaptive immune response by migrating to local lymph nodes, where they function as 14 professional APCs. Peptides from measles virus are presented to naïve T cells to induce differentiation and expansion into effector T cell subsets (58, 59). The inflammatory environment established by innate immune cells allows for the expansion and differentiation of antigen-specific effector T cells that encompasses a large portion of the adaptive immune response. Antibody response Measles virus infection elicits several adaptive immune mechanisms to resolve the infection. Measles virus-specific antibody and T cell responses appear at the same time as the characteristic rash (Figure 1). B cells are a major component of the adaptive immune response, and are activated through the interaction of their surface antibody B cell receptor and helper T cells (74). Measles virus-specific IgM antibody is the sole isotype produced for approximately a week, between 10-17 days, and then persists for about 2 months (70). The majority of these early antibodies are specific to the nucleocapsid protein (71). Measles virus-specific B cells then undergo classswitch recombination in germinal centers to produce measles virus-specific IgG and IgA antibodies. Somatic hypermutation in germinal centers allows for selection of B cells that produce antibodies with high affinity for measles virus antigens (73). N-specific antibodies are the predominant specificity. The IgG and IgA antibodies specific to measles virus H and F glycoproteins can be neutralizing 15 and protective (70). M protein-specific antibodies are detected at low levels (71), and are not neutralizing (70). T lymphocyte response Biopsies of the measles virus rash show CD4+ and CD8+ lymphocyte infiltration into areas of skin epithelium that are infected with measles virus (Figure 5). The CD8+ cellular response is also present in the blood during this time, and these cells are thought to be most important for clearance of infectious measles virus (64). This is supported by studies in rhesus macaques where CD8+ T cell depletion (65), but not to B cell depletion (66), leads to prolonged viremia after measles virus infection. CD8+ T cells have two types of effector mechanisms that contribute to clearance of measles virus, including cytotoxicity and IFN-γ production (1). Clearance of infectious measles virus occurs shortly after the rash fades (Figure 6). Though infectious measles virus has been cleared by approximately two weeks post-infection, measles virus RNA persists in peripheral blood mononuclear cells (PBMCs), urine and/or nasopharyngeal aspirates of hospitalized children (67, 68) and rhesus macaques (69) for months following measles virus infection. It is thought that measles virus RNA remains present after recovery due to slow clearance by the immune response (1). Effector CD4+ T lymphocytes T-helper subsets are effector cells with varying immune functions. They are activated by the interaction with antigen presented in MHC class II 16 molecules by APCs (73). These CD4+ T helper lymphocytes can differentiate into Th1, Th2, Th17, T regulatory cells (Tregs), or T follicular helper cells (Tfh). Differentiation to a specific T helper cell lineage is a result of the surrounding immune environment. Induction of Th1 cells are promoted by IL-12, Th2 by IL-4, Th17 by IL-23, TGF-β for Tregs, and IL-6 and IL-21 for Tfh cells (77, 83). In response to measles virus infection, the roles of Th17 and Tfh cell subsets have not yet been elucidated. Before and during the rash due to measles virus infection, a Th1 response dominates the CD4+ T lymphocyte population. Th1 cells function by producing high levels of IFN-γ and IL-2, and are observed during the rash (75, 79). Th1 cells are considered an important host defense mechanism to protect against and clear viral infection (73). After the rash subsides, Th1 cytokines return to normal levels and plasma IL-4 levels increase along with the Th2 subset (75). Th2 cells are characterized by their ability to produce cytokines IL-4, IL-5, and IL-13 (73). IL-4 can remain elevated in some patient plasma samples for seven weeks after measles virus infection (75). A mixed Th1/Th2 response has also been reported with significant IL-10 production. IL-10 may be a product of monocytes, macrophages or CD4+ CD25+ Tregs (50). A significant increase in the numbers of T regulatory cells was observed in these patients (50). 17 Immunosuppression following measles virus infection A state of immune suppression is another clinical feature that follows measles virus infection. There are several factors that contribute to this period of immunosuppression that are not completely understood. Following measles virus infection, little, if any type I interferon is produced (84, 85). There are abnormalities in the number and function of lymphocytes, due to apoptosis and impaired proliferation (85). Furthermore, impairment of maturation and antigen presentation by dendritic cells may lead to decreased T cell activation (84, 85). The interaction of measles virus H protein with its receptor, CD150, results in the inhibition of IL-12 production by dendritic cells (86). Additionally, cross-linking the CD46 receptor decreases the production of IL12 by monocytes (85). The suppression of the IL-12 response could lead to a decrease in induction of Th1 cells (85, 86). Furthermore, the effector cytokine IFN-γ produced by Th1 cells inhibits the proliferation of the Th2 subset, and conversely IL-4 and IL-10 produced by Th2 cells inhibit Th1 cytokine production (80, 81). The shift from a Th1 to a Th2 response early after infection will suppress activation of macrophages and proliferation of T cells, and may prevent the host from mounting an effective Th1 response upon subsequent exposure to new pathogens, leaving the host more vulnerable to these exposures (85, 76). 18 Furthermore, a prolonged presence of IL-10 and regulatory T cells could play a role in immune suppression as well (50). Tregs contribute to a favorable environment for opportunistic infections, and also can suppress T cell responses that clear viral infections (82). There are probably multiple mechanisms that play a role in suppression of the immune response following measles virus infection (Figure 7). The decreased ability of the immune response to clear virus may also be contributing to the persistence of measles virus RNA in cells for months after infection. 19 Figure 1. Measles virus infection and pathogenesis. (a) Diagram outlines spread of measles virus and associated viral titer, as measles virus spreads from the initial site of infection, the respiratory epithelium, to the local lymph nodes and blood, from which measles virus is spread systemically. (b) Clinical symptoms are outlined in this panel as they appear following measles virus infection. (c) This diagram summarizes the immune response to measles virus infection over time [7]. 20 Figure 2. 2012 immunization coverage rates with measles-containing vaccines in infants. The figure was compiled using data provided by the WHO (2012). Figure 3. Measles virus structure, RNA genome, and replication. (a) Picture detailing the organization of measles virus and the association of the structural proteins as labeled in the table. (b) Measles virus single stranded, negative sense RNA genome, with genes in different colors and letters indicating proteins made by the labeled gene. (c) Diagram depicting measles virus entry, transcription and translation, and replication and budding [7]. 21 Figure 4. Defective interfering particle formation from the measles virus genome. 5’ copy-back mechanism is outlined during replication of the negative strand RNA genome, the polymerase becomes detached from the antigenome template strand and resumes replication on the nascent chain. This results in a final DI RNA form with a stem-loop structure [156]. Figure 5. Biopsies of the measles virus rash show CD4+ and CD8+ lymphocyte infiltration. Rash (a) due to measles virus infection of the skin epithelium. (b) Hematoxylin and eosin staining shows immune cell infiltration in regions infected epithelial cells, with an arrow pointing to mononuclear cells. Immunoperoxidase staining of biopsy samples show CD4+ (c) and CD8+ (d) T cells in brown [1]. 22 Figure 6. Measles virus RNA levels over the course of infection. Early after measles virus infection, viremia is established with relatively high levels of infectious measles virus in the blood. Following the rash phase, this is cleared, but non-infectious measles virus RNA remains persistent for several weeks and is slowly cleared [1]. Figure 7. Potential mechanisms leading to measles virus-induced immune suppression. These include apoptosis of lymphocytes, impaired lymphoproliferation, increase in production of immunomodulatory cytokines IL-4 and IL-10 by monocytes, downregulation of IL-12 production in monocytes, and impaired differentiation and antigen presentation by dendritic cells (85). 23 Chapter Two: Comparision of in vitro immune responses to wild-type measles virus with C and V protein-knock out strains; wild-type and vaccine strains of measles virus 24 Introduction Infection with measles virus leads to a well-established sequence of events following the incubation period, progressing through the prodromal phase and transforming into the characteristic rash, followed by a period of immune suppression. Though these generalized phases are well established, the immune responses during the early innate period have been elusive. This is due in part to difficulty in study of phases prior to rash in vivo because infection is not recognized at this stage and the confounding of in vitro studies by DI in virus stocks. Many viruses encode proteins involved in evading the host immune response, and measles virus has this ability as well. Measles virus immune evasion Like other morbilliviruses, measles virus encodes nonstructural proteins within the P gene, the V and C proteins. These proteins counter host innate defenses to measles virus infection (88, 89, 90). Most notably, they prevent the production and signaling of type I interferons, the main innate cytokines produced during most viral infections (87). Block of type I interferon production IFNα/β transcription is regulated primarily by IRF3, IRF7 and NFkB transcription factors. Measles virus interferes with the activities of these transcription factors to suppress target gene expression and subsequent production of type I interferon. The nuclear factor κB (NF-κB) transcription factors play a role in the regulation and efficiency of IFN-β transcription, as 25 well as several other innate immune system responses (91, 92). Measles virus proteins P, V, and C interfere with gene expression dependent on NF-κB, in response to activation of several immune receptors, including the tumor necrosis factor (TNF) receptor, RIG-I-like receptors or TLR receptors (93). The measles virus V protein has the most robust ability to suppress NF-κB activity, whereas the presence of P and C proteins lead to a moderate NF-κB inhibition (93). Some studies have suggested that the measles virus C protein can inhibit the interferon response (41, 94), whereas others have not found this result (15). The V protein specifically interacts with the NF-κB subunit p65. This interaction prevents the translocation of the transcription factor complex to the nucleus. The NF-κB complex is maintained in the cytoplasm by the V protein, which also binds STAT2, IRF7 and MDA-5 via its cysteine-rich Cterminal domain. The V protein C-terminal domain interactions with these host molecules are sufficient for the inhibition of gene transcription by NF-κB (93). Furthermore, the measles virus V protein can interfere with the signaling of TLR7 and TLR9 by binding IκB kinase alpha and transcription factor IRF7, which is necessary for type I interferon production (35). MDA-5 and RIG-I are cytoplasmic PRRs used by the host in the recognition of viral nucleic acids (35). The V protein encoded by measles virus can bind MDA-5 and interfere with recognition of viral RNA and downstream activation of the interferon β promoter (35, 39). RIG-I is important for 26 production of type I interferon in response to RNA virus infection (40), and RIG-I recognizes measles virus RNA (35). However, the lack of a type I interferon response due to measles infection suggests interference with signaling after RIG-I activation. Downstream in this pathway, IRF-7 is normally activated through phosphorylation, but it is bound by the measles virus V protein, which prevents translocation of this transcription factor to the nucleus important for type I interferon transcription (6, 35). Block of type I interferon signaling Measles virus V protein also targets interferon signaling pathways (87, 106). Recently, the V proteins from several morbilliviruses were explored for their ability to block the responses to type I and type II interferons. Measles virus V protein blocks the activity and phosphorylation of Tyk2 necessary for signaling of type I interferon (106). Co-immunoprecipitation studies have shown that both Tyk2 and Jak1 interact with the measles virus V protein to prevent the subsequent activation of the STAT transcription factors necessary for type I interferon signaling and induction of an antiviral state (106). Strains of measles virus have slight differences in their P, V and C protein sequences, and therefore have varying abilities to interfere with the signaling of type I interferons (106). The IC-B wild-type strain of measles virus inhibits interferon α signaling by means of the common N-terminal domain of the P and V proteins (87). The IC-B wild-type measles virus C 27 protein does not interfere with Jak-Stat pathway of type I interferon signaling (41, 87, 89, 90), although the C protein of the Edmonston measles virus vaccine strain does inhibit this signaling pathway (94). The measles virus C protein is thought to regulate the synthesis of viral RNA (89). It is possible that the C protein of measles virus complexes with the ribonucleoprotein (RNP) complex, comprised of the N, P and L proteins, however data on this are inconsistent (95, 96). Either way, the role of the C protein in regulating synthesis of measles virus RNA could be an indirect mechanism to suppress induction of interferon (89). It is likely that measles virus requires the varying mechanisms of the V and C proteins to sustain viremia, and fully evade the host type I interferon response (89, 97). Interferon-stimulated genes (ISGs) and virus stress-induced genes (VSIGs) ISGs include hundreds of genes whose expression is induced by the action of interferon (98). However, some genes referred to as ISGs also fall into a category of virus stress-inducible genes (VSIGs), that are stimulated using different mechanisms (98). Viral infection and dsRNA can induce certain VSIGs by an interferon-independent mechanism (153) (Figure 1). One important VSIG is IFIT1, also known as ISG56. IFIT1 transcription is induced by viral stress quickly and transiently (99). IFIT1 binds and sequesters single stranded viral PPP-RNA, decreasing viral replication (154, 155). Another VSIG is Mx1, an interferon-induced GTPase 28 localized in the cytoplasm that restricts replication of negative stranded RNA viruses (100). Mx1 has been implicated in antiviral protection against paramyxoviruses, and measles virus replication is sensitive to inhibition by Mx1 in a cell type-specific manner (101). Role of dendritic cells in measles virus infection Dendritic cells are an important determinant of the host immune response to measles virus infection. CD150+ dendritic cells are implicated early in infection and can become infected directly by measles virus or through phagocytosis of infected cells in the airway (61, 63, 77). They contribute to the innate immune response by producing pro-inflammatory cytokines, and establish a specific cytokine environment for antigen-specific effector T cells to develop during the adaptive immune response. Mature dendritic cells function as professional APCs in lymph nodes, where they present measles virus peptides in MHC class II molecules to naïve CD4 T cells to induce differentiation and expansion into effector T cells (58, 59). To study the immune response to measles virus infection, in vitro studies have used monocyte-derived dendritic cells (61). Defective interfering (DI) particles DI particles are formed through a defective replication, where the polymerase detaches from the antigenome template while forming the negative strand measles virus genome and then copies back on the nascent chain it is forming (55). DI particles can efficiently induce the production of 29 type I interferon (57). The presence of DI RNA in measles virus stocks used for in vitro studies have complicated reports of the immune response to measles virus infection (1). Th17 response to viral infection IL-17-producing cells are produced in response to HIV infection in humans (146), and to herpes simplex virus and respiratory syncytial virus infections in mice (147, 148). IL-17 plays a role in regulating the inflammatory response to these viral infections. Th17 cells promote viral persistence in chronic virus infections, through an IL-17-mediated upregulation of anti-apoptotic molecules, which promote cell survival of virusinfected cells and confer resistance to cytotoxic T cells (152). Th17 response to measles virus infection It is currently unknown whether the Th17 effector T lymphocyte population is involved in the immune response to measles virus. Bi-phasic development of measles virus-specific IL-17-producing cells has recently been described in infected vaccinated and unvaccinated rhesus macaques (102). Th17 cells differentiate in the presence of TGF-β in an inflammatory environment, consisting of IL-6 or IL-1β, and IL-21 that promotes Th17 cell differentiation through a feedback mechanism (77, 103). The development of Th17 cells is inhibited by the Th1 effector cytokine IFN-γ (150). The production and signaling of type I interferons has a similar antagonistic effect on Th17 cell development (151). Additionally, IL-27 produced by 30 dendritic cells, monocytes or endothelial cells can suppress the development of Th17 cells (105, 106). Differentiation of Th17 cells leads to the upregulation of the receptor for IL-23 (IL-23R), allowing for the signaling of the cytokine IL-23, which is necessary for their survival (77). The production of the anti-inflammatory cytokine IL-10 inhibits IL-23 production and therefore the establishment of permanent Th17 cells (104). RORγt is the transcription factor associated with the Th17 cell lineage, and is necessary for the expression of IL-23R and the production of the Th17 cytokines, IL-17 and IL-22 (77). Th17 cells that produce IL-22 have been permanently differentiated through signaling by IL23 (77). The cytokine IL-17 promotes the inflammatory response, by inducing mediators of inflammation and leading to recruitment of neutrophils (149). Materials and Methods Viruses The wild-type measles virus strain IC-B and Edmonston measles vaccine strain were used to create the corresponding C and V knockout strains using site-directed mutagenesis of the infectious cDNA by Dr. Roberto Cattaneo’s lab (97). The C protein knockout strains were created using sitedirected mutagenesis to eliminate the AUG start codon by mutating this sequence to ACG, as well as inserting a UAG stop codon downstream of the start site (Figure 2). The V protein knockout strains were created by mutating the RNA editing site from AAAAAGGG to AAAGAGGG, causing 31 this site to become nonfunctional, and inserting a stop codon downstream of that site (Figure 2). These six measles virus strains were provided to our laboratory by Dr. Cattaneo for further experimentation. Sequencing To sequence each virus, viral RNA was isolated using the QIAamp® Viral RNA Mini Kit (Qiagen). The SuperScript® III One-Step RT-PCR with Platinum® Taq (Invitrogen) was then used to amplify a 1,681 base pair sequence, using the MVP 1745 forward primer and the MVP 3426 reverse primer to flank the P gene (Table 1). The PCR products from each reaction were purified using the QIAquick® PCR Purification Kit (Qiagen), following the manufacturer’s protocols. The NanoDrop® ND-1000 spectrophotometer was used to measure DNA concentrations and 150 ng of PCR product was used as the template for sequencing reactions. The primer MVP1745 was used to hybridize just upstream of the C protein start codon, and MVP2373 hybridizes just upstream of the RNA editing site for V protein synthesis (Figure 3). Primer sequences can be found in Table 1. Two separate sequencing reactions were done on the wild-type measles virus IC-B and Edmonston virus strains, to sequence both of these areas. The C protein knockout strains were amplified only with the MVP1745, whereas the V protein knockout strains were amplified only with MVP2373. The products of these reactions were sequenced by the JHMI Synthesis and Sequencing Facility. 32 Cells Vero, Vero/hSLAM, and WI-38 cells, human lung fibroblast cell line (ATCC), were grown in Dulbecco’s modified Eagle’s medium (DMEM, Gibco®) and B95a cells (145) were grown in Roswell Park Memorial Institute 1640 (RPMI-1640, Gibco®). Cells were grown in incubators at 37°C, 5% CO2, and all media were supplemented with 10% heat inactivated fetal bovine serum (FBS), 1% penicillin/streptomycin and 1% L-glutamine. Detection of DI RNA Viral RNA was isolated from each virus stock, using the QIAamp® Viral RNA Mini Kit (Qiagen). The SuperScript® III One-Step RT-PCR with Platinum Taq (Invitrogen) was used according to the manufacturer’s directions, using primers (JM396, JM402) specific for the measles virus standard genome and for (JM396, JM403) the measles virus 5’ copy-back DI RNA genome (Table 1). A 1% UltraPure™ Agarose (Invitrogen) gel was used to run PCR products stained with 5X dye, in relation to the I KB Plus ladder (Invitrogen). Purification of DI-free viruses All six original virus stocks were tested and found to be positive for defective interfering (DI) particles using reverse-transcriptase PCR (Figure 5). Plaque purification was used to purify DI-free virus from stocks that contained the DI genome. Six-well plates were infected in triplicate with each virus using serial dilutions from 10-1 to 10-7 for one hour and overlayed with a 33 mixture of one part 1.2% bacterioagar and one part 2X MEM supplemented with 2% FBS, 1% penicillin/streptomycin and 1% L-glutamine, before incubating for 6 days at 37°C, 5% CO2. Plaques were detected by eye, and individual plaques were isolated and added to a T25 flask with a confluent monolayer of Vero/hSLAM cells. The amount of plaqued virus added to each flask varied, ranging from 3 plaques per flask to 1/16th of a plaque. Fractions of a plaque were added to flasks by first dissociating a plaque in 1 ml of media and adding only a portion of that media to the flask to be infected. Virus was propagated for five to ten days, until 70-80% of the flask exhibited cytopathic effects, as observed by syncytia formation, cell clearings or dead floating cells. All cells and media were used for the new virus aliquots. Cells were separated from the media, and then subjected to three cycles of 15minute exposure to dry ice, each followed by thawing. After the last thaw, media was added to resuspend any cell-associated virus that was freed in this process and pooled with the original media supernatant from the flask, then aliquoted as a new virus stock. Each new virus stock was tested for DI particles. Virus strains that continued to show the presence of DIs after several rounds of plaque purification, were then grown in different cell lines thought to be less permissive to DI formation. Rather than using Vero/hSLAM for in vitro growth of the wild-type viruses, a semi-adherent subline of the B95a marmoset B-lymphoblastoid cell line, B95-8 susceptible to infection with WT 34 measles virus, was used (2). The vaccine strain does not rely on the SLAM (CD150) receptor for entry and was grown in Vero cells, which contain the receptor CD46 necessary for the binding of measles vaccine strains (30). In an attempt to obtain DI-free stocks, virus was also grown in the WI-38 cell line, a human fibroblast cell line derived from embryonic lung tissue (56). PBMC isolation Leukopaks were obtained from healthy human adult donors at the Johns Hopkins Hospital Blood Bank. Blood was diluted 1:3 with 1X PBS and layered on top of Ficoll-Paque PLUS (GE Healthcare) for gradient centrifugation. PBMCs were isolated from this gradient and any remaining red blood cells were lysed during a 5-minute incubation with ACK Lysing Buffer (Quality Biological, Inc). PBMCs were resuspended in 1X RPMI-1640 4% heat inactivated human AB serum (Lonza), 1% penicillin/streptomycin, 1% 200 mM L-glutamine, 1% 100 mM sodium pyruvate. Collection of monocytes and differentiation to dendritic cells PBMCs were fractionated using the AutoMACS pro cell sorter (Miltenyi Biotec) and human anti-CD14 microbeads (Miltenyi Biotec) to positively select for monocytes. Monocytes were differentiated by culturing 1 million cells/ml with 500 U/ml of recombinant human GM-CSF (R&D Systems) and 1,000 U/ml of recombinant human IL-4 (R&D Systems) for 6 days at 37°C, 5% CO2 to create monocyte-derived dendritic cells (moDCs). 35 Infection of monocyte-derived dendritic cells Wild-type measles virus and its C- and V-protein knockout strains Infections of moDCs were done in Costar 96-well round bottom plates (Corning) with 2x105 cells per well. DI-free wild-type measles virus with its respective C- and V-protein knockout viruses obtained from plaque purification were used for these infections. Variations of this experiment were conducted four times. Cells were infected with each virus strain at multiplicities of infection (MOIs) of 0.1 or 0.01, in duplicate or triplicate. Samples were collected 12- and 24-hours following infection, with one experiment including samples collected at 2- and 48-hours post-infection as well. All samples were frozen at -80°C. Cells were collected as pellets, with replicate wells pooled. Supernatant fluids were also gathered, with one experiment pooling duplicate samples and the remaining three experiments collecting each well’s supernatant individually. Each experiment included non-infected controls, where samples were collected in the same manner. Edmonston vaccine and Bilthoven wild-type measles virus strains PBMC isolation, positive selection of monocytes, and differentiation of monocytes to dendritic cells was done by Dr. Rupak Shivakoti using the same methods described above. He followed the same infection protocols, but infected with the Edmonston vaccine strain or the Bilthoven wild-type strain of measles virus, each at MOIs of 0.4 and 4.0. Post-infection time points were collected at 2, 12, 24 and 48 hours, also using uninfected moDCs as controls. 36 Cells were collected as pellets, with replicate wells pooled into one sample. These samples were archived by Rupak and were used for the rest of the experiments as described. Measurement of mRNAs Cell pellets from all moDC infections were used for RNA isolation using the RNeasy® Plus MicroKit (Qiagen). The Taqman® RNA-to-CT™ 1Step Kit (Applied Biosystems) was used according to directions of the manufacturer. Taqman® Gene Expression Assays and PrimeTime® Std qPCR Assays were used to detect GAPDH, IL-28 (IFNλ2), IL-29 (IFNλ1), IFN-β, and ISG56 (IFIT1) and Mx1 mRNAs. The RNAs from Rupak’s measles virus infected moDCs, and RNA from one of the replicate experiments, were analyzed using the Taqman® Gene Expression Assays and PrimeTime® Std qPCR Assays to detect GAPDH, IL-28 (IFNλ2), IL-29 (IFNλ1), ISG56 (IFIT1), Mx1, IL-23A, IL-6, IL-1β, IL-27, and IL-10. Plates were read using the 7500 Real Time PCR System and relative quantification was done using the 7500 System Software with GAPDH as the endogenous control for amplification. The presence of mRNA for these genes was calculated using Ct values and reported as fold-change relative to noninfected conditions. Interferon bioassays Supernatant fluids were analyzed for interferon production by bioassays. Vero cells were grown overnight in Costar 96-well flat-bottom 37 plates (Corning). Vero cells were then incubated with either supernatant samples, recombinant human interferon alpha A (rhIFN-α 2A, PBL Interferon Source) or recombinant human interferon beta (IFN-β 1A, PBL Interferon Source) for 24 hours at 37°C, 5% CO2. The following day, cells were challenged with vesicular stomatitis virus expressing green fluorescent protein (VSV-GFP, a gift from Sean Whelan at Harvard Medical School, Boston, Massachusetts) at an MOI of 1.0 for 24 hours at 37°C, 5% CO2. The negative control wells included cells that were incubated in media only and were not challenged with VSV-GFP, and the positive control wells were incubated in media only and were challenged with VSV-GFP. Following infection, cells were trypsinized and washed with PBS 1% FBS before being fixed in PBS 1% FBS 1% formaldehyde. Results were analyzed using the BD FACS Canto II™ flow cytometer by reading signals of VSV-GFP-infected cells on the FITC channel using BD FACSDiva Software. Interferon standards were run as controls to show protection from infection by inducing an antiviral state in the cells. Four different dilutions were used for each standard; IFN-α 2A was used at 500, 50, 5, and 0.5 units/well, and IFN-β 1A was used at concentrations of 500, 5, 0.5, and 0.05 units/well. Results Sequencing and DI-status of measles virus stocks Virus stocks obtained from the lab of Dr. Roberto Cattaneo (Mayo, Rochester, MN) were sequenced to confirm the presence of the desired 38 mutations in knock out (KO) strains and the absence of mutations in the wild-type (Wt) and vaccine measles virus strains (Figure 4). These viruses were created by site-directed mutagenesis (97). The start codon of the measles C protein was mutated (AUG ACG) to prevent translation. Additionally, a stop codon was introduced slightly downstream of this site (UGG UAG), leading to the C protein KO (C KO) measles virus strain. The measles virus V protein knockout (V KO) strains were developed by mutating the RNA editing site (AAAAAGGG AAAGAGGG) to prevent editing of the P gene to produce the V protein, while retaining the ability to translate the full length P protein. A stop codon was also introduced slightly downstream in V protein frame (AGA UGA) (97). All measles virus strains had the correct sequence (Figure 4). Both the Wt measles virus strain and Edmonston measles vaccine strain (Edm) preserved the C protein start codon and V protein RNA editing site, with no insertions of stop codons downstream of these sites. Wt C KO and Edm C KO strains contained mutations in the start codon sequence and a stop codon in the C protein reading frame. Sequencing of Wt V KO and Edm V KO strains confirmed the mutations to the RNA editing sites and the premature stop codon in the V protein reading frame. Each measles virus strain was tested for the presence of DI particles by PCR, and 5 of 6 virus strains were contaminated, with only the Edm strain being DI-free (Figure 5). All six strains of measles virus were plaque 39 purified in order to obtain measles virus stocks free of DI particles and subsequently grown to increase the stock viral titer, by infecting at a low MOI (0.003-0.0001). Four measles virus strains: Wt MV, Wt C KO, Wt V KO, and Edm, were successfully isolated without DI particle contamination. The DI-free Wt MV stock used for experiments was isolated after two rounds of plaque purification and the DI-free Wt C KO virus stock used was isolated after one round of plaque purification. These viruses grew to titers between 104 and 107 pfu/ml. The Wt V KO strain was more difficult to isolate free of DI particles, at high enough titers. For these reasons, three different Wt V KO stocks were used for in vitro experiments. An initial plaque purification of Wt V KO strains yielded a low titer, DI-free virus that was subsequently grown at MOIs of 0.0003 and 0.0001 on Vero/hSLAM cells, which yielded DI-containing virus stocks. After one more round of plaque purification, two DI-free Wt V KO stocks were isolated. The last DI-free Wt V KO stock was isolated after two more rounds of plaque purification. Figure 6 shows gels with PCR products for the measles standard genome and the DI genome from each virus used for in vitro assays. Infection of monocyte-derived dendritic cells Interferon assays To determine the effects of measles virus infection with and without C and V on interferon production and interferon-stimulated gene (ISG) 40 induction, moDCs were infected with Wt, Wt V KO and Wt V KO strains. Data on mRNA expression of type III interferons λ1 and λ2, also known as IL28 and IL-29, were not reported, because the results were highly variable and no trends were observed (data not shown). Primers used for IFN-β did not differentiate between genomic RNA and mRNA. Although a control sample without reverse transcriptase was used, the signal from IFN-β began to amplify at a very similar Ct value compared to the normal RT-PCR conditions, making it difficult to determine if the reaction was amplifying mRNA (data not shown). Interferon bioassays were done to detect the presence of type I interferon in supernatants from the four study replicates. No significant protection was observed (data not shown). Although this suggests that no interferon was present in the supernatants, the control recombinant interferon samples prevented infection with VSV-GFP only at the higher concentrations so sensitivity of the assay was an issue. Recombinant IFN-β 1A provided complete protection from infection at 500 units/well and partial protection at 5 units/well, whereas IFN-α 2A conferred only partial protection at the highest dilution of 500 units/well. It is possible that interferon α/β is present in supernatant samples, but below the limit of detection with this assay. mRNA gene expression changes in monocyte-derived dendritic cells Induction of cytokine and ISG mRNAs was explored by qPCR on RNA 41 isolated from infected monocyte-derived dendritic cells, specifically investigating ISGs/VSIGs IFIT1 and Mx1, as well as positive (IL-1β, IL-6, IL23) and negative regulators (IL-27, IL-10) of Th17 differentiation. Time points at 12- and 24-hours post-infection were collected for four replicates of infection using Wt, Wt CKO, and Wt V KO at MOIs of 0.1 and 0.01. For infections using Edm and Bilt at MOIs of 4.0 and 0.4, samples were collected following infection at 2, 12, 24, and 48 hours and were done in duplicate. Interferon-stimulated genes (ISGs) and viral stress-induced genes (VSIGs) Edmonston vaccine and Bilthoven wild-type measles virus strains For both Edm and Bilt at both MOIs, IFIT1 mRNA was upregulated approximately 30-fold at 2 hours post-infection (Figure 7). For Edm and Bilt at high MOIs (4.0), IFIT1 was further upregulated at 12, 24, and 48 hours post-infection. At 24 hours, IFIT1 mRNA expression peaked, and was higher for an MOI of 4 than 0.4. Edm had a more robust response than Bilt, with upregulation at an MOI of 0.4 as well as 4.0 (3000X), whereas Bilt increased FIT1 primarily at an MOI of 4 (1000X) (Figure 7a). Mx1 mRNA was not increased until 12 hours and was maximal at 24 hours. A virus dose-response was exhibited for each strain of measles virus, though none achieved statistical significance. Overall, infection with Edm exhibited higher expression, with Mx1 mRNA upregulated approximately 400-fold at an MOI of 4, while Bilt infection led to a 100-fold Mx1 mRNA 42 upregulation (Figure 7b). Overall, the Mx1 pattern was similar to that of IFIT1. Wild-type measles virus and its C- and V-protein-knockout strains For wild-type measles virus and the corresponding C- and V-protein KO strains, only lower MOIS of 0.1 and 0.01 could be used for infection. The patterns of IFIT1 and Mx1 mRNA increases at both MOIs were similar (Figure 8). IFIT1 mRNA levels were not increased from baseline at 12 hours but by 24 hours post-infection, all viruses upregulated IFIT1 mRNA except Wt V KO at the low (0.01) MOI. Mx1 mRNA expression was upregulated approximately 5-fold at the 24-hour time point for each Wt virus at the higher MOI (0.1), although only the Wt V KO strain showed a statistically significant difference from baseline (Figure 8b). An increase in Mx1 mRNA expression was not observed at low MOIs (0.01) for Wt or KO infection. Statistically significant increases in IFIT1 or Mx1 gene expression were observed only in the absence of C and V proteins at the high MOI (0.1) (Figure 8). Positive regulators of Th17 differentiation Edmonston vaccine and Bilthoven wild-type measles virus strains Expression of positive and negative regulators of Th17 differentiation was investigated in the RNA samples from Edm- and Bilt-infected moDCs (Figure 9, 11) and Wt-, Wt C KO-, and Wt V KO-infected moDC mRNAs (Figures 10, 12). 43 mRNA expression of IL-1β, IL-23A, and IL-6, positive regulators of Th17 differentiation, were explored following measles virus infection with Edm and Bilt (Figure 9). IL-1β mRNA was upregulated only in response to Edm at a high MOI (4.0) where a 6-fold increase in IL-1β mRNA expression was observed at 48 hours, though this was not statistically significant (Figure 9a). The primers used to detect IL-23A detected genomic DNA and mRNA. For Edm and Bilt infections, large enough differences in the Ct values were observed between the control lacking reverse transcriptase, and the normal RT-PCR conditions to be confident that induction could have been detected (Figure 9b). At 12 hours post-infection, with the high MOI (4.0) of Bilt, IL23A transcripts were significantly downregulated, otherwise there was little evidence that either Edm or Bilt altered IL-23A mRNA expression (Figure 9b). A robust increase in expression occurred of IL-6 mRNA after infection with Edm and Bilt at both MOIs, but none reached statistical significance (Figure 9c). At 2 hours post-infection, all viruses showed approximately 30fold upregulation of IL-6 mRNA. By 48 hours post-infection, Edm infection led to a substantial increase in IL-6 mRNA expression with the high MOI inducing IL-6 mRNA approximately 106-fold and the lower MOI approximately 104-fold compared to levels in mock-infected moDCs. There was variability in the IL-6 mRNA expression following infection with Bilt and overall little evidence of induction. 44 Wild-type measles virus and its C and V protein-knockout strains The effects of the C and V protein deletion on induction of positive and negative regulators for Th17 expression were also investigated a tMOIs of 0.1 and 0.01 (Figure 10, 12). IL-1β and IL-6 mRNAs were downregulated at 2 hours but were generally back to baseline by 12 hours (Figure 10). Upregulation of IL-1β mRNAs were observed at 12 or 24 hours post-infection, for infection at the high MOI (0.1) (Figure 10a). The Wt measles virus had an IL-1β mRNA fold-induction around 30 at 24 hours post-infection. The C and V protein-KO measles virus strains showed lower levels, ranging between 6and 12-fold, respectively. IL-6 mRNA expression demonstrated the same trends, with similar fold changes as for IL-1β (Figure 10b). The numbers of replicates did not allow for any statistical analyses. Negative regulators of Th17 differentiation Edmonston vaccine and Bilthoven wild-type measles virus strains RNA expression of IL-27 and IL-10, negative regulatory cytokines for the development of Th17 cells, were explored (Figure 11, 12). IL-27 mRNA expression was increased compared to mock-infected moDCs (Figure 11a), while IL-10 mRNA was not (Figure 11B). For both Edm and Bilt measles virus strains, the high MOI (4.0) infection increased IL-27 transcripts 2 hours post-infection to approximately 30-fold, peaked at 24 hours, and subsequently declined at 48 hours post-infection. At 24 hours post-infection, IL-27 mRNA 45 was significantly upregulated 1000-fold in response to Edm infection (p< 0.01), and 100-fold in response to Bilt infection (not statistically significant). Wild-type measles virus and its C and V protein-knockout strains IL-27 transcript levels were variable for all Wt and KO measles virus strains (Figure 12a) with no clear dose-response and high variation between samples making it difficult to conclude if and how IL-27 expression was regulated by measles virus infection in moDCs. There was a downregulation of IL-10 transcripts at 2 hours post-infection, followed by a 5- to 20-fold upregulation 12- and 24-hours post infection at high MOI infections (Figure 12b). The Wt C KO showed similar IL-10 regulation at both MOIs, whereas Wt and Wt V KO viruses showed a dose-dependent response. Trends for Th17 negative regulator mRNAs were not statistically significant when compared to mock-infected moDCs (Figure 12). Discussion In these studies we were unable to detect type I interferon production by measles virus-infected moDCs, but demonstrated MOI-dependent induction of ISGs/VSIGs, IFIT1 and Mx1. IFIT1 and Mx1 mRNAs were significantly increased by Wt V KO, and IFIT1 mRNAs were also significantly increased by Wt C KO and Edm. For all strains of measles virus tested, IFIT1 was upregulated to a greater extent than Mx1. Over the first 48 hours following infection, cytokines induced would inhibit a Th17 response, as IL-23A mRNA is downregulated and IL-27 mRNA is upregulated. It 46 appears that a proinflammatory (IL-1β, IL-6) response may be occurring as well, but this did not reach significance. Type I interferons were not detected Little or no biologically active type I interferon as detected from the supernatants of measles virus-infected moDCs, using the Wt, Wt C KO and Wt V KO strains. It was expected that some may be present in the Wt C KO and Wt V KO conditions, due to the absence of immunomodulatory C and V proteins that have the ability to inhibit type I interferon production. The sensitivity of this assay was low, and there may have been small amounts of interferon α/β present, below detectable levels. Similarly, previous studies done by Dr. Rupak Shivakoti showed that in the absence of DI RNA, Bilt infection of moDCs produced no functional type I interferon. Although Edm produced low levels of interferons α/β, this effect returns to a similar level when levels of infection were adjusted between Edm and Bilt (data not shown). ISGs/VSIGs were upregulated in response to measles virus infection IFIT1 and Mx1 both function to decrease viral replication (100, 154, 155). IFIT1 mRNA increased to a greater degree to measles virus infection than Mx1 mRNA and was more highly upregulated by Edm than Bilt. Upregulation continually increases up to 24 hours, and then begins to decrease. This result agrees with previous obervations with dsRNA that IFIT1 transcription is induced quickly and is transient (99). Although 47 upregulation of Mx1 transcripts did not reach statistical significance, because of the low number of replicates (2) and increase in Mx1 is likely to be biologically significant, because measles virus glycoprotein synthesis and replications are sensitive to the inhibitory effects of Mx1(159). Absence of measles virus C and V proteins may enhance induction of ISGs/VSIGs The regulation of transcription of ISGs/ VSIGs in response to measles virus infection and the role of the nonstructural proteins, C and V, have not been explored, although these proteins contribute to evasion of the host interferon α/β response (34, 41, 97, 161). Our data suggest that the C and V proteins may play a role in regulating the induction of ISGs/VSIGs. Upregulation of IFIT1 and Mx1 mRNA transcripts were observed 24 hours after infection at an MOI of 0.1 for the Wt V KO infection, and Wt C KOinduced Mx1 transcripts. at high MOI conditions (0.1) at 24 hours postinfection for the the Wt V KO-induced upregulation of IFIT1 and Mx1 transcripts, and the Wt C KO-induced upregulation of Mx1 transcripts. Although statistically significant upregulation of IFIT1 and Mx1 occurred with C KO and V KO infections compared to mock infected baseline expression, the increase was not significantly different than the upregulation after Wt infection. Coupled with the lack of detection of interferon, upregulation of IFIT1 and Mx1 are likely due to activation of the viral stressinduced, interferon-independent pathway by infection. ISG/VSIG 48 upregulation by Wt C KO and Wt V KO infections, supports the idea that IFIT1 and Mx1 can be induced by more than one mechanism. However, the ability of our bioassay to detect interferon suggests the need to increase the sensitivity of this assay to detect low levels of type I interferons. This is supported by studies done with the same Wt C KO and Wt V KO viruses in vivo, where interferon α/β as detected at day 7 and day 14 following infection with these viruses in rhesus macaques (97). The C and V proteins are important for inhibition of interferon production, and the V protein plays an additional role in inhibition of interferon cell signaling (87, 93, 106). This additional ability of the V protein to interfere with interferon cell signaling, may have increased induction of ISG/VSIG mRNAs for IFIT1 and Mx1. Inhibition of Th17 cell differentiation early after measles virus infection The Th17 effector cell subset is regulated by several cytokines produced by APCs and innate immune cells (103). Differentiation occurs in an inflammatory environment, but the survival and expansion of Th17 cells requires IL-23A signaling (77). Our results suggest that measles virus infection tends to upregulate pro-inflammatory cytokines IL-1β and IL-6. The upregulation of IL-1β, although it did not reach significance in our results, is supported by the detection of IL-1β in plasma of children infected with measles (144). However, there was a significant downregulation of IL-23A mRNA which is needed to establish long-lasting Th17 cells (77). Though 49 there is an early downregulation, IL-23A could play an important role in development of a Th17 response at later time points following measles virus infection. IL-10 transcripts did not vary significantly from baseline following measles virus infection of moDCs. This is not surprising early following viral infection. Although IL-10 plays a role in preventing the formation of Th17 cells through inhibition of IL-23 production (104), it also inhibits T cell proliferation and suppresses the production of pro-inflammatory cytokines necessary for the effector responses to measles virus infection (160). Furthermore, Th17 cell differentiation is inhibited by IL-27 (105, 106), and a statistically significant increase in IL-27 transcripts at 24 hours following infection with Edm was observed. A similar trend was observed in response to Bilt infection at a lower magnitude. IL-27 upregulation early in response to measles virus infection may support Th1 cell differentiation, which assists in the clearance of infectious measles virus (138). Overall, the downregulation of IL-23A and upregulation of IL-27 support inhibition of the Th17 cell differentiation early after infection. Conditions, limitations, and future directions DI-free viruses were difficult to grow at high titers. Virus stocks with high titers were often contaminated with DI particles and could not be used for these experiments. Due to the low titer of virus stocks, infections were only done at low MOIs and results might be different with infections at 50 higher MOIs. Nevertheless, significant upregulation of IFIT1 and Mx1 mRNAs were observed. Importantly, we can confirm that ISG/VSIG induction was not a result of aberrant activation of innate immune responses leading to the production of type I interferon. In future studies, it would be beneficial to use higher MOIs to try to elucidate clear trends in the regulation of ISGs/VSIGs and cytokines. To do this, viruses must be isolated at higher titers that are DI-free. This is especially important for comparison of the Wt, Wt C KO, and Wt V KO viruses and the Edm, Edm C KO, and Edm V KO measles viruses to more definitiviely determine the roles of V and C in modulating the responses of moDCs to infection with Wt and vaccine strains of measles virus. Preparation of DI-free KO viruses were important because the knockout system of a measles virus IC-B represents a complete system for determining accurate physiological effects of measles virus infection, with no confounding by DI particles. Other studies using whole measles virus KO strains have been used, and support our findings. In rhesus macaques, Wt C KO and Wt V KO viruses induced interferons α and β and spread less efficiently, but still induced strong adaptive immune responses (97). Tober et al. also detected a decrease in titers in cotton rats with a measles virus V KO virus (161), and Yanagi et al. used measles virus C KO in A549/hSLAM cells and measles virus V KO in H358 cells to confirm the roles of the C and V in inhibition of interferon induction (35, 41). With more time, the limited 51 analysis of mRNAs for Th17 can be further expanded with more replicates of Wt, Wt C KO, and Wt V KO infections. Conclusions Although little or no biologically active type I IFNs were detected in response to MV infection of moDCs, there may be trace amounts produced by infection with Wt C KO and Wt V KO viruses that were below the limit of detection. In the future, it would be beneficial to increase the sensitivity of interferon assays to detect low levels of interferons α/β. IFIT1 and Mx1 transcript levels are likely induced by the viral stress-induced pathway in response to Wt measles virus infection, whereas the Wt C KO and Wt V KO viruses may also induce the transcription of IFIT1 and Mx1 through the interferon-stimulated pathway. The absence of C and V proteins during virus replication likely lessens the ability to interfere with production and signaling of interferon, though interference with these pathways may not be complete with the KO of only one immunomodulatory protein. It would be interesting to study ISG/VSIG mRNA regulation in response to infection with a measles virus C- and V-protein double knockout strain. IFIT1 appears to be more vigorously induced in response to measles virus infection than Mx1. Furthermore, early after measles virus infection, moDCs do not support the formation of long-lasting Th17 cells. Current and future studies will help us delineate the role of C and V protein and the immune response to measles virus infection, as well as Th17 cytokine regulation. 52 Table 1. PCR primers, target and cycling conditions for P gene sequencing, and detection of measles virus standard and defective genomes. Primers for Sequencing P gene (Biosource International) Sequencing Primer and Sequence Target Measles virus V protein RNA editing site MVP 2373F 5’-GATCCACGAGCTCCTGAGAC-3’ MVP 3426R 5’-GGAGGCAATCACTTTGCTCCTAAG-3’ Measles virus C protein start codon MVP 1745F 5’-CTTAGGAACCAGGTCCACACAGCC-3’ MVP 3426R 5’-GGAGGCAATCACTTTGCTCCTAAG-3’ Cycling Conditions 45°C, 30 min 94°C, 2 min [94°C, 30 s; 55°C, 30 s; 68°C, 2 min for 40 cycles] 68°C, 5 min; 4°C, hold 45°C, 30 min 94°C, 2 min [94°C, 30 s; 55°C, 30 s; 68°C, 2 min for 40 cycles] 68°C, 5 min; 4°C, hold Primers for Detection of Measles Virus Standard and Defective Genomes (IDT) Target Primer and Sequence Measles virus standard genome JM 396 5’-TATAAGCTTACCAGACAAAGCTGG GAATAGAAACTTCG-3’ JM 402 5’-TTTATCCAGAATCTCAAGTCCGG-3’ Measles virus 5’ copyback DI genome JM 396 5’-TATAAGCTTACCAGACAAAGCTGG GAATAGAAACTTCG-3’ JM 403 5’-CGAAGATATTCTGGTGTAAGTCTA GTA-3’ 53 Cycling Conditions 45°C, 30 min 94°C, 2 min [94°C, 30 s; 53°C, 30 s; 68°C, 2 min for 40 cycles] 68°C, 5 min; 4°C, hold 45°C, 30 min 94°C, 2 min [94°C, 30 s; 53°C, 30 s; 68°C, 2 min for 40 cycles] 68°C, 5 min; 4°C, hold Figure 1. Signaling pathways leading to virus stress-inducible gene (VSIG) induction. Type I interferon interacts with the IFN receptor (IFNAR) and activates the Jak-STAT signaling pathway, leading to the activation of the interferon-sensitive response element (ISRE), which is present in interferon stimulated gene (ISG) promoters. TLR3 is activated by dsRNA, which activates transcription factors NFκB and IRF3 to induce VSIGs. Additionally, a TLR3- and IFN-independent pathway exists that activates transcription factors ATF2, IRF3, and NFκB [98]. 54 Figure 2. Generation of wild-type measles virus defective for the C or V protein. Two mutations were used to silence C protein expression, or V protein expression, creating two new measles virus strains. For the wild-type measles virus C knockout (KO) strain, the nucleotide sequence was altered to render the start codon nonfunctional and also introduce a stop codon slightly downstream of the start site. The wild-type measles virus V KO strain, was altered to inactivate the RNA editing sequence necessary for V protein expression and add a stop codon downstream of that site [97]. Figure 3. Primer binding sites for sequencing. Blue arrows correspond to locations on or near the P gene where primers hybridize to amplify RNA for sequencing of viruses. The arrows facing right, from left to right, are MVP 1745F and MVP 2373F, and were used to amplify the start sequence of the C gene and the latter half of the V gene, respectively. The left-facing arrow, primer MVP 3426R was used in both PCR conditions. Adapted from [93]. 55 Figure 4. Sequencing confirms correct viral sequences. Wild-type (Wt) measles virus RNA genome sequences are boxed in blue. (a) Measles virus (Cprotein knockout (C KO) mutation [97]. In red, the triplet sequence targeted for mutations is boxed. Below, sequencing of Wt and Edm strains confirmed the wild-type sequence, and Wt C KO and Edm C KO strains confirmed the existence of mutations. (b) Wt V-protein knockout (V KO) mutations as done by the laboratory of Dr. Roberto Cattaneo [97]. In red, the triplet sequence targeted for mutations is boxed. Below, sequencing of Wt and MV Edm strains confirmed the wild-type sequence, and Wt V KO and Edm V KO strains confirmed the existence of mutations. 56 Figure 5. Gel of measles virus stocks. (a) PCR products of the measles virus N gene (primers JM396, JM402), indicative of the presence of the measles virus standard genome. (b) PCR products of the measles virus 5’ copy-back DI RNA genome (primers JM396, JM403). All molecular weight standards and measles virus PCR products were run on the same gel. Lane 1 has 1 KB Plus molecular weight standards (Invitrogen). Lane 2 contains PCR products with no template measles virus. Lanes 3-8 corresponded to a specific virus strain: lane 3 – Wt measles virus IC-B, lane 4 – Wt C KO, lane 5 – Wt V KO, lane 6 – Edmonston (Edm) measles virus vaccine, lane 7 – Edm C KO, lane 8 – Edm V KO. 57 Figure 6. Measles virus standard and DI genome PCR products of measles virus stocks used in in vitro experiments. Lane 1 of each figure is 1 KB Plus molecular weight standards (Invitrogen). (a,b) Lanes 2 and 5 have no measles virus template in the PCR product, Lanes 3 and 4 detected the presence of the measles virus standard genome, whereas lanes 6 and 7 did not detect the presence of the measles virus DI genome. (a) contains Wt and Wt C KO viruses and (b) contains two Wt V KO stocks. (c) Lanes 2 and 4 have no measles virus template, lane 3 detects the measles virus standard genome, and lane 5 detects the measles virus DI genome. The small band in (b) lane 6 is indicative of extra primers. 58 Figure 7. mRNAs for interferon-stimulated genes, IFIT1 and Mx1, are upregulated in moDCs by the Edmonston measles virus vaccine strain and Bilt Wt strain. IFIT (a) and Mx1 (b) mRNA fold changes were log transformed and compared to the baseline expression of mock-infected moDCs using a two-way ANOVA, with a Bonferroni multiple comparisons correction. (*) p < 0.05, (**) p < 0.01, (***) p < 0.001, and (****) p < 0.0001. Figure 8. Interferon-stimulated genes, IFIT1 and Mx1, are comparably upregulated in monocyte derived dendritic cells (moDCs) in response to Wt measles virus, and its respective C- and Vprotein KO strains at MOIs of 0.01 and 0.1. IFIT (a) and Mx1 (b) mRNA fold changes were log transformed and compared to the baseline expression of mock-infected moDCs using a two-way ANOVA, with a Bonferroni multiple comparisons correction. (*) p < 0.05. 59 Figure 9. Positive regulators of Th17 cell differentiation, IL-1β, IL23A and IL-6, expression levels from moDCs in response to Edmonston and Bilthoven measles virus infection at MOIs of 0.4 and 4.0. IL-1β (a), IL-23A (b), and IL-6 (c) mRNA fold changes were log transformed and compared to the baseline expression of mock-infected moDCs using a two-way ANOVA, with a Bonferroni multiple comparisons correction. (**) p < 0.01. 60 Figure 10. Positive regulators of Th17 cell differentiation, IL-1β and IL-6, expression levels from moDCs in response to wild-type measles virus and its respective C- and V-protein knock out strains at MOIs of 0.01 and 0.1. IL-1β (a) and IL-6 (b) mRNA fold changes were log transformed and compared to the baseline expression of mock-infected moDCs using a two-way ANOVA, with a Bonferroni multiple comparisons correction. None of the changes in transcript levels were significant. Figure 11. Negative regulators of Th17 cell differentiation, IL-27 and IL-10, mRNA expression levels from moDCs in response to Edmonston and Bilthoven measles virus infection at MOIs of 0.4 and 4.0. IL-27 (a) and IL-10 (b) mRNA fold changes were log transformed and compared to the baseline expression of mock-infected moDCs using a two-way ANOVA, with a Bonferroni multiple comparisons correction. (**) p < 0.01. 61 Figure 12. Negative regulators of Th17 cell differentiation, IL-27 and IL-10, mRNA expression levels from moDCs in response to wild-type measles virus and its respective C- and V-protein knock out strains at MOIs of 0.01 and 0.1. IL-27 (a) and IL-10 (b) mRNA fold changes were log transformed and compared to the baseline expression of mock-infected moDCs using a two-way ANOVA, with a Bonferroni multiple comparisons correction. None of the changes in transcript levels were significant. 62 Chapter Three: Effects of vitamin A supplementation on the immune response and the Th17 response to measles virus infection in rhesus macaques 63 Introduction Immunization is the most effective way to reduce the morbidity and mortality due to measles virus infection. However, many regions of the world do not have the resources to efficiently deliver two doses of vaccine to their populations. To reduce the morbidity and mortality in children that develop measles, vitamin A supplementation is recommended by the World Health Organization and the United Nations Children’s Fund (119, 120). Vitamin A therapy is inexpensive to administer at just $0.35 in US dollars per patient (122). Additionally, this supplementation decreases the amount of money spent on healthcare by shortening the time spent in the hospital due to measles by 2.9 days on average (122). However, the mechanism by which vitamin A protects against severe measles virus-induced disease is currently unknown. Vitamin A and measles virus infection Vitamin A is obtained from the diet in several forms and can be acquired from fruits, vegetables and leafy greens as β-carotene, or from animal-based food products, such as meat and dairy, that offer preformed vitamin A. (141, 142) Vitamin A is an essential vitamin that is involved in a wide range of biological processes, including vision, immunity, growth and development, as well as cellular differentiation (143). For the immune system vitamin A is necessary for B and T cell proliferation, T cell activation, and enhances the antigen-presenting ability and maturation of dendritic cells 64 (139). In its bioavailable form, vitamin A is found in the body as serum retinol bound to retinol binding protein (RBP) (114). Serum retinol is transferred to peripheral tissues from the liver, where it is stored. Serum retinol levels can be depleted during the acute phase of infection with many pathogens and epidemiologic data indicate that infection with measles virus often precedes vitamin A deficiency (27, 108, 115, 122, 123, 124). Importantly, hyporetinolemia due to measles occurs regardless of vitamin A status of the individuals before infection, and this has been observed in both vitamin A sufficient and deficient populations (116, 117). Plasma vitamin A depletion may be due to increased metabolic utilization of vitamin A in peripheral tissues (26, 108, 119), less hepatic RBP production (118), or increased losses of RBP and retinol through urinary excretion (119121) (Figure 1). Increased urinary excretion is associated with decreased reabsorption of fat-soluble vitamins and low-molecular weight proteins such as RBP after these molecules are filtered through the glomerulus of the kidney, due to damaged proximal tubular epithelium (119). Renal tubular damage can be mediated by inflammatory cytokines, such as IL-1, IL-6, and TNF, released during acute phase responses to infection (128). It is important to note that, serum retinol levels may not correlate with body stores of vitamin A (109). In healthy human adults fed a vitamin Adeficient diet, vitamin A levels can remain stable through usage of hepatic stores for months before an effect is noticed (129). However, after several 65 days of continued retinol loss, liver vitamin A stores may be significantly diminished (119). A diet high in vitamin A is not necessary for healthy adults, due to the storage capacity of the liver. However, the continued loss of vitamin A that may accompany measles virus infection could deplete liver stores, leading to low serum retinol levels. While a sharp drop in vitamin A levels has been observed (Figure 2), during the acute phase of measles virus infection in rhesus macaques, this is reversible (108, 115-117). As children with measles recover and enter the convalescent phase, vitamin A levels in their plasma begin to return to normal levels (113). Peripheral tissues may then have access to normal amounts of circulating vitamin A. However, total body stores may still be depleted for months post-infection, as has been observed in cases of influenza in children (132). This is problematic, because vitamin A deficiency increases the severity of disease, resulting from bacterial, viral or parasitic infections (131), and children in developing countries show increased levels of morbidity and mortality due to respiratory infections and diarrhea for a year after apparent recovery from measles virus infection (133, 134, 135, 136). Role of vitamin A in CD4+ T cell differentiation Vitamin A supplementation has been associated with improved clinical outcomes in patients with measles when given during the acute rash phase of disease (27-29, 122, 123). However, there is no evidence for a direct effect of this supplementation on cytokine production or lymphocyte activation (162). 66 Though, it does appear that in vaccination models, signaling of retinoic acid occurs in the presence of an inflammatory environment (163). The metabolites of vitamin A play critical roles in the differentiation of T helper cell subsets. Retinoic acid plays a direct role in suppression of the Th1 response, while simultaneously enhancing Th2 development (165-167). The interaction of vitamin A with differentiating regulatory T cells and Th17 cells is slightly more complex. Activated CD4+ T cells in the context of TGF-β differentiate into T regulatory cells, unless IL-6, IL-1β, and IL-23 or IL-21 are also present (168). Low retinoic acid concentrations are necessary for Th17 cell differentiation (169). However, all-trans retinoic acid has been implicated in inhibition of the Th17 response, and promotion of transcription factor Foxp3 expression in Treg cells in vitro (163, 164). After a certain threshold in vitamin A concentration is reached, CD4+ T cells will differentiate down the T regulatory cell pathway in the context of TGF-β, regardless of the presence of an inflammatory environment (169). It has been hypothesized that immune tolerance in mucosal tissues, could provide beneficial immune effects for the outcome of measles (170). Vitamin A supplementation Vitamin A supplementation to mitigate infection-induced deficiency is thought to be one of the safest, most efficacious and affordable therapeutic approaches to decreasing measles mortality (140). In the early 1930’s, vitamin A supplementation was first found to positively affect the outcome of 67 measles virus infection in children less than five years of age, by leading to a decrease in deaths and less severe pulmonary complications (107). The beneficial effects of vitamin A in children with measles are numerous (27, 121,122). Vitamin A supplementation during the acute phase of measles virus infection leads to better health outcomes (27-29, 122, 123) and does not increase retinol or retinyl acetate excretion (119), suggesting that vitamin A supplementation is an effective way to increase retinol levels. A study of children in New York City with measles in 1992 demonstrated that nearly 25% were hyporetinolemic, although vitamin A deficiency is rarely seen in this location. In this study, low plasma vitamin A levels were associated with more severe measles, as indicated by higher and more prolonged fever and a longer hospital stay (113). A study of children in Milwaukee, Wisconsin also associated depressed vitamin A levels with increased severity of measles (159). In Zaire, low serum vitamin A levels were associated with increased measles mortality in children less than 2 years old (13). Because many studies demonstrate that vitamin A supplementation decreases the risk of mortality in children less than 2 years of age, risk from low vitamin A levels may be age-dependent. However, the specific function and mechanism by which vitamin A alters the course of infection with measles virus is unknown. 68 Vitamin A: Potential roles in improving measles outcome Repair of lung epithelium It is possible that vitamin A functions in a multi-faceted way to protect against measles virus-associated morbidity and mortality. Vitamin A may mitigate measles virus-induced damage to respiratory epithelial surfaces (108). During measles virus infection of the respiratory tract, the lung shows epithelial changes similar to those that occur during vitamin A-deficiency (107). This may lead to a need for additional vitamin A for repair and healing of the lung epithelium following infection with measles virus (108). Effect on lymphopenia and T cell-mediated viral clearance The effects of borderline vitamin A deficiency on immune function was explored by Clive West, using Newcastle disease virus, a paramyxovirus that infects poultry with many characteristics in common with measles virus. In this study, vitamin A deficiency was associated with lymphopenia, which was further exacerbated by infection (137). Lymphopenia describes a condition where the number of circulating lymphocytes are decreased compared to normal and is a characteristic of the febrile stage of measles virus infection (138). Measles-induced lymphopenia may be due to an elevated cortisol response due to stress and the acute phase reaction, to altered trafficking and sequestration of lymphocytes in peripheral lymphoid tissues, or to destruction of measles virus-infected T and B cells (130). Thus, vitamin A 69 deficiency could further exacerbate measles-related lymphopenia, which suggests that vitamin A supplementation may lessen lymphocyte loss. It is possible that measles- or vitamin A-related lymphopenia could weaken the T cell response to infection through a decrease in circulating T lymphocytes. Clearance of infectious measles virus and RNA is accomplished by the adaptive cellular immune responses (1). Viremia in measles virus infections lasts until approximately two weeks post-infection (68). When CD8+ T cells are depleted, rhesus monkey studies show that peak viral titers are elevated and the period of viremia is extended (65, 66). Inhibition of viral replication Vitamin A supplementation could also have effects on viral replication and clearance. In vitro, retinoids induce type I interferon and directly inhibit replication of measles virus (110, 111). However, in vivo there is little evidence that type I interferons (α/β) are produced during the early stage of disease (144) and vitamin A supplementation had no effect on the viral load of a related morbillivirus, canine distemper virus in ferrets (112). Once infectious virus has been cleared, measles virus RNA can persist for several months in the respiratory tract, urine, PBMCs and lymphoid tissues (1, 68, 125) (Figure 3). Sequencing suggests that the prolonged presence of measles virus RNA is due to slow clearance rather than mutation to evade the host immune response (68). Clearance of measles virus is 70 delayed in cases of malnutrition, supporting the idea that vitamin A supplementation may facilitate viral clearance (126, 127). Enhanced antibody response Vitamin A could also play a role in improving the host antibody response, as it is required for the development of both B cells and T helper cells (137). In children with measles, low plasma retinol levels are correlated with a low measles virus-specific antibody response (113) and decreased levels of measles virus antibodies have been associated with increased measles mortality (130). This suggests that vitamin A deficiency may exacerbate measles, by preventing a robust antibody response to infection. Measles virus-specific IgG antibody concentrations correlate closely with disease outcome, with higher antibody responses associated with less severe morbidity and mortality (29). A randomized trial of vitamin A supplementation in African children two years or younger showed higher measles virus-specific IgG antibody concentrations compared to the nontreated group at day 8 after onset of rash, as well as a significant reduction in mortality (29). Th17 response to measles virus infection It has been noted that measles induces an early CD8+ T cell response along with a CD4+ Th1 polarized response, which later shifts towards a CD4+ Th2 response (138). However, it has not been determined how CD4+ Th17 cells may contribute to the measles immune response. Th17 cells are a 71 unique lineage of CD4+ T helper cells, defined by their ability to secrete IL17A, IL-17F, and IL-17AF (103). Recently, measles virus-specific IL-17producing cells were shown to display a bi-phasic pattern with peaks at day 18 and day 35 in response to measles virus challenge in a naïve monkey and at day 10 and day 35 in monkeys vaccinated with a poorly protective vaccine (102). It is currently unknown whether or not Th17 cells play a role in the immune response to natural measles virus infection in humans, or if vitamin A plays a role in this process. Materials and methods Animals Six male 3-year-old juvenile rhesus macaques (Macaca mulatta) that were measles naïve (14Y, 17Y, 24Y, 31Y, 46Y, and 50Y) were obtained from the Johns Hopkins Primate Breeding Facility. All studies were performed in accordance with experimental protocols approved by the Animal Care and Use Committee for Johns Hopkins University and animals were maintained within these guidelines. Before infection, animals were shaved and baseline measurements were taken for all PBMC and plasma parameters to be assessed after infection. Measles virus infection and vitamin A supplementation The DI-free stock of wild-type Bilthoven measles virus (a gift from Albert Osterhaus at Erasmus University, Rotterdam, Netherlands) used to infect the monkeys was grown in human cord blood cells by Wendy Lin, 72 assayed by plaque formation on Vero/hSLAM cells, and stored at -80°C. All six monkeys were challenged intratracheally with 104 plaque-forming units (pfu) in 1 ml PBS. After development of the characteristic measles rash, three monkeys (14Y, 24Y, and 50Y) were given vitamin A in the form of retinol palmitate (Vitamin Angels, Parsippany, NJ). The oral liquid contents from each 100,000 IU capsule were mixed with strawberry jam and two doses were given to monkeys at the time of the rash, days 10 and 11 post-infection. Monkeys 17Y, 31Y and 46Y received the strawberry jam without vitamin A. In collaboration with the laboratory of Dr. Richard Semba, HPLC analysis was done on plasma samples from all monkeys to monitor retinol levels before and after infection. Sample collection For all procedures, monkeys were anesthetized with 10-15 mg/kg body weight of ketamine. Approximately 5 ml of heparinized blood was collected from the femoral vein of each animal before infection and on days 7, 10, 18, 21, 28, 35, 52, 56, 71/72, and 84 after infection. Comprehensive complete blood counts (CBCs) were done on each animal from day 10, onwards. Peripheral blood mononuclear cells (PBMCs) and plasma were isolated from blood by whole blood gradient centrifugation on Lympholyte®-Mammal (Cedarlane Labs). Plasma samples were stored at -20°C and 2 million PBMCs were flash frozen for RNA isolation at -80°C. Bone marrow biopsies were 73 collected at days 14, 28, 39, 56, and 84. Mononuclear cells were isolated from bone marrow using a Lympholyte®-Mammal (Cedarlane Labs) gradient centrifugation. Any mononuclear cells from blood or bone marrow that were not used fresh in assays were transferred to 1 ml of cold freezing media (90% FBS, 10% DMSO) and stored in a Nalgene Mr. Frosty™ Freezing Container for 1 hour at 4°C and -80°C overnight before moving to permanent storage in liquid nitrogen. Nasal secretions were collected using one sterile cotton swab per nostril on days 0, 7, 10, 14, 17, 21 and 39 to monitor measles virus shedding from the respiratory tract. Swabs were immersed in PBS, which was centrifuged to collect the cell and supernatant portions and stored at -80°C. Skin biopsies of the rash were obtained using a skin punch, collected into PBS, transferred to 4% paraformaldehyde and stored at 4°C for several days before transferring back to PBS for long-term storage at 4°C. Lymph node biopsies were collected from 3 monkeys (14Y, 17Y, 24Y) on day 71 and the remaining 3 monkeys (31Y, 46Y, 50Y) on day 78. Virus infection assays The amount of infectious virus present in the blood was measured by co-cultivating serially diluted fresh PBMCs (105 to 100) with Vero/hSLAM cells. After incubation for 5-6 days at 37°C 5% CO2, the tissue culture infectious dose 50 (TCID50) was determined by assessing the cytopathic 74 effects, observed as cell clearing, syncytia, or dead floating cells. Viremia was then expressed as the number of infected cells per million PBMCs. Nasal swab RNA analyses Debra Hauer did the following RNA analyses, using the RNeasy® Plus Micro Kit (Qiagen) to isolate RNA from nasal swabs. RNA was eluted in 30 ul of RNase-free water (Qiagen), and 10 ng or 10 ul were used for RT-PCR. Primers MV41 and MV42 were used to amplify a 350 base pair sequence within the measles virus N gene (Table 1), and human β-actin RT-PCR primers (Agilent) were used as a control. Skin punch and lymph node biopsies Skin punch biopsies were taken of an area of rash on the belly of each monkey on day 10. Biopsies were fixed in 4% PFA at 4°C for 48 hours and stored in PBS. Skin samples were sent to the Johns Hopkins Hospital Reference Histology Lab for paraffin embedding, sectioning, and hematoxylin and eosin staining. Pathology was read by Victoria Baxter, DVM. Portions of lymph nodes from each monkey were fixed in 4% PFA at 4°C overnight, and stored longer term in PBS at 4°C prior to sending lymph nodes to the Johns Hopkins Hospital Reference Histology Lab for paraffin embedding, sectioning, and hematoxylin and eosin staining. Antibody and cytokine assays Plaque reduction neutralization tests (PRNTs) were done to measure the amount of antibody in plasma samples needed to reduce plaque formation 75 by a DI-free stock of the Edmonston measles virus vaccine strain on Vero cells by 50%. Plasma was serially diluted from 1:3 to 1:30,000 in DMEM supplemented with 10% FBS, 1% L-glutamine, and 1% penicillin/streptomycin. Each dilution was incubated with 250 pfu of Edmonston measles vaccine strain virus in 200 ul, plated in a 6-well plate, and incubated in triplicate at 37°C, 5% CO2 for one hour, shaking plates every 20 minutes. Each well was then overlayed with a mixture of one part 1.2% bacterioagar and one part 2X MEM supplemented with 2% FBS, 1% penicillin/streptomycin and 1% L-glutamine, before incubating for 5 days at 37°C, 5% CO2. Data are expressed as the geometric mean of the titer. Enzyme immunoassays (EIAs) were conducted to determine the levels of measles virus-specific IgG and IL-17 in plasma. For the measles virusspecific IgG EIA, Nunc 96-well Maxisorp plates were coated with 50 ul of virus antigen (Rubeola/Measles Edmonston Strain Inactivated Vero Cell Extract; ABI) at a 1:25 dilution and incubated overnight at 4°C. The next day, the plate was blocked with 1% bovine serum albumin (BSA) in PBS for 2 hours. Plasma was added to wells neat, diluted 1:25 in PBS 10% FBS and then serially diluted two-fold to 1:25,600 for another overnight incubation at 4°C. On day three, anti-monkey horseradish peroxidase (HRP)-conjugated IgG antibody (Southern Biotech) was diluted 1:5000 in PBS 10% FBS and 50 ul was added to each well and incubated at 37°C for one hour. The plates were then developed in the dark with 3,39,5,59-tetramethylbenzidine (TMB) 76 substrate solution. After 5-7 minutes, sulfuric acid (2M) stop solution was added and the optical density was read at 450 nm. Data are presented as the reciprocal of the highest dilution with an OD absorption value greater than two times the average background. IL-17A EIAs were done using the ELISA for Monkey IL-17A kit (Mabtech). Corning Costar ELISA plates were coated with monoclonal capture antibodies and incubated overnight at 4°C. The next day, the plate was blocked with PBS 0.05% Tween 20 0.1% BSA for one hour at room temperature. Standard curves were established by using 2-fold dilutions of recombinant human IL-17A (Mabtech) from 1000 pg/ml to 7.5 pg/ml, accounting for background levels at 0 pg/ml. Plasma samples were diluted 2fold from 1:30 to 1:3840, added to the plate, and incubated at room temperature for 2 hours. Biotinylated monoclonal detection antibody (Mabtech) was then added to each well and the plate was incubated at room temperature for one hour. Streptavidin-HRP (Mabtech) was diluted 1:1000 and added to the plate, then incubated at room temperature for 1 hour. After washing, the plate was developed at room temperature by adding TMB substrate solution in the dark for 15-30 minutes. Sulfuric acid (2M) stop solution was added and the plate was read immediately at 450 nm. Because concentrations were too low to extrapolate from the human recombinant IL17 standard curve results were reported as a positive or negative for plasma neat or for the 1:30 dilution. 77 Antibody-secreting cell assays Mononuclear cells from the blood and bone marrow were used to measure antibody-secreting cells (ASC). A Multiscreen® HTS HA Opaque 96well filtration plate (Millipore) was coated overnight with either Rubeola/Measles (Edmonston Strain) Inactivated Vero Cell Extract (ABI) at a 1:25 dilution, or anti-monkey IgG, IgA, IgM (Sigma) at a 1:50 dilution and then stored overnight at 4°C. The plate was blocked the next day with RPMI media/10% FBS for 1 hour at 37°C. This was then replaced with fresh RPMI/10% FBS and 5x105 fresh PBMCs, and serial two-fold PBMC dilutions. If bone marrow mononuclear cells were used, the same dilutions were used for the total Ig. To detect the measles virus-specific ASC response; 5x105 cells were added to replicate wells. Plates were then incubated for 5-6 hours at 37°C 5% CO2. Goat anti-monkey IgG-HRP conjugated antibody (Nordic MUbio) was added to each well and incubated overnight at 4°C. The next day, the plate was developed with stable diaminobenzidine (DAB) solution (Invitrogen) for 5-7 minutes in the dark. The plate was thoroughly rinsed with DI water and left to dry before being read with the ImmunoSpot® plate reader. ImmunoSpot® 5.0 software was used for counting spots and quality control analysis to provide final counts, which are reported as measles virusspecific ASCs or spot-forming cells (SFCs)/106 PBMCS or ASCs/106 bone marrow mononuclear cells. 78 T cell assays Enzyme-linked Immunosorbent Spot (ELIspot) Assays were used to monitor T cells producing IFN-γ and IL-17. Multiscreen® HTS HA Opaque 96-well filtration plates (Millipore) were coated with purified mouse antihuman IFN-γ antibody (BD Biosciences) at 2ug/ml or anti-human IL-17A (eBioscience) at 5 ug/ml overnight at 4°C. The plates were blocked for an hour at 37°C with RPMI 10% FBS blocking media. Peptides or cell stimulants were then added to the plate. IFN-γ assays were in duplicate and were either non-stimulated, or stimulated with 1 ug/ml of pooled H, N, or F measles virus overlapping peptides or 5 ug/ml of concanavalin A (ConA). IL-17 ELIspot assays were done in duplicate and were either non-stimulated or stimulated with 5.8 ug/ml ABI measles virus-infected Vero cell lysate, or 5 ug/ml ConA. PBMCs were then added, with 105 PBMCs for IFN-γ and ConA and 5x105 PBMCs for IL-17 non-stimulated and measles virus lysate wells. Plates were then incubated at 37°C, 5% CO2 for 40-42 hours. Biotinylated anti-human IFN-γ antibody (Mabtech AB) was diluted in PBS 2% FBS 0.05% Tween, to 1 ug/ml and biotinylated anti-human IL-17A antibody (eBioscience) was diluted to 2 ug/ml and added to appropriate wells before incubating at 37°C for two hours. Avidin-HRP (BD Biosciences) diluted 1:2000 was added to all wells and incubated at 37°C for one hour. Stable DAB substrate (Invitrogen) was then added and incubated in the dark at room temperature for 5-7 minutes. After rinsing the plate thoroughly with DI water and allowing it to dry 79 completely, the plates were read and analyzed using the ImmunoSpot plate reader and ImmunoSpot 5.0 software. Data are presented as SFCs/106 PBMCs for spontaneous production and for measles virus-specific SFCs after subtracting the no cell/no in vitro stimulation SFCs. Flow Cytometry Flow cytometry was used to evaluate the Th17 response in PBMCs from day 0, 10, 18, 28, 39, 56, and 84. PBMCs were stimulated under four separate conditions with peptide mixes for measles virus-specific H protein and N protein, dimethyl sulfoxide (DMSO) as a negative control or staphylococcal enterotoxin B (SEB) as a positive control. Anti-CD28 and antiCD49 were included with the H and N peptides and DMSO. All stimulation mixes included GolgiStop (BD Biosciences) and GolgiPlug (BD Biosciences). The Th17 panel surface markers assessed varied slightly, but stimulation conditions and intracellular staining targets remained the same. Live/Dead® Fixable Violet Dead cell Stain Kit (Invitrogen) was used to stain and gate out dead cells. Prior to surface staining, cells were incubated with a human FcR binding inhibitor (eBiosciences). Surface markers that remained constant in all Th17 panels were anti-CD4 and anti-CD3. For earlier time points a ―dump gate‖ was used to gate out CD14- and CD20-positive cells and anti-CD8 was present in the panel. Intracellular staining was done following fixation and permeabilization of cells. For early time points the Cytofix/Cytoperm™ Fixation and 80 Permeabilization Kit (BD Biosciences) was used according to directions. At later time points, the Foxp3 Staining Buffer Set (eBioscience) was used. Intracellular staining was done to detect the transcription factor RORγt as well as IL-17A and IL-21 cytokines. Samples were run on the BD FACS Canto II™ flow cytometer and were analyzed using BD FACSDiva and FlowJo software. Results Measles virus infection of monkeys Six naïve rhesus macaques were successfully infected with measles virus after intratracheal challenge with 104 pfu of DI-free wild-type Bilthoven measles virus. Monkeys 14Y, 24Y and 50Y received vitamin A supplementation at days 10 and 11 post-infection, while monkeys 17Y, 31Y and 46Y did not. The presence of infectious virus in the blood was monitored using PBMC co-cultivation. All six monkeys established viremia by day 7 post-infection, and cleared infectious virus from PBMCs by day 18 (Figure 4). Nasal swabs were used to collect RNA from respiratory secretions, and RT-PCR confirmed that measles virus N gene RNA was present in the nasal passages of all monkeys (Table 2). All monkeys were shedding measles virus in respiratory secretions early in infection, though the duration of shedding varied. Monkeys 17Y and 50Y were positive for measles virus in respiratory secretions from day 7 to day 21, whereas, for 24Y, measles virus was only detected on day 14 post-infection. 81 Over the course of infection, the weights of monkeys were monitored as a measure of morbidity, and were reported as percent change from day 0 (Figure 5). Weights fluctuated over time between individual monkeys, but most exhibited weight gain throughout day 70. By day 84, two of the six monkeys returned to their original weight at time of infection. Two of the three monkeys supplemented with vitamin A gained approximately 10% of their body weight, while the remaining two monkeys (one treated, and one non-treated with vitamin A) lost about 4% of weight by day 84. Measles virus maculopapular rash The characteristic maculopapular rash also developed around day 10 in all monkeys, but to varying degrees (Figure 6). Rash biopsies confirmed a mild to moderate infiltration of inflammatory cells into the skin epithelium at this time (Figure 7). Monkeys that shed measles virus for longer periods of time (17Y, 50Y) exhibited a more extensive rash and more severe inflammatory infiltration in the skin biopsy sections. Monkeys 17Y, 14Y, and 50Y showed mild-to-moderate infiltration of inflammatory cells into the dermis, consisting primarily of mononuclear cells (lymphocytes and macrophages), with some eosinophils and neutrophils (Figure 7a, 7b, 7c). Severe inflammation was evident in 17Y by cells forming large aggregates or found individually across the dermis. Perivascular cuffing by macrophages was also seen in the biopsy section of monkey 14Y. All three of the 82 aforementioned monkeys had a notable amount of debris, suggesting necrosis of the superficial dermis, which correlates with the severity of their rash. Monkeys 46Y, 31Y, and 24Y showed mild infiltration of inflammatory cells into the dermis, listed in decreasing order of severity. Of the inflammatory cells present, most were lymphocytes and macrophages, with occasional eosinophils. Inflammatory aggregates were observed in rash biopsies of 46Y and 31Y. Some perivascular cuffing, without major aggregation was observed in the biopsy of 24Y (Figure 7d). While none of the rash biospies showed inflammatory infiltration into the epidermis, a majority of the inflammatory cells were present in the superficial dermis in 31Y. Lymph node biopsies are depicted for vitamin A-supplemented monkeys 14Y (day 71) and 50Y (day 78) (Figure 8). Lymph nodes were reactive, with increased cellularity that included plasma cells and macrophages in addition to lymphocytes. Lymph node histology was comparable between vitamin A-supplemented and non-supplemented monkeys at these days. Levels of vitamin A Three monkeys (14Y, 24Y, and 50Y) were supplemented with vitamin A (100,000 IU) on days 10 and 11 post-infection. Vitamin A levels were monitored through day 21 post-infection (Figure 9). At this point the serum retinol levels in the vitamin A-supplemented and non-supplemented groups of monkeys begin to diverge (Figure 9b). It is expected that the vitamin A 83 levels in the non-supplemented group of monkeys (17Y, 31Y, and 46Y) will continue downward until approximately day 50, as this was previously observed in two non-supplemented rhesus macaques following measles virus infection (Figure 2). The limited data obtained demonstrated a statistically significant decrease in serum retinol at day 21 in the non-supplemented group of monkeys. This was not observed in the vitamin A supplemented group. Plasma retinol levels at subsequent times will be determined when all samples have been collected at the end of the study period (day 180). Comprehensive blood counts Comprehensive blood counts were done on day 10 and on every bleed thereafter to assess changes in leukocyte populations. Cell counts were reported for white blood cells (WBCs), lymphocytes, and neutrophils, as well as the lymphocyte:neutrophil ratio (Figure 10). There was a high level of individual variation in WBCs among the monkeys (Figure 10a). Monkey 50Y (VA+) exhibited high WBC and lymphocyte counts, that remained above the normal range for most time points (Figure 10a, 10c). However, monkey 14Y (VA+) had below normal WBC and lymphocyte counts (Figure 10a, 10c). Overall, the average WBC and lymphocyte counts remained in the normal range (Figure 10b, 10d), with individual increases after the rash (Figure 10a, 10c). There was considerable variation in neutrophil counts (Figure 10e), and the average number showed a transient increase post-rash (Figure 10f). The lymphocyte:neutrophil ratios were elevated above the expected value of 1, for 84 most monkeys and for all days following rash (Figure 10g, 10h). Differences between vitamin A-supplemented and non-supplemented monkeys were not identified. Frequency of CD4+ and CD8+ cells CD4+ and CD8+ T cell frequencies were reported as the percentage of the live, non-monocyte (CD14-), non-B cell (CD20-) population after gating on lymphocyte and single cell populations (Figure 11). The frequency of CD4+ cells for most monkeys were approximately 50% during the first month postinfection, but decreased approximately 10% for vitamin A-supplemented and non-supplemented groups of monkeys at day 39 (Figure 11a, 11b). The average CD4+ frequency increased in the vitamin A-supplemented group at day 56 (not significant), and returned to a similar frequency as the nonsupplemented group by day 84, though this difference was not statistically significant. All monkeys, except monkey 46Y, maintained similar frequencies of CD8+ cells throughout 3 months post-infection. Monkey 46Y exhibited a high frequency of CD8+ and low frequency of CD4+ cells for the first month. The CD4:CD8 ratios of monkeys are typically 2. These ratios increased for some monkeys early after measles virus infection, but were not identifiably different between the groups (Figures 11e, 11f). IFN-γ T cell response ELISPOT assays were used to detect IFN-γ-producing T cells specific for measles virus proteins H, N, and F (Figure 12). At day 21 post-infection, 85 all six monkeys had developed a measles virus H-specific IFN-γ response (Figure 12a, 12b), and monkeys 14Y, 46Y, and 50Y showed N- and F-specific IFN-γ responses (Figures 12c, 12e). Though individual differences were evident on the robustness of each response, the vitamin A-supplemented group and non-supplemented groups had similar average responses for each measles virus protein (Figures 12b, 12d, 12f). At day 35, the measles-specific IFN-γ-producing T cell response decreased to baseline, with the exception of an H-specific IFN-γ response detected for monkey 14Y at day 52. Th17 cell characterization by Flow Cytometry To determine if vitamin A supplementation impacted the Th17 effector cell population, the levels of IL-17 and IL-21 cytokine expression were determined from gated CD4+ T cell populations using ICS and flow cytometry (Figure 13, 14). Frequencies of IL-17+ and IL-21+ CD4+ T cells differed depending on the stimulation condition. Measles virus peptides from the H and N proteins were used to detect a measles virus-specific response, and staphylococcus enterotoxin B (SEB) was used to stimulate all PBMCs as a positive control. Frequencies of measles virus H-specific CD4+ IL-17+ cells increased in all monkeys from less than 0.2% at days 0 and 10, to 0.5%-3.25% at day 18 and then remained below 1% from day 28 (Figure 15a, 15b). Measles virus N-specific IL-17+ T cells were detected in monkey 31Y of approximately 3% 86 at day 18, and increased in monkey 17Y to over 1% between day 18 and day 56 (Figure 15c, 15d). The measles virus H-specific IL-21+ frequencies increased from an initial level below 0.15% on days 0 and 10, to between 1-4% for all monkeys at day 18 (Figure 16a, 16b). Subsequently, the response fluctuated with a decrease at day 28 for all monkeys and an increase at day 39 for half of the monkeys. The vitamin A-supplemented group demonstrated a higher Hspecific IL-21+ frequency at day 39 compared to the non-supplemented group of monkeys. At day 56, frequencies of H-specific IL-21+ cells were still elevated in some monkeys compared to their naïve state. These H-specific IL21+ frequency changes remained above their baseline frequencies through all time points, but there were notable differences between the magnitude and days of responses in individual monkeys, that were not specific to vitamin Asupplemented or non-supplemented monkeys. N-specific IL-21+ frequencies of CD4+ T cells varied between monkeys (Figure 16c). Monkey 50Y increased to 0.8% at day 10, and monkeys 31Y and 14Y exhibited a more robust increase at day 18, ranging between 4-5.5%. The remaining monkeys exhibited N-specific IL-21+ frequencies above their naïve states, but were variable and remained below 2%. However, monkey averages indicated a clear biphasic increase in N-specific IL-21+ frequencies at day 18 and day 39 (Figure 16d). A difference between vitamin Asupplemented and non-supplemented monkeys was not observed. 87 To further investigate Th17 cells and their response in vitamin Asupplemented and non-supplemented monkeys, transcription factor expression and cytokine production were investigated by comparing Th17 and non-Th17 populations. Th17 cells were defined as CD4+ IL-17+ T cells in this panel, and non-Th17 cells were CD4+ IL-17- T cells. These populations were plotted together as histograms of RORγt transcription factor expression, at days 0, 18, and 56 (Figure 17). At day 0, prior to infection, IL-17- and IL17+ populations had very low levels of RORγt expression. However, by day 18, both IL-17- and IL-17+ populations increased in RORγt expression, but the Th17 cells exhibited a more positive RORγt signal. At day 56, two distinct populations were evident and stimulated and unstimulated Th17 populations expressed higher levels of the transcription factor RORγt. Due to the rarity of double positive IL-17+IL-21+ cells, IL-17+ cells versus IL-17- cells were also plotted at days 0, 18, and 56 to compare the amount of IL-21 production between these two populations (Figure 18). At day 0, IL-17+ cells produced similar levels of IL-21 compared to the IL-17cells. However, by day 18, IL-17+ cells exhibited a slight increase in IL-21 production, but there was still considerable overlap with the IL-17- cells. By day 56 IL-17+ cells produced much higher levels of IL-21 than the IL-17cells. Detection of IL-17-producing T cells in response to measles virus infection was further explored with an ELISPOT assay (Figure 19). The 88 unstimulated response was not further stimulated ex vivo, so IL-17 spot forming cells in the unstimulated condition were due to in vivo stimulation in the infected monkey (Figure 19a, 19b). Measles virus-specific IL-17 T cell responses that were present in wells stimulated by measles virus infected Vero cell lysate (ABI) (Figure 19c, 19d). To provide a picture of the measles virus-only specific IL-17-producing T cell response the unstimulated responses were subtracted from the measles-specific responses (Figure 19e, 19f). In all monkeys, small measles virus-specific IL-17+ T cells were detected at day 14, but was more apparent at day 52 and decreased by day 71/72. There were no significant differences between the vitamin A-supplemented and non-supplemented monkey groups. To detect IL-17A cytokine in the monkey plasma, IL-17A ELISAs were performed (Table 3). Values were deemed significant if they were two times the background. Monkeys 17Y and 50Y exhibited higher and more consistent levels of IL-17A compared to the other monkeys, with 14Y and 31Y showing little to no detectable IL-17A in the plasma, despite the robust Th17 responses that were detected in other assays. Antibody response The measles-specific IgG binding antibody response was assayed by EIA using measles virus-infected Vero cell lysate (ABI) and anti-monkey HRP conjugated IgG antibody (Figure 20). Five of the monkeys developed a measles virus-specific antibody response, although monkey 24Y did not, 89 despite the presence of viremia and detection of measles virus shedding in nasal secretions indicating an infection occurred (Table 2, Figure 4). Data are reported as the reciprocal of the highest dilution with a value two times the background. For each monkey, the highest measles virus-specific IgG response was observed at day 52 (Figure 20a). There was no significant difference observed in the antibody response to measles virus infection in the vitamin A-supplemented and non-supplemented monkeys, however, there was a high standard deviation in the vitamin A-supplemented group as monkey 24Y did not have a detectable response (Figure 20b). The number of antibody secreting cells (ASCs) in PBMCs and bone marrow were measured. Total antibody-secreting cells increased in circulation early in response to measles virus infection, with a distinct peak at day 14 (Figure 21a, 21b), but few were identified as producing antibody to measles virus (Figure 21c, 21d). Measles virus-specific ASC were detected in blood in large numbers at day 52 (Figure 21c, 21d). Bone marrow (BM) was collected twice a month for the first two months, and once near the end of the third month. Total numbers of IgM, IgA, and IgG-ASCs from BM remained relatively stable until day 56, when monkeys displayed an increase in BM ASCs, which plateaued by day 84 (Figure 22a, 22b). At day 28, measles virus-specific ASCs were unable to be counted because of a signal that exceeded the countable threshold in monkeys 24Y and 50Y, though they were increased in monkeys 14Y, 17Y, 31Y, and 90 46Y. They then decreased at day 40, and subsequently increased again at day 56. Peaks of measles virus-specific ASCs were found at day 28 and day 56-84 (Figures 22c, 22d). For the two monkeys (17Y, 24Y) with data at day 84, this upward trend of measles virus-specific bone marrow ASCs continued. The non-supplemented group displayed higher measles virus-specific ASC in BM at day 28 and day 84. However, this was not significant and some points were unable to be calculated due to too many spots, or background levels that were too high to count the spots on the plate. Therefore, missing data points may change averages significantly. Neutralizing antibodies were detected by PRNTs in five of the six monkeys (Figure 23a). Monkey 24Y did not mount a neutralizing antibody response. All other monkeys showed a dramatic increase in neutralizing antibodies at day 14 and day 18, that remained elevated through day 84 (Figure 23b). Discussion Vitamin A supplementation has been associated with improved clinical outcomes in patients with measles when given during the acute rash phase (27-29, 122, 123). There are several theories for the potential ways in which vitamin A may improve the health of patients with measles. This study of vitamin A-supplementation of rhesus macaques was done to elucidate the effects of vitamin A on the immune response to measles infection. Measurement of T cell (Th1, Th17) and antibody (EIA, ASC, PRNT) through 91 day 84 has not shown a difference between vitamin A-supplemented and nonsupplemented groups. However, none of the hypotheses have been discounted due to small groups and missing data. In our study, differences in plasma retinol levels begin to diverge at day 21 between the vitamin A-supplemented and non-supplemented monkeys (Figure 9), and so differences observed due to vitamin A treatment are hypothesized to occur between days 21-50, or slightly thereafter, as this is when plasma retinol levels previously showed a decrease in non-supplemented monkeys (Figure 2). We also have yet to confirm that the amount of vitamin A supplementation given prevents this decrease after measles. Vitamin A and lymphopenia Measles virus-induced lymphopenia is transient, and lymphocytes return to normal or elevated levels in both vitamin A-supplemented and nonsupplemented monkeys shortly after rash subsides (Figure 10b). This occurs before the divergence of vitamin A levels is observed between the two monkey groups at day 21, and therefore, vitamin A supplementation will not affect measles virus-induced lymphopenia. However, the frequencies of CD4+ T cells dropped approximately 10% at day 39 for unknown reasons. The vitamin A-supplemented monkeys recovered more quickly (by day 56) than the nonsupplemented monkeys, who do not recover from this until day 84. This finding approached statistical significance (p < 0.1). It is possible that nonsupplemented monkeys have a vitamin A deficiency-induced lymphopenia 92 between day 39 and 84 and that larger groups of monkeys are needed to identify this difference (137). Vitamin A, measles virus replication, and clearance of measles virus persistent infection Vitamin A has also been previously suggested to inhibit measles virus replication in vitro (110, 111). However, viremia is established prior to vitamin A supplementation and non-supplemented monkeys (Figure 4), and is cleared by day 21 when retinol levels begin to drop. Future studies will determine whether vitamin A enhances the clearance of measles virus RNA. An increase in IL-21 producing T cells observed at day 39, was higher in the vitamin A-supplemented monkeys than the non-supplemented monkeys, and approached statistical significance (p < 0.1). This increase in IL-21-producing T cells in vitamin A-supplemented monkeys could increase Th17 cell differentiation (77, 103). This idea is supported by the increase in measles virus-specific IL-17-producing T cells at day 52 (Figure 19). IL-21 could also function to control persistent measles virus infection (171, 172). If this is the case, vitamin A may play a role in clearance of persistently infected cells. This mechanism still needs to be explored further in larger groups of monkeys and with quantitation of measles virus RNA. Vitamin A and the measles virus-specific antibody response Another theory of vitamin A function that may mitigate the severity of disease and death due to measles is that it plays a role in enhancing the 93 antibody response. However, monkey 24Y was supplemented with vitamin A, and over the course of infection did not appear to have a detectable IgG or neutralizing antibody response (Figure 20, 23), but did have detectable ASCs (Figure 21, 23). For EIA, this discrepancy may be due to lack of crossreactivity of the anti-human Ab and monkey IgG. Interestingly, the shedding of measles virus was only detected on one time point from monkey 24Y (Table 2). It is possible that no antibody class switching occurred, and any measles virus-specific antibody response mounted by monkey 24Y was a different isotype, though this has not been confirmed. Trends of ASCs in the peripheral blood and bone marrow show that 24Y has a decreased number of ASCs, though the variation in numbers over time follows the same trends as monkeys in both vitamin A-supplemented and non-supplemented groups (Figure 21, 22). Further investigation is needed to resolve this issue. Vitamin A supplementation in a randomized clinical trial in African children two years or younger showed higher measles virus-specific IgG antibodies compared to the non-treated group, as well as a significant reduction in mortality (29). At present, our data do not support this, but technical issues with detecting monkey IgG and with counting large numbers of ASCs need to be resolved. Measles virus-specific IFN-γ response The measles virus-specific IFN-γ response was predominantly Hspecific, and developed around day 21 post-infection along with small 94 numbers of N- and F-specific IFN-γ producing T cells. This response, along with cytotoxic CD8+ T cells, likely contributes to the rash and clearance of infectious virus (75, 79, 138), and the response wanes quickly. It appears that there is potential for a transient reappearance of this response later in the course of infection, as monkey 14Y exhibited a recurrence of H-specific IFN-γproducing T cells at day 52. Future studies will determine whether this is associated with an increase in viral RNA. Vitamin A had no identifiable effect on altering this response. Th17 biphasic response to measles virus infection Measles virus-specific IL-17-producing T cells were detected by ELISPOT with peaks at day 18 and day 56 following infection. This biphasic IL-17 producing T cell response was also identified by intracellular cytokine staining and flow cytometry, which confirmed increases in CD4+IL-17+ T cells at these days. Th17 cells were further characterized as having increased RORγt expression and higher levels of IL-21 production, compared to nonTh17 cells. Conditions, limitations, and future directions of vitamin A analysis No statistically siginificant differences in immune responses to measles virus infection were observed between the vitamin A-supplemented and non-supplemented monkey groups. Observed differences between the vitamin A-supplemented and non-supplemented groups had large, overlapping standard deviations among the averages for each group. The 95 vitamin A group included monkey 24Y, which did not mount detectable antibody or Th17 immune responses as well as monkey 14Y, which consistently had very high immune responses compared to other monkeys. These studies will be repeated to increase the numbers of monkeys in each group. It may be of interest to challenge 24Y again. Conclusion Thus far, the specific role of vitamin A in host response to measles virus infection still remains unclear. Vitamin A does not play a role in measles virus-induced lymphopenia, but may prevent later formation of lymphopenia that is induced by vitamin A-deficiency. Vitamin A may play a role in enhancing clearance of measles virus RNA from persistently infected cells and samples have been collected to determine this. We did not confirm a correlation between increased vitamin A levels and increased measles-specific antibody titers, and vice versa (29, 113, 130). Major differences were not observed in the IFN-γ-producing T cell response between vitamin Asupplemented and non-supplemented monkeys. Furthermore, this study has allowed the characterization of the Th17 response up to three months following measles virus infection in vivo, with major peaks of Th17 cells present around day 18 and day 56. Further analysis of samples from this study will explore the Th1 and Tfh responses, and other potential differences due to vitamin A supplementation. Three months of the study remain, and samples have been 96 archived so there is much data to be collected and analyzed yet. Additionally, qualitative imaging should be done using immunohistochemistry on the skin biopsies to confirm and localize measles virus in rash biopsy samples. The evaluation of measles virus RNA clearance will include studies to detect measles virus RNA in PBMCs, bone marrow, and lymph node cells (B cell, T cell, and monocyte populations), to determine the type of cell harboring measles virus RNA. This study is the first step towards elucidation of the role of vitamin A in providing better health outcomes following measles virus infection. Overall, the immune responses to measles virus infection varied between individual monkeys, as it does in the human population. The number of monkeys used needs to be larger to identify small differences due to vitamin A supplementation. 97 Table 1. PCR primers, target and cycling conditions for detection of the measles virus N gene. Primers for Detection of Measles Virus N Gene (IDT) Cycling Target Primer and Sequence Conditions 50°C, 30 min 94°C, 2 min 350 bp MV 41 94°C, 30 s; 55°C, 30 amplicon of 5’- CATTACATCAGGATCCGG -3’ s; 68°C, 45 s for 40 the measles MV 42 cycles virus N gene 5’- GTATTGGTCCGCCTCATC -3’ 68°C, 5 min; 4°C, hold Table 2. Measles virus shedding in respiratory secretions. Virus shedding was monitored by RT-PCR detection of measles virus N gene in RNA isolated from nasal swabs. (+) indicates a positive result, (-) indicates there was no amplified PCR product. (VA+) indicates monkeys supplemented with vitamin A at the time of rash (day 10, 11). Presence of Measles Virus RNA Day 14Y (VA+) 17Y 24Y (VA+) 31Y 46Y 50Y (VA+) 7 - + - - - + 10 + + - + + + 14 + + + + + + 18 - + - + - + 21 - + - - - + 39 - - - - - - 98 Table 3. IL-17A ELISAs. IL-17A ELISAs (Mabtech) were done on the plasma samples. Wells were considered to be positive if the absorbance was greater than two times the background absorbance levels. (+) plasma only, (++) 1:30 dilution, (-) no IL-17A detected. (VA+) indicates monkeys supplemented with vitamin A at the time of rash (day 10, 11). IL-17A ELISA Results Day 14Y (VA+) 17Y 24Y (VA+) 31Y 46Y 50Y (VA+) 0 - - - - + - 7 - + - - - - 10 - + - + + + 14 - + - - - + 18 - + + - + - 21 - + - - - + 28 - + - - - ++ 35 - + - - - - 40 - + - - - - 52 - + - - - ++ 56 - + - - - + 71/72 - - - - - - 84 - - - - - - 99 Figure 1. Vitamin A (retinol) status and usage is impaired during infection. During infection, decreased food intake can lead to lower vitamin A uptake. During infection, less retinol is absorbed and more is excreted in urine. Other hypotheses leading to vitamin A impairment include impaired transport of stores from liver to peripheral tissues or by increasing requirements at sites of inflammation [137]. Figure 2. Plasma retinol levels averaged between two rhesus macaques after measles virus infection [Lin, W.H, and Griffin, D.E., unpublished]. 100 Figure 3. Time course of measles virus clearance. Infectious virus shown in blue, viral RNA outlined by the dashed black line, in reference to the characteristic rash (red box) that occurs around day 10 [1]. Figure 4. Viremia is present by day 7, and is cleared in all animals by day 18. Viremia was measured by tissue culture infectious dose 50 (TCID50) assays, by co-cultivation of PBMCs from each animal on a monolayer of Vero/hSLAM cells. (a) Individual monkeys and (b) averages between the vitamin A-supplemented monkeys (14Y, 24Y, 50Y; dashed line) and the nonsupplemented monkeys (17Y, 31Y, 50Y; solid line) were compared using a two-way ANOVA and a Bonferroni multiple comparison correction. There were no statistically significant differences between the two monkey groups. 101 Figure 5. Change in total body weight over course of measles virus infection. (a) Changes in weight reported as the percent change from day 0 for each monkey. (b) Averages between the vitamin A-supplemented monkeys (14Y, 24Y, 50Y; dashed line) and the non-supplemented monkeys (17Y, 31Y, 50Y; solid line) were compared using a two-way ANOVA and a Bonferroni multiple comparison correction. There were no statistically significant differences between the two monkey groups. Figure 6. Maculopapular rash was very robust on monkey 50Y on day 10 post-infection. The upper limbs (a) and trunk (b) of 50Y showed the most extensive rash. 102 Figure 7. Rash histology of skin biopsies. Hematoxylin and eosin staining of skin punch biopsies. Rash severity was graded and monkeys were ordered from most severe to least severe: 17Y (a), 14Y (b), 50Y (c), 46Y (not pictured), 31Y (not pictured), and 24Y (d). Yellow boxes highlight areas of cellular debris and edema (a, c). White arrows point out eosinophils (a, b), grey arrows point out lymphocytes (a, b), and black arrows point out macrophages (a, b). Scale bars are 100 um in (a, b, d) and 50 um in (c). 400X. 103 a b c d Figure 8. Histology of lymph node biopsies. Lymph node biopsies are pictured for vitamin A-supplemented monkeys 14Y (day 71) at 2X (a) and 20X (b) and monkey 50Y (day 78) at 2X (c) and 20X (d). Lymph nodes are reactive, with other cells in addition to lymphocytes seen at 20X. Lymph node reactivity was assessed by Tori Baxter, DVM and Diane Griffin, MD, PhD. Scale bars are 1 mm (a, c) and 100 um (b, d). 104 Figure 9. Vitamin A levels begin to drop at day 21 in the nonsupplemented group of monkeys (17Y, 31Y, 46Y) but remain more stable in vitamin A-supplemented monkeys (14Y, 24Y, 50Y). (a) Retinol concentrations were determined for each monkey by HPLC analysis of plasma, by the laboratory of Dr. Richard Semba. (b) Averages between the vitamin A-supplemented monkeys (14Y, 24Y, 50Y; dashed line) and the nonsupplemented monkeys (17Y, 31Y, 50Y’ solid line) were compared using a two-way ANOVA and a Bonferroni multiple comparison correction. Results were significant at day 21. (**) p < 0.0 105 Figure 10. Comprehensive blood counts and differential leukocyte counts following rash. Space between the horizontal/dashed lines in each graph indicates the normal range of cell counts for rhesus macaques between 3-4 years old: 7,700-13,300 WBCs/ul, 2,671-8,350 lymphocytes/ul, and 2,6715,147 neutrophils/ul [54], and the normal lymphocyte:neutrophil ratio is 1 (a, c, e, g). Averages between the vitamin A-supplemented monkeys (14Y, 24Y, 50Y; dashed line) and the non-supplemented monkeys (17Y, 31Y, 50Y; solid line) were compared using a two-way ANOVA and a Bonferroni multiple comparison correction (b, d, f, h). There were no statistically significant differences between the two monkey groups. 106 Figure 11. Frequency of CD4+ and CD8+ cells within the CD14-CD20live cell population, and CD4:CD8 cell ratio. CD4+ and CD8+ T cell frequencies were determined by flow cytometry. Cells were first gated by lymphocytes and single cells, and frequencies were assessed in the nonmonocyte, non-B cell populations (a, c, e). Averages between the vitamin Asupplemented monkeys (14Y, 24Y, 50Y; dashed line) and the nonsupplemented monkeys (17Y, 31Y, 50Y; solid line) were compared using a ttest for data on each day (b, d, f). There were no statistically significant differences between the two monkey groups. 107 Figure 12. Measles virus H, N, and F protein-specific IFN-γ secreting T cells peak at 21 days post-infection. IFN-γ secreting T cells were detected by ELISPOT (a, c, e). Averages between the vitamin Asupplemented monkeys (14Y, 24Y, 50Y; dashed line) and the nonsupplemented monkeys (17Y, 31Y, 50Y; solid line) were compared using a two-way ANOVA and a Bonferroni multiple comparison correction (b, d, f). There were no statistically significant differences between the two monkey groups. 108 Figure 13. Intracellular staining for IL-17A. Gating scheme for IL-17 from CD4+ cells on day 0 (a), 18 (b) and 56 (c) post-infection is shown. On days 0 and 18 CD4+ cells were defined as CD14-CD20-CD3+CD8- and from day 28 onward as CD14-CD20-CD3+CD4+. Plots are shown for one vitamin A-supplemented monkey (50Y) in the top row, and one non-supplemented monkey (46Y) in the bottom row. Cells were stimulated with SEB. IL-17+ populations are the top square in each plot, and IL-17- populations are in the bottom square. Percentages of IL-17+ and IL-17- from CD4+ populations are indicated in their respective boxes. 109 Figure 14. Intracellular staining for IL-21. Gating scheme for IL-21 from CD4+ cells on day 0 (a), 18 (b) and 56 (c) post-infection is shown. On days 0 and 18 CD4+ cells were defined as CD14-CD20-CD3+CD8- and from day 28 onward as CD14-CD20-CD3+CD4+. Plots are shown for one vitamin Asupplemented monkey (50Y) in the top row, and one non-supplemented monkey (46Y) in the bottom row. Cells were stimulated with SEB. IL-21+ populations are selected in each plot, with percentages IL-21+ from CD4+ cells indicated in their respective boxes. 110 Figure 15. Frequency of IL-17+ cells as a percentage of total CD4+ T cells peaked at day 18. IL-17+ frequencies were determined by intracellular staining and flow cytometry (a, c, e). Averages between the vitamin A-supplemented monkeys (14Y, 24Y, 50Y; dashed line) and the nonsupplemented monkeys (17Y, 31Y, 50Y; solid line) were compared using a ttest for data on each day (b, d, f). There were no statistically significant differences between the two monkey groups. 111 Figure 16. Frequency of IL-21+ cells as a percentage of total CD4+ T cells showed peaks at day 18 and day 39 post-infection. IL-21+ frequencies were determined by intracellular staining and flow cytometry (a, c, e). Averages between the vitamin A-supplemented monkeys (14Y, 24Y, 50Y; dashed line) and the non-supplemented monkeys (17Y, 31Y, 50Y; solid line) were compared using a t-test for data on each day (b, d, f). There were no statistically significant differences between the two monkey groups. 112 Figure 17. RORγt expression was upregulated in CD4+ T cells by day 18 post-infection, and is higher in IL-17+ cells than IL-17- cells. The level of RORγt expression was detected using intracellular staining and flow cytometry and was evaluated on IL-17+ and IL-17- CD4+ T cells on day 0 (a), day 18 (b), and day 56 (c). CD4+IL-17- populations are in red and CD4+IL17+ cell populations are in blue. For a, b and c, RORγt shift data from nonsupplemented monkey 46Y is shown in the left column, and vitamin Asupplemented monkey 50Y is shown on the right for each day. The top row is from the DMSO negative control condition, followed by measles virus Hstimulated, N-stimulated and the bottom row depicts SEB-stimulated conditions. 113 Figure 18. IL-21 production begins to increase by day 18, and is much greater by day 56 post-infection in IL-17+ cells than IL-17- cells. The level of IL-21 expression was detected using intracellular staining and flow cytometry and was evaluated on IL-17+ and IL-17- CD4+ T cells on day 0 (a), day 18 (b), and day 56 (c). CD4+IL-17- populations are in red and CD4+IL17+ cell populations are in blue. A shift was used to compare IL-21 expression between IL-17+ and IL-17- populations, rather than looking at double positive populations, because the number of events were low for IL-17+ and IL-21+ populations. For a, b and c, IL-21 shift data from non-supplemented monkey 46Y is shown in the left column, and vitamin A-supplemented monkey 50Y is shown on the right for each day. The top row is from the DMSO negative control condition, followed by measles virus H-stimulated, Nstimulated and the bottom row depicts SEB-stimulated conditions. 114 Figure 19. IL-17A-secreting T cells are present in a biphasic response, with an early peak at day 14 and a late peak at day 52. IL17A secreting T cells were detected by ELISPOT assays (a, c, e). Unstimulated condition was not stimulated ex vivo (a, b). Measles virusspecific IL-17A T cells were detected by ex vivo stimulation with measles virus-infected Vero cell lysate (ABI) (c, d). The unstimulated response was subtracted from the measles virus-specific response to remove background and observe the measles virus-stimulated response only (e, f). Averages between the vitamin A-supplemented monkeys (14Y, 24Y, 50Y; dashed line) and the non-supplemented monkeys (17Y, 31Y, 50Y; solid line) were compared using a t-test for data on each day (b, d, f). There were no statistically significant differences between the two monkey groups. 115 Figure 20. Measles virus-specific IgG as detected by ELISA. Plasma from each monkey was used for an EIA detecting IgG that bound measles virus antigen. Results are plotted at the highest consecutive dilution two times above the background (a). Averages between the vitamin Asupplemented monkeys (14Y, 24Y, 50Y; dashed line) and the nonsupplemented monkeys (17Y, 31Y, 50Y; solid line) were compared using a ttest for data on each day (b). There were no statistically significant differences between the two monkey groups. 116 Figure 21. Total antibody- and measles virus-specific antibodysecreting cells in PBMCs as detected by ASC assays. ASCs detected to (a) total antibody or (c) measles virus-specific antibody in PBMCs. Averages between the vitamin A-supplemented monkeys (14Y, 24Y, 50Y; dashed line) and the non-supplemented monkeys (17Y, 31Y, 50Y; solid line) were compared using a t-test for data on each day (b, d). There were no statistically significant differences between the two monkey groups. 117 Figure 22. Total antibody- and measles virus-specific antibodysecreting cells in BM as detected by ASC assays. ASCs detected to (a) total antibody or (c) measles virus-specific antibody in bone marrow mononuclear cells. At day 28, measles virus-specific ASCs (c) were unable to be counted because of a signal that exceeded the countable threshold in monkeys 24Y and 50Y. Averages between the vitamin A-supplemented monkeys (14Y, 24Y, 50Y; dashed line) and the non-supplemented monkeys (17Y, 31Y, 50Y; solid line) were compared using a t-test for data on each day (b, d). There were no statistically significant differences between the two monkey groups. 118 Figure 23. Neutralizing antibody response as detected by PRNTs. Reciprocal titers of neutralizing antibodies were measured by 50% plaque reduction of a DI-free strain of Edmonston infection on Vero cells (a). Averages between the vitamin A-supplemented monkeys (14Y, 24Y, 50Y; dashed line) and the non-supplemented monkeys (17Y, 31Y, 50Y; solid line) were compared using a t-test for data on each day (b). There were no statistically significant differences between the two monkey groups. 119 Chapter Four: Discussion of the immune responses to measles virus infection in vitro and in vivo 120 The innate immune response to measles virus infection Understanding the immune response to measles virus infection in humans has been a difficult process. Individual differences in the immune response, as well as immune response differences due different strains of measles virus have complicated analysis and synthesis of an overall picture. The innate immune response was analyzed using several measles virus strains for in vitro infections of monocyte-derived dendritic cells. Dendritic cells play an important role in the innate immune response, but also provide the proper environment for the development of adaptive immune responses. The role of measles virus C and V proteins were explored using an infection model of moDCs to examine their role in type I interferon induction and how that may affect ISG/VSIG expression Type I interferon In these experiments, no type I interferon was detected after infection of moDCs by DI-free measles vaccine or Wt strains. Because the V and C proteins inhibit type I interferon induction and responses, the Wt C KO and Wt V KO strains of measles virus were expected to induce type I interferon expression. This was not observed in our studies. However, a previous study was able to detect interferon α and β following Wt C KO and Wt V KO measles virus infection in vivo (97). A more sensitive assay should be explored to provide definitive data on whether or not type I interferons are induced by the measles virus infections of moDCs. 121 The role of measles virus and its C and V proteins on interferonstimulated gene (ISG) and viral stress-induced gene (VSIG) expression Measles virus C- and V-KO viruses exhibited the ability to enhance the induction of IFIT1, with the V protein KO virus also enhancing Mx1 transcription. Under the assumption that the Wt C KO and Wt V KO viruses have produced low biologically active type I interferons, that are below our limit of detection, it is likely that these measles virus KO strains were able to activate the transcription of these genes through multiple mechanisms, including the viral stress-induced and interferon stimulated pathways. Contrastingly, the Wt measles virus, with the C and V proteins intact, were likely only to activate the viral stress-induced pathway alone. IFIT1 mRNA appears to be more efficiently induced than Mx1 in the first 48 hours following measles virus infection. Differences in ISG/VSIG expression showed that Edm vaccine strain exhibited a much greater upregulation of expression of these antiviral protein genes than the Wt strains. In the future, it would be interesting to include a comparison with a double C- and V-protein KO and to expand the analysis to Edm and its DI-free C- and V-deleted vaccine strains. The early adaptive immune response to measles virus infection Measles virus infection of rhesus macaques has provided the opportunity to evaluate development of the adaptive immune processes to infection. All monkeys displayed hallmark characteristics of measles virus 122 infection, with development of viremia and rash. All monkeys had mounted a measles virus-specific IFN-γ-producing T cell response around day 21 postinfection. The IFN-γ response correlated with clearance of infectious measles virus from blood between days 10 and 18. After viremia had been cleared, most IFN-γ-producing T cells disappeared from circulation by day 35 postinfection. These IFN-γ-producing T cells, and their role in the Th1 response, will be confirmed by flow cytometry analysis and intracellular cytokine staining to detect IFN-γ, IL-2, and TNF-α in CD4+ T cells. Data from in vitro measles virus infection show a significant upregulation of IL-27 transcripts in moDCs at 24 hours post-infection, which would support the differentiation of Th1 T effector cells. Th17 regulatory cytokine expression to measles virus infection Production of cytokines by moDCs that influence the adaptive immune response, favor the inhibition of Th17 cell differentiation. Cytokines that lead to a favorable environment for Th17 cell differentiation and survival are IL-6, IL-1β, and IL-23A, and although a non-significant trend of upregulation of pro-inflammatory cytokine transcripts (IL-1β and IL-6) was present, a significant downregulation of IL-23A mRNA and upregulation of IL-27 mRNA are predicted to prevent long-term differentiation of Th17 cells early in infection. Although mRNA expression of Th17 regulatory cytokines favors the moDC inhibition of Th17 cell differentiation within the first 48 hours following measles virus infection, a robust Th17 response was detected in 123 vivo at later time points. In the future it would be interest to test for the presence of these cytokines and examine APC function from samples in vivo, to cover more extensive time points because it is clear that a Th17 response is mounted in all monkeys. The Th17 response to measles virus infection The Th17 response to measles virus infection in monkeys is biphasic, with a small peak between weeks 2 and 3 post-infection, and a much larger response between weeks 5 and 6 post-infection. Detection of the early IL-17 response did not depend on ex vivo stimulation with measles virus antigens and this returns to baseline. These cells were likely stimulated in vivo. A second wave of the measles virus-specific Th17 response detected by ex vivo stimulation was established by day 52. Flow cytometry analysis of IL-17+ cells by intracellular cytokine staining showed that they also expressed RORγt and IL-21. The role of this response in maturation of the antibody response requires further study. The measles virus antibody response All monkeys had a large increase in circulating ASCs at day 14, but most were not producing detectable antibody to measles virus. However, large numbers of measles virus-specific ASC were present in the bone marrow at day 28. These decreased by day 40, and increased again by day 56. Measles virus-specific ASCs in PBMCs were detected at day 52, which likely trafficked to the bone marrow to establish a measles virus-specific, long-lived 124 plasma cell population. Measles virus-specific IgG was detected in plasma beginning at day 14, and continued to increase until day 52 when this response peaked. Avidity of this antibody has not yet been measured. The role of vitamin A on the immune response to measles virus infection Vitamin A was supplemented at the time of the rash, at day 10 and 11. Plasma retinol levels in the non-supplemented group of monkeys were first decreased at day 21 so it is expected that the major effects of vitamin A supplementation would not manifest until day 21 or later. It is likely that vitamin A plays no role in the prevention of the measles virus-induced viremia or lymphopenia that occurs early following infection, before vitamin A levels diverge. Furthermore, data from this study does not currently support previous findings that vitamin A correlates with a higher measles virus-specific IgG antibody response. The vitamin A-supplemented group did exhibit some changes that approached statistical significance. Monkeys that were supplemented with vitamin A recovered from a decrease in CD4+ T cells more quickly than nonsupplemented monkeys. In addition, higher numbers of IL-21-producing T cells were present at day 39 in the vitamin A-supplemented monkeys function to control persistent measles virus infected cells (171, 172). These observations and mechanism needs to be further explored. 125 Three months of this study remain and many samples have been archived, so much data has yet to be collected and analyzed. Vitamin A plays a role in differentiation of CD4+ T effector cell subsets, and data that has yet to be analyzed through flow cytometry and intracellular cytokine staining will further explore development of the Th1 and Tfh cells lineages. This experiment will also be repeated to increase the number of monkeys in each group. 126 References 1. Griffin, D. E., W. H. Lin, and C. H. Pan. 2012. Measles virus, immune control, and persistence. FEMS microbiology reviews 36(3): 649-662. 2. Moss, W. J., and D. E. Griffin. 2006. Global measles elimination. Nature reviews. Microbiology 4: 900–8. 3. Nandy, R., T. Handzel, M. Zaneidou, J. Biey, R. Z. Coddy, R. Perry, P. Strebel, and L. Cairns. 2006. Case-fatality rate during a measles outbreak in eastern Niger in 2003. Clinical infectious diseases 42(3): 322-328. 4. Wolfson, L. J., R. F. Grais, F. J. Luquero, M. E. Birmingham, and P. M. Strebel. 2009. Estimates of measles case fatality ratios: a comprehensive review of community-based studies. International journal of epidemiology 38(1): 192-205. 5. Bryce, J., C. Boschi-Pinto, K. Shibuya, and R. E. Black. 2005. WHO estimates of the causes of death in children. The Lancet 365(9465): 1147-1152. 6. Moss, W. J., and D. E. Griffin. 2011. Measles. The Lancet 379(9811): 153-164. 7. Moss, W. J. and D. E. Griffin. 2006. Global measles elimination. Nature Reviews Microbiology 4(12): 900-908. 8. WHO: Measles deaths decline, but elimination progress stalls in some regions. http://www.who.int/mediacentre/news/notes/2013/measles_20130117 /en/. 9. Loening, W. E. K., and H. M. Coovadia. 1983. Age-specific occurrence rates of measles in urban, peri-urban and rural environments: implications for time of vaccination. The Lancet 2: 324-6. 10. Aaby, P. 1988. Malnutrition and overcrowding/intensive expo- sure in severe measles infection: review of community studies. Rev Infect Dis 10: 478-91. 11. Coovadia, H. M., P. Brain, A. F. Hallet, A. Wesley, L. G. Henderson, and G. H. Vos. 1977. Immunoparesis and outcome in measles. The Lancet 1: 619-21. 127 12. Morley, D. 1969. Severe measles in the tropics. I. British medical journal 1(5639): 297. 13. Markowitz, L. E, N. Nzilambi, W. J. Driskell, M. G. Sension, E. Z. Rovira, P. Nieburg, and R. W. Ryder. 1989. Vitamin A levels and mortality among hospitalized measles patients, Kinshasa, Zaire. J Trop Paed 35: 109-112. 14. Grais, R. F., C. Dubray, S. Gerstl, J. P. Guthmann, A. Djibo, K. D. Nargaye, J. Coker, K. P. Alberti, A. Cochet, C. Ihekweazu, N. Nathan, L. Payne, K. Porten, D. Sauvageot, B. Schimmer, F. Fermon, M. E. Burny, B. S. Hersh and P. J. Guerin. 2007. Unacceptably high mortality related to measles epidemics in Niger, Nigeria, and Chad. PLoS medicine 4(1): e16. 15. Gay, N. J. 2004. The theory of measles elimination: implications for the design of elimination strategies. J Infect Dis. 13: S27–S35. 16. Dallaire, F., G. De Serres, F. W. Tremblay, F. Markowski, and G. Tipples. 2009. Long-lasting measles outbreak affecting several unrelated networks of unvaccinated persons. Journal of Infectious Diseases 200(10): 1602-1605. 17. Muscat, M., H. Bang, J. Wohlfahrt, S. Glismann, and K. Mølbak. 2009. Measles in Europe: an epidemiological assessment. The Lancet 373(9661): 383-389. 18. Richard, J. L. and S. V. Masserey. 2008. Large measles epidemic in Switzerland from 2006 to 2009: consequences for the elimination of measles in Europe. Euro surveillance: bulletin Europeen sur les maladies transmissibles= European communicable disease bulletin 14(50): 298-298. 19. Enders, J., and T. Peebles. 1954. Propagation in tissue cultures of cytopathogenic agents from patients with measles. Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine (New York, N.Y.) 86: 277–86. 20. Cutts, F. T, M. Grabowsky, and L. E. Markowitz. 1995. The effect of dose and strain of live attenuated measles vaccines on serological responses in young infants. Biologicals 23: 95–106. 21. WHO 1988. Joint WHO/UNICEF Statement on Vitamin A for Measles. Int. Nurs. Rev. 35:21. 128 22. WHO/UNICEF Joint Statement on reducing measles. http://www.unicef.org/publications/files/WHO_UNICEF_Measles_Emer gencies.pdf. 23. Hussey, G.D., and M. Klein. 1990. A randomized, controlled trial of vitamin A in children with severe measles. N. Engl. J. Med. 323:160164. 24. Fulginiti, V.A., J. J. Eller, A. W. Downie, and C. H. Kempe. 1967. Altered reactivity to measles virus: Atypical measles in children previously immunized with inactivated measles virus vaccines. JAMA 202: 1075. 25. Hussey, G.D., and M. Klein. 1993. Routine high-dose vitamin A therapy for children hospitalized with measles. J. Trop. Ped. 39: 342345. 26. Barclay, A. J. C., A. Foster, and A. Sommer. 1987. Vitamin A supplements and mortality related to measles: a randomised clinical trial. Br Med J 294: 294-6. 27. Reddy V., P. Bhaskaram, N, Raghuramulu, R. C. Milton, V. Rao, J. Madhusudan, and K. V. Krishna. 1986. Relationship between measles, malnutrition and blindness: a prospective study in Indian children. Am I Clin Nutr 44: 924-30. 28. Bluhm, D. P., and R. S. Summers. 1993. Plasma vitamin A levels in measles and malnourished pediatric patients and their implications in therapeutics. Journal of tropical pediatrics 39(3): 179-182. 29. Coutsoudis, A., P. Kiepiela, H. M. Coovadia, and M. Broughton. 1992. Vitamin A supplementation enhances specific IgG antibody levels and total lymphocyte numbers while improving morbidity in measles. The Pediatric infectious disease journal 11(3): 203-208. 30. Dorig, R. E., A. Marcil, A. Chopra, and C. D. Richardson. 1993. The human CD46 molecule is a receptor for measles virus (Edmonston strain). Cell 75: 295–305. 31. Sidorenko, S. P. and E. A. Clark. 2003. The dual-function CD150 receptor subfamily: the viral attraction. Nat Immunol 4: 19– 24. 32. Sinn, P.L., G. Williams, S. Vongpunsawad, R. Cattaneo, and P. B. McCray Jr. 2002. Measles virus preferentially transduces the basolateral surface of well-differentiated human airway epithelia. J Virol 76: 2403–2409. 129 33. Shirogane Y, M.Takeda, M. Tahara, S. Ikegame, T. Nakamura and Y. Yanagi. 2010. Epithelial-mesenchymal transition abolishes the susceptibility of polarized epithelial cell lines to measles virus. J Biol Chem 285: 20882–20890. 34. Chinnakannan, S. K., S. K. Nanda, and M. D. Baron. 2013. Morbillivirus V Proteins Exhibit Multiple Mechanisms to Block Type 1 and Type 2 Interferon Signalling Pathways. PloS one 8(2): e57063. 35. Ikegame, S., M. Takeda, S. Ohno, Y. Nakatsu, Y. Nakanishi, and Y. Yanagi. 2010. Both RIG-I and MDA5 RNA helicases contribute to the induction of alpha/beta interferon in measles virus-infected human cells. Journal of virology 84(1): 372-379. 36. Hornung, V., J. Ellegast, S. Kim, K. Brzózka, A. Jung, H. Kato, H. Poeck, S. Akira, K. K. Conzelmann, M. Schlee, S. Endres, and G. Hartmann. 2006. 5'-Triphosphate RNA is the ligand for RIG-I. Science 314(5801): 994-997. 37. Pichlmair, A., O. Schulz, C. P. Tan, T. I. Näslund, P. Liljeström, F. Weber, and C. R. e Sousa. 2006. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5'-phosphates. Science 314(5801): 9971001. 38. Kato, H., O. Takeuchi, E. Mikamo-Satoh, R. Hirai, T. Kawai, K. Matsushita, A. Hiiragi, T. S. Dermody, T. Fujita and S. Akira. 2008. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid–inducible gene-I and melanoma differentiation– associated gene 5. The Journal of experimental medicine 205(7): 16011610. 39. Akira, S., S. Uematsu, and O. Takeuchi. 2006. Pathogen recognition and innate immunity. Cell 124(4): 783-801. 40. Kato, H., S. Sato, M. Yoneyama, M. Yamamoto, S. Uematsu, K. Matsui, T. Tsujimura, K. Takeda, T. Fujita, O. Takeuchi, and S. Akira. 2005. Cell type-specific involvement of RIG-I in antiviral response. Immunity 23: 19–28. 41. Nakatsu, Y., M. Takeda, S. Ohno, R. Koga, and Y. Yanagi. 2006. Translational inhibition and increased interferon induction in cells infected with C protein-deficient measles virus. Journal of virology 80(23): 11861-11867. 42. Katze, M. G., Y. He, and M. Gale. 2002. Viruses and interferon: a fight for supremacy. Nature Reviews Immunology 2(9): 675-687. 130 43. González-Navajas, J. M., J. Lee, M. David, and E. Raz. 2012. Immunomodulatory functions of type I interferons. Nature Reviews Immunology 12(2): 125-135. 44. Goodbourn, S., L. Didcock, and R. E. Randall. 2000. Interferons: cell signalling, immune modulation, antiviral response and virus countermeasures. Journal of General Virology 81(10): 2341-2364. 45. MacMicking, J. D. 2004. IFN-inducible GTPases and immunity to intracellular pathogens. Trends in immunology 25(11): 601-609. 46. Katayama, Y., A. Hirano, and T. C. Wong. 2000. Human receptor for measles virus (CD46) enhances nitric oxide production and restricts virus replication in mouse macrophages by modulating production of alpha/beta interferon.Journal of virology 74(3): 1252-1257. 47. Sato, H., R. Honma, M. Yoneda, R. Miura, K. Tsukiyama-Kohara, F. Ikeda, T. Seki, S. Watanabe, and C. Kai. 2008. Measles virus induces cell-type specific changes in gene expression. Virology 375(2): 321-330. 48. Duhen, T., F. Herschke, O. Azocar, J. Druelle, S. Plumet, C. Delprat, S. Schicklin, T. F. Wild, C. Rabourdin-Combe, D. Gerlier, and H. Valentin. 2010. Cellular receptors, differentiation and endocytosis requirements are key factors for type I IFN response by human epithelial, conventional and plasmacytoid dendritic infected cells by measles virus. Virus research 152(1): 115-125. 49. Griffin, D. E., B. J. Ward, E. Jauregui, R. T. Johnson, and A. Vaisberg. 1990. Natural killer cell activity during measles. Clinical and experimental immunology 81: 218–24. 50. Yu, X. L., Y. M. Cheng, B. S. Shi, F. X. Qian, F. B. Wang, X. N. Liu, H. Y. Yang, Q. N. Xu, T. K. Qi, L. J. Zha, Z. H. Yuan, and R. Ghildyal. 2008. Measles virus infection in adults induces production of IL-10 and is associated with increased CD4+ CD25+ regulatory T cells. The Journal of Immunology 181(10): 7356-7366. 51. Leopardi, R., T. Hypia, and R. Vainionpaa. 1992. Effect of interferon-α on measles virus replication in human peripheral blood mononuclear cells. APMIS 100: 125–131. 52. Bieback, K., E. Lien, E., I. M. Klagge, E, Avota, J. SchneiderSchaulies, W. P. Duprex, H. Wagner, C. J. Kirschning, V. ter Meulen, and S. Schneider-Schaulies. 2002. Hemagglutinin protein of wild-type measles virus activates toll-like receptor 2 signaling. Journal of virology 76(17): 8729-8736. 131 53. Servant, M. J., N. Grandvaux, R. Lin, and J. Hiscott. 2002. Recognition of the measles virus nucleocapsid as a mechanism of IRF-3 activation. Journal of virology 76(8): 3659-3669. 54. Bernacky, B. J., S. V. Gibson, M. E. Keeling, and C. R. Abee. Nonhuman Primates in Laboratory Animal Medicine, 2nd ed. Fox, J. G., L. C. Andersno, F. M. Loew, and F. W. Quimby, editors. 2002. Elsevier Science, USA, ISBN:0-12-263951-0. 55. Perrault, J. 1981. Origin and replication of defective interfering particles. In Initiation Signals in Viral Gene Expression (pp. 151-207). Springer Berlin Heidelberg. 56. Whistler, T., W. J. Bellini, and P. A. Rota. 1996. Generation of defective interfering particles by two vaccine strains of measles virus. Virology 220(2): 480-484. 57. Yount, J. S., L. Gitlin, T. M. Moran, and C. B. López. 2008. MDA5 participates in the detection of paramyxovirus infection and is essential for the early activation of dendritic cells in response to Sendai virus defective interfering particles. The Journal of Immunology 180(7): 4910-4918. 58. Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392(6673): 245-252. 59. Mellman, I., and R. M. Steinman. 2001. Dendritic Cells-Specialized and Regulated Antigen Processing Machines. Cell 106(3): 255-258. 60. Klingele, M., H. K. Hartter, F. Adu, W. Ammerlaan, W. Ikusika, and C. P. Muller. 2000. Resistance of recent measles virus wild‐type isolates to antibody‐mediated neutralization by vaccinees with antibody. Journal of medical virology 62(1): 91-98. 61. De Swart, R. L., M. Ludlow, L. De Witte, Y. Yanagi, G. Van Amerongen, S. McQuaid, S. Yuksel, T. B. H. Geijenbeek, W. P. Duprex, and A. D. E. Osterhaus. 2007. Predominant infection of CD150+ lymphocytes and dendritic cells during measles virus infection of macaques. PLoS pathogens 3(11): e178. 62. Sakaguchi, M., Y. Yoshikawa, K. Yamanouchi, T. Sata, K. Nagashima, and K. Takeda. 1986. Growth of measles virus in epithelial and lymphoid tissues of cynomolgus monkeys. Microbiology and immunology 30(10): 1067-1073. 132 63. Ferreira, C. S. A., M. Frenzke, V. H. Leonard, G. G. Welstead, C. D. Richardson, and R. Cattaneo. 2010. Measles virus infection of alveolar macrophages and dendritic cells precedes spread to lymphatic organs in transgenic mice expressing human signaling lymphocytic activation molecule (SLAM, CD150). Journal of virology 84(6): 3033-3042. 64. Mongkolsapaya, J., A. Jaye, M. F. Callan, A. F. Magnusen, A. J. McMichael, and H. C. Whittle. 1999. Antigen-specific expansion of cytotoxic T lymphocytes in acute measles virus infection. Journal of virology 73(1): 67-71. 65. Permar, S. R., S. A. Klumpp, K. G. Mansfield, W. K. Kim, D. A. Gorgone, M. A. Lifton, K. C. Williams, J. E. Schmitz, K. A. Reimann, M. K. Axthelm, F. P. Polack, D. E. Griffin, and N. L. Letvin. 2003. Role of CD8+ lymphocytes in control and clearance of measles virus infection of rhesus monkeys. Journal of virology 77(7): 4396-4400. 66. Permar, S. R., S. A. Klumpp, K. G. Mansfield, A. A. Carville, D. A. Gorgone, M. A. Lifton, J. E. Schmitz, K. A. Reimann, F. P. Polack, D. E. Griffin, and N. L. Letvin. 2004. Limited contribution of humoral immunity to the clearance of measles viremia in rhesus monkeys. Journal of Infectious Diseases 190(5): 998-1005. 67. Permar, S. R., W. J. Moss, J. J. Ryon, M. Monze, F. Cutts, T. C. Quinn, and D. E. Griffin. 2001. Prolonged Measles Virus Shedding in Human Immunodeficiency Virus—Infected Children, Detected by Reverse Transcriptase—Polymerase Chain Reaction. Journal of Infectious Diseases 183(4): 532-538. 68. Riddell, M. A., W. J. Moss, D. Hauer, M. Monze, and D. E. Griffin. 2007. Slow clearance of measles virus RNA after acute infection. Journal of clinical virology 39(4): 312-317. 69. Pan, C. H., A. Valsamakis, T. Colella, N. Nair, R. J. Adams, F. P. Polack, C. E. Greer, S. Perri, J. M. Polo, and D. E. Griffin. 2005. Modulation of disease, T cell responses, and measles virus clearance in monkeys vaccinated with H-encoding alphavirus replicon particles. Proceedings of the National Academy of Sciences of the United States of America 102(33): 11581-11588. 70. Bouche, F. B., O. T. Ertl, and C. P. Muller. 2002. Neutralizing B cell response in measles. Viral immunology 15: 451–71. 71. Graves, M., D. E. Griffin, R. T. Johnson, R. L. Hirsch, I. L. de Soriano, S. Roedenbeck, and A. Vaisberg. 1984. Development of antibody to measles virus polypeptides during complicated and uncomplicated 133 measles virus infections. Journal of virology 49: 409–12. 72. Christenson, B., and M. Böttiger. 1994. Measles antibody: comparison of long-term vaccination titres, early vaccination titres and naturally acquired immunity to and booster effects on the measles virus. Vaccine 12(2): 129-133. 73. Janeway, C. 2001. Immunobiology, 5th ed. 74. Parker, D. C. 1993. T cell-dependent B cell activation. Annual review of immunology 11(1): 331-360. 75. Griffin, D. E., and B. J. Ward. 1993. Differential CD4 T cell activation in measles. Journal of Infectious Diseases 168(2): 275-281. 76. Ryon, J. J., W. J. Moss, M. Monze, and D. E. Griffin. 2002. Functional and phenotypic changes in circulating lymphocytes from hospitalized Zambian children with measles. Clinical and diagnostic laboratory immunology 9(5): 994-1003. 77. Lyakh, L., G. Trinchieri, L. Provezza, G. Carra, and F. Gerosa. 2008. Regulation of interleukin‐12/interleukin‐23 production and the T‐helper 17 response in humans. Immunological reviews 226(1): 112131. 78. Zhou, L. J., and T. F. Tedder. 1996. Cd14+ blood monocytes can differentiation into functionally mature CD83+ dendritic cells. Proceedings of the National Academy of Sciences of the United States of America 93: 2588-92. 79. Griffin, D. E., B. J. Ward, E. Jauregui, R. T. Johnson, and A. Vaisberg. 1989. Immune activation in measles. The New England journal of medicine 320: 1667–72. 80. Fiorentino, D. F., M. W. Bond, and T. R. Mosmann. 1989. Two types of mouse T helper cell IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med 170: 2081-95. ] 81. Fiorentino, D. F., A. Zlotnik, P. Vierira, T. R. Mosmann, M. Howard, K. W. Moore, and A. O’Garra. 1991. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol 146: 344451. 82. Mills, K. H. 2004. Regulatory T cells: friend or foe in immunity to infection? Nat. Rev. Immunol. 4: 841–855. 134 83. Ma, C. S., E. K. Deenick, M. Batten, and S. G. Tangye. 2012. The origins, function, and regulation of T follicular helper cells. The Journal of experimental medicine 209(7): 1241-1253. 84. Griffin, D. E. 2010. Measles virus-induced suppression of immune responses. Immunological reviews 236: 176-89. 85. Moss, W. J., M. O. Ota, and D. E. Griffin. 2004. Measles: immune suppression and immune responses. The international journal of biochemistry & cell biology 36: 1380-5. 86. Hahm, B., J. H. Cho, and M. Oldstone. 2007. Measles virus–dendritic cell interaction via SLAM inhibits innate immunity: selective signaling through TLR4 but not other TLRs mediates suppression of IL-12 synthesis. Virology 358(2): 251-257. 87. Ohno, S., N. Ono, M. Takeda, K. Takeuchi, and Y. Yanagi. 2004. Dissection of measles virus V protein in relation to its ability to block alpha/beta interferon signal transduction. Journal of general virology 85(10): 2991-2999. 88. Devaux, P., and R. Cattaneo. 2004. Measles virus phosphoprotein gene products: conformational flexibility of the P/V protein amino-terminal domain and C protein infectivity factor function. Journal of virology 78(21) 11632-11640. 89. Nakatsu, Y., M. Takeda, S. Ohno, Y. Shirogane, M. Iwasaki, and Y. Yanagi. 2008. Measles virus circumvents the host interferon response by different actions of the C and V proteins. Journal of virology 82(17): 8296-8306. 90. Takeuchi, K., M. Takeda, N. Miyajima, Y. Ami, N. Nagata, Y. Suzaki, J. Shahnewaz, S. I. Kadota, and K. Nagata. 2005. Stringent requirement for the C protein of wild-type measles virus for growth both in vitro and in macaques. Journal of virology 79(12): 7838-7844. 91. Lenardo, M. J., C. M. Fan, T. Maniatis, and D. Baltimore. 1989. The involvement of NF-κB in β-interferon gene regulation reveals its role as widely inducible mediator of signal transduction. Cell 57(2): 287-94. 92. Xiao, C., and S. Ghosh. 2005. NF-κB, an evolutionarily conserved mediator of immune and inflammatory responses. In Mechanisms of Lymphocyte Activation and Immune Regulation X (pp. 41-45). Springer US. 135 93. Schuhmann, K. M., C. K. Pfaller, and K. K, Conzelmann. (2011). The measles virus V protein binds to p65 (RelA) to suppress NF-κB activity. Journal of virology 85(7): 3162-3171. 94. Shaffer, J. A., W. J. Bellini, and P. A. Rota. 2003. The C protein of measles virus inhibits the type I interferon response. Virology 315: 389-397. 95. Bellini, W. J., G. Englung, S. Rozenblatt, H. Arnheiter, and C. D. Richardson. 1985. Measles virus P gene codes for two proteins. J. Virol. 53: 908-919. 96. Liston, P., C. DiFlumeri, and D. J. Briedis. 1995. Protein interactions entered into by the measles virus P, V and C proteins. Virus Res. 38: 214-259. 97. Devaux, P., G. Hodge, M. B. McChesney, and R. Cattaneo. 2008. Attenuation of V-or C-defective measles viruses: infection control by the inflammatory and interferon responses of rhesus monkeys. Journal of virology 82(11): 5359-5367. 98. Sarkar, S. N., and G. C. Sen. 2004. Novel functions of proteins encoded by viral stress-inducible genes. Pharmacology & therapeutics 103(3): 245-259. 99. Bandyopadhyay, S. K., G. T. Leonard, T. Bandyopadhyay, G. R. Stark, and G. C. Sen. 1995. Transcriptional induction by double-stranded RNA is mediated by interferon-stimulated response elements without activation of interferon-stimulated gene factor 3. Journal of Biological Chemistry 270(33): 19624-19629. 100. Haller, O., and G. Kochs. 2002. Interferon‐induced Mx proteins: Dynamin‐like GTPases with antiviral activity. Traffic 3(10): 710-717. 101. Schneider-Schaulies S., J. Schneider-Schaulies A. Schuster A, M. Bayer, J. Pavlovic, and V. Ter Meulen. 1994. Cell type-specific MxA mediated inhibition of measles virus transcription in human brain cells. J Virol 68: 6910–6917. 102. Lin, W. H. W., A. Vilalta, R. J. Adams, A. Rolland, S. M. Sullivan, and D. E. Griffin. 2013. Vaxfectin adjuvant improves antibody responses of juvenile rhesus macaques to a DNA vaccine encoding the measles virus hemagglutinin and fusion proteins. Journal of virology 87(12): 65606568. 103. Korn, T., E. Bettelli, M. Oukka, and V. K. Kuchroo. 2009. IL-17 and 136 Th17 Cells. Annual review of immunology 27: 485-517. 104. McGeachy, M. J., K. S. Bak-Jensen, Y. I. Chen, C. M. Tato, W. Blumenschein, T. McClanahan, and D. J. Cua. 2007. TGF-β and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain TH-17 cell–mediated pathology. Nature immunology 8(12): 1390-1397. 105. Neufert, C., C. Becker, S. Wirtz, M. C. Fantini, B. Weigmann, P. R. Galle, and M. F. Neurath. 2007. IL‐27 controls the development of inducible regulatory T cells and Th17 cells via differential effects on STAT1. European journal of immunology 37(7): 1809-1816. 106. Villarino, A. V., E. Huang, and C. A. Hunter. 2004. Understanding the pro-and anti-inflammatory properties of IL-27. The Journal of Immunology 173(2): 715-720. 107. Ellison, J. B. 1932. Intensive vitamin therapy in measles. British medical journal 2(3745): 708. 108. Neuzil, K. M., W. C. Gruber, F. Chytil, M. T. Stahlman, B. Engelhardt, and B. S. Graham. 1994. Serum vitamin A levels in respiratory syncytial virus infection. The Journal of pediatrics 124(3): 433-436. 109. WHO/UNICEF/IVACG Task Force, International Vitamin A Consultative Group, UNICEF, and World Health Organization. 1997. Vitamin A supplements: a guide to their use in the treatment and prevention of vitamin A deficiency and xerophthalmia. World Health Organization. 110. Trottier, C., M. Colombo, K. K. Mann, W. H. Miller Jr., and B. J. Ward. 2009. Retinoids inhibit measles virus through a type I IFNdependent bystander effect. FASEB J 23: 3203-3212. 111. Trottier, C., S. Chabot, K. K. Mann, M. Colombo, A. Chatterjee, W. H. Miller Jr., and B. J. Ward. 2008. Retinoids inhibit measles virus in vitro via nuclear retinoid receptor signaling pathways. Antiviral Res 80: 45-53. 112. Rodeheffer, C., V. von Messling, S. Milot, F. Lepine, A. R. Manges, and B. J. Ward. 2007. Disease manifestations of canine distemper virus infection in ferrets are modulated by vitamin A status. J Nutr. 137: 1916-1922. 113. Frieden, T.R., A. L. Sowell, K. J. Henning, D. L. Huff, and R. A. Gunn. 1992. Vitamin A levels and severity of measles: New York City. 137 Amer. J. Dis. Child. 146: 182-186. 114. Kanai, M., A. Raz, and D. S. Goodman. 1968. Retinol-binding protein: the transport protein for vitamin A in human plasma. Journal of Clinical Investigation 47(9): 2025. 115. Stephensen, C. B. 2000. When does hyporetinolemia mean vitamin A deficiency?. The American Journal of Clinical Nutrition 72(1): 1-2. 116. Christian, P., K. Schulze, R. J. Stoltzfus, and K. P. West. 1998. Hyporetinolemia, illness symptoms, and acute phase protein response in pregnant women with and without night blindness. The American journal of clinical nutrition 67(6): 1237-1243. 117. Semba, R. D., K. P. West, G. Natadisastra, W. Eisinger, Y. Lan, and A. Sommer. 2000. Hyporetinolemia and acute phase proteins in children with and without xerophthalmia. The American journal of clinical nutrition 72(1): 146-153. 118. Thurnham, D. I., and R. Singkamani. 1991.The acute phase protein response and vitamin A status in malaria. Trans R Soc Trop Med Hyg 85: 194–9. 119. Stephensen, C. B., J. O. Alvarez, J. Kohatsu, R. Hardmeier, J. I. Kennedy Jr., and R. B. Gammon Jr. 1994. Vitamin A is excreted in the urine during acute infection. Am J Clin Nutr 60: 388–92. 120. Alvarez, J.O., E. Salazar-Lindo, J. Kohatsu, P. Miranda, and C. B. Stephensen. 1995. Urinary excretion of retinol in children with acute diarrhea. Am J Clin Nutr 61: 1273–6. 121. Mitra, A. K., J. O. Alvarez, M. A. Wahed, C. J. Fuchs, and C. B. Stephensen. 1998. Predictors of serum retinol in children with shigellosis. Am J Clin Nutr 68: 1088–94. 122. Foster, A., and A. Sommer. 1987. Corneal ulceration, measles, and childhood blindness in Tanzania. Br I Ophthalmol 71: 331-43. 123. Khan, M. V., E. Hague, and M. R. Khan.1984. Nutritional ocular diseases and their association with diarrhoea in Matlab, Bangladesh. Br I Nutr 52: 1-9. 124. Cohen, N., H. Rahman, I. Sprague, M. A. Jalil, E. Leemhuis de Regt, and A. M. Mitra. 1985. Prevalence and determinants of nutritional blindness in Bangladeshi children. World Health Stat Q 38: 317-30. 138 125. Lin, W. H., R. D. Kouyos, R. J. Adams, B. T. Grenfell, and D. E. Griffin. 2012. Prolonged persistence of measles virus RNA is characteristic of primary infection dynamics. PNAS. 109: 14989-14994. 126. Dossetor, J., H. C. Whittle, and B. M. Greenwood. 1977. Persistent measles infection in malnourished children. British medical journal 1(6077): 1633. 127. Scheifele, D. W., and C. E. Forbes. 1972. Prolonged giant cell excretion in severe African measles. Pediatrics 50(6): 867-873. 128. Warren, J. S. 1990. Interleukins and tumor necrosis factor in inflammation. Crit Rev Clin Lab Sci 28: 37-59. 129. Hume, E. M., and H. A. Krebs. 1949. Vitamin A requirement of human adults: an experimental study of vitamin A deprivation in man. MRC Spec Rep. 264: 1-145. 130. Coovadia, H. M., A. Wesley, and P. Brain. 1978. Immunological events in acute measles influencing outcome. Arh Dis Child. 53: 861-867. 131. Milton, R. C., V. Reddy, and A. N. Naidu. 1987. Mild vitamin A deficiency and childhood morbidity: an Indian experience. Am J Clin Nutr. 46: 827-829. 132. Campos, F.A., H. Flores, and B. A. Underwood.1987. Effect of an infection on vitamin A status of children as measured bythe relativedose response (RDR). Am J Clin Nutr. 46: 91-94. 133. Shahid, N. S., P. Clauquin, K. Shaikh, and S. Zimicki. 1984. Longtermcomplications of measles in rural Bangladesh. J Trop Med Hyg. 86: 77-80. 134. Bhaskaram, P., V. Reddy, S. Raj, and R. C. Bhatnager. 1984. Effect ofmeasles on the nutritional status of preschool children. J TropMed Hyg. 87: 21-25. 135. Hull, R. F., P. J. Williams, and F. Oldfield. 1983. Measles mortality andvaccine efficacy in rural West Africa. Lancet. 1: 972-975. 136. Aaby, P., J. Bukh , D. Kronborg, I. M. Lisse, and M. Clotilde da Silva. 1990. Delayed excess mortality after exposure to measles during the first six months of life. Am J Epidemiol. 132: 211-219. 137. Stephensen, C. B. 2001. Vitamin A, infection, and immune function. Annu. Rev. Nutr. 21: 167-92. 139 138. Griffin, D. E., B. J. Ward, and L. M. Esolen. 1994. Pathogenesis of measles virus infection: An hypothesis for altered immune responses. J. Infect. Dis. 170: S24-S31. 139. Mora, J. R., M. Iwata, and U. H. von Andrian. 2008. Vitamin effects on the immune system: vitamins A and D take centre stage. Nature Reviews Immunology 8(9): 685-698. 140. Humphrey, J. H., K. P. West Jr, and A. Sommer. 1992. Vitamin A deficiency and attributable mortality among under-5-year-olds. Bull World Health Organ 70(2): 225-232. 141. Ross, C. A. 2010. Vitamin A. In: Coates, P. M., Betz, J. M., Blackman, M. R., et al., eds. Encyclopedia of Dietary Supplements. 2nd ed. London and New York: Informa Healthcare, 778-91. 142. Sommer A., and F. R. Davidson. 2002. Assessment and control of vitamin A deficiency: the annecy accords. J Nutr 132: 2845S–2850S. 143. Moise, A. R., N. Noy, K. Palczewski, and W. S. Blaner. 2007. Delivery of retinoid-based therapies to target tissues. Biochemistry 46(15): 44494458. 144. Zilliox, M. J., W. J. Moss, and D. E. Griffin. 2007. Gene expression changes in peripheral blood mononuclear cells during measles virus infection. Clinical and vaccine immunology 14: 918–23. 145. Kobune, F., H. Sakata, and A. Sugiura. 1990. Marmoset lymphoblastoid cells as a sensitive host for isolation of measles virus. Journal of Virology 64(2): 700-705. 146. Maek-A-Nantawat, W., S. Buranapraditkun, J. Klaeswongkram, and K. Ruxrungthem. 2007. Increased interleukin-17 production both in helper T cell subset Th17 and CD4-negative T cells in human immunodeficiency virus infection. Viral Immunol 20: 66-75. 147. Molesworth-Kenyon, S. J., R. Yin, J. E. Oakes, and R. N. Laush. 2008. IL-17 receptor signaling influences virus-induced corneal inflammation. J Leukoc. Biol. 83: 401-408. 148. Hashimoto, K., J. E. Durbin, W. Zhou, R. D. Collins, S. B. Ho, J. K. Kolls, P. J. Dubin, J. R. Sheller, K. Gleniewska, and J. F. O’Neal. 2005. Respiratory syncytial virus infection in the absence of STAT 1 results in airway dysfunction, airway mucus, and augmented IL-17 levels. J. Allergy Clin. Immunol. 116: 550-557. 140 149. Witowski, J., K. Ksiazek, and A. Jorres. 2004. Interleukin-17: a mediator of inflammatory responses Cell. Mol. Life Sci. 61: 567-579. 150. Steinman, L. 2007. A brief history of TH17, the first major revision in the TH1/TH2 hypothesis of T cell–mediated tissue damage. Nature medicine 13(1):139-145. 151. Shinohara, M. L., J. H. Kim, V. A. Garcia, and H. Cantor. 2008. Engagement of the type I interferon receptor on dendritic cells inhibits T helper 17 cell development: role of intracellular osteopontin. Immunity 29(1): 68-78. 152. Hou, W., H. S. Kang, and B. S. Kim. 2009. Th17 cells enhance viral persistence and inhibit T cell cytotoxicity in a model of chronic virus infection. The Journal of experimental medicine 206(2): 313-328. 153. Guo, J., K. L. Peters, and G. C Sen. 2000. Induction of the human protein P56 by interferon, double-stranded RNA, or virus infection. Virology 267(2): 209-219. 154. Abbas, Y. M., A. Pichlmair, M. W. Górna, G. Superti-Furga, G., and B. Nagar. 2013. Structural basis for viral 5 [prime]-PPP-RNA recognition by human IFIT proteins. Nature. 155. Pichlmair, A., C. Lassnig, C. A. Eberle, M. W. Górna, C. L. Baumann, T. R. Burkard, T. Burckstummer, A. Stefanovic, S. Krieger, K. I. Bennett, T. Rulicke, F. Weber, J. Colinge, M. Muller, and G. SupertiFurga. 2011. IFIT1 is an antiviral protein that recognizes 5 [prime]triphosphate RNA. Nature immunology 12(7): 624-630. 156. Shingai, M., T. Ebihara, N. A. Begum, A. Kato, T. Honma, K. Matsumoto, H. Saito, H. Ogura, M. Matsumoto, and T. Seya. 2007. Differential type I IFN-inducing abilities of wild-type versus vaccine strains of measles virus. Journal of immunology 179: 6123-33. 157. Choe, J., M. S. Kelker, and I. A Wilson. 2005. Crystal structure of human toll-like receptor 3 (TLR3) ectodomain. Science 309(5734): 581585. 158. Diebold, S. S., T. Kaisho, H. Hemmi, S. Akira, and C. R. e Sousa. 2004. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303(5663): 1529-1531. 159. Butler, J. C., P. L. Havens, S. E. Day, M. J. Chusid, A. L. Soweel, D. L. Huff, D. E. Peterson, R. A. Bennin, R. Circo, and J. P. Davis. 1993. 141 Measles severity and serum retinol (vitamin A) concentration among children in the United States. Pediatrics 91(6): 1176-1181. 160. Chernoff, A. E., E. V. Granowitz, L. Shapiro, E. Vannier, G. Lonnemann, J. B. Angel, J. S. Kennedy, A. R. Rabson, S. M. Wolff, and C. A. Dinarello, C. A. 1995. A randomized, controlled trial of IL-10 in humans. Inhibition of inflammatory cytokine production and immune responses. The Journal of Immunology 154(10): 5492-5499. 161. Tober, C., M. Seufert, H. Schneider, M. A. Billeter, I. C. Johnston, S. Niewiesk, V. ter Muelen, and S. Schneider-Schaulies. 1998. Expression of measles virus V protein is associated with pathogenicity and control of viral RNA synthesis. Journal of virology 72(10): 8124-8132. 162. Villamor, E., and W. W. Fawzi. 2005. Effects of vitamin A supplementation on immune responses and correlation with clinical outcomes. Clinical microbiology reviews 18(3): 446-464. 163. Pino-Lagos, K., Y. Guo, C. Brown, C., M. P. Alexander, R. Elgueta, K. A. Bennett, V. De Vries, E. Nowak, R. Blomhoff, S. Sockanathan, R. A. Chandraratna, E. Dmitrovsky, and R. J. Noelle. 2011. A retinoic acid– dependent checkpoint in the development of CD4+ T cell–mediated immunity. The Journal of experimental medicine 208(9): 1767-1775. 164. Elias, K. M., A. Laurence, T. S. Davidson, G. Stephens, Y. Kanno, E. M. Shevach, and J. J. O'Shea. 2008. Retinoic acid inhibits Th17 polarization and enhances FoxP3 expression through a Stat-3/Stat-5 independent signaling pathway. Blood 111(3): 1013-1020. 165. Iwata, M., Y. Eshima, and H. Kagechika. 2003. Retinoic acids exert direct effects on T cells to suppress Th1 development and enhance Th2 development via retinoic acid receptors. International immunology 15(8): 1017-1025. 166. Iwata M, Y. Eshima, and H. Kagechika. 2003. Retinoic acids exert direct effects on T cells to suppress Th1 development and enhance Th2 development via retinoic acid receptors. Int Immunol 15: 1017–1025. 167. Lovett-Racke, A. E., and M. K. Racke. 2002. Retinoic acid promotes the development of Th2-like human myelin basic protein-reactive T cells. Cell Immunol 215: 54–60. 168. Bettelli, E., Y. Carrier, W. Gao, T. Korn, T. B. Strom, M. Oukka, H. L. Weiner, and V. K. Kuchroo. 2006. Reciprocal developmental pathways 142 for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441: 235–238. 169. Uematsu, S., K. Fujimoto, M. H. Jang, B. G. Yang, Y. J. Jung, M. Nishiyama, S. Sato, T. Tsujimura, M/ Yamamoto, Y. Yokota, H. Kiyono, M. Miyasaka, K. J. Ishii, and S. Akira. 2008. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nature Immunol 9: 769–776. 170. Eisenstein, E. M., and C. B. Williams. 2009. The Treg/Th17 cell balance: a new paradigm for autoimmunity. Pediatric research 65: 26R31R. 171. Elsaesser, H., K. Sauer, and D. G. Brooks. 2009. IL-21 is required to control chronic viral infection. Science 324(5934): 1569-1572. 172. John, S. Y., M. Du, and A. J. Zajac. 2009. A vital role for interleukin-21 in the control of a chronic viral infection. Science 324(5934): 1572-1576. 143 Curriculum vitae Nicole E. Putnam (920) 216-6835 - [email protected] EDUCATION Master of Science in Molecular Microbiology & Immunology Expected May 2014 Johns Hopkins Bloomberg School of Public Health, Baltimore, MD Thesis: The innate and adaptive immune response to measles virus Advisor: Dr. Diane E. Griffin Certificate in Public Health Preparedness Expected May 2014 Johns Hopkins Bloomberg School of Public Health, Baltimore, MD Bachelor of Science in Biochemistry and Psychology December 2010 University of Wisconsin-La Crosse, La Crosse, WI RESEARCH EXPERIENCE ScM Thesis Research 2012-2014 Dr. Diane Griffin, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD Determination of innate immunological effects in vitro of the V and C protein in wild type and vaccine strains of measles virus using virus growth, plaque assays, plaque purification, PCR and agarose gel electrophoresis, qPCR, RTPCR, interferon bioassays, isolation of PBMCs, and generation of monocyte derived dendritic cells. Determination of effects of vitamin A supplementation on wild-type measles infection in rhesus macaque models using TCID50, EIAs, ASCs, ELISPOTs, PRNTs, and multiparameter flow cytometry. Pharmaceutical Research and Development Intern 2010 Dr. Raghavan Rajagopalan, Covidien, St. Louis, MO Used synthetic chemistry to make novel photosensitive compounds, bioconjugation of photosensitive and fluorescent compounds to antibodies and peptides to target ovarian and colon cancer cell lines using LC-MS, NMR, ESR, bioconjugation of compounds to peptides and antibodies, limited confocal microscopy, and in vitro cytotoxicity assays. Laboratory Manager 2009-2010 Dr. Alex O’Brien, Dr. Bart VanVoorhis, La Crosse, WI Scheduled lab assistants for data collection and experiment times, managed informed consent paperwork, while maintaining duties of research assistant. 144 Research Assistant 2008-2009 Dr. Alex O’Brien, Dr. Bart VanVoorhis, La Crosse, WI Assisted in experiment development using SuperLab program, collected data biweekly, and participated in journal club. Undergraduate Researcher 2008-2009 Dr. Aaron Monte, La Crosse, WI Assisted in the organic synthesis of the beta-alkaloid compound tetrahydroharmine. HONORS AND AWARDS MSCI Scholarship 2013-2014 SOURCE Service Scholar in community outreach 2013-2014 SOURCE Recognition award 2014 First Place in the 2010 Covidien Intern Poster Symposium High Honor Award 2010 from Psi Chi International Honor Society Officer 2009 for Eta Phi Alpha Honors Fraternity Society Memberships: Golden Key International Honor Society, Psi Chi International Honor Society, Eta Phi Alpha Honors Fraternity, American Chemical Society, American Society for Microbiology PUBLICATIONS AND PRESENTATIONS Rajagopalan, R., A. R. Poreddy, A. Karwa, B. Asmelash, N. E. Putnam, L. Chinen, M. Nickols, J. J. Shieh, and R. B.Dorshow. (2011, January 22-27). Folate receptor targeted Type 1 photosensitizer bioconjugates for tumor visualization and phototherapy. Presented at the ―Optical Methods for Tumor Treatment and Detection: Mechanisms and Techniques in Photodynamic Therapy XX‖ conference, part of the SPIE BiOS: Biomedical Optics Symposium. http://dx.doi.org/10.1117/12.875166. Rajagopalan, R., T. Lin, A. Karwa, A. Poreddy, B. Asmelash, N. Putnam, D. Lin, and R. Dorshow. (2011, May 10-14). Discovery and development of novel thiaza and thioxa type 1 photosensitizers. ―13th World Congress of the International Photodynamic Association‖ conference. 145