* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 200 µmol /L is far too low a concentration of ammonium to affect
Survey
Document related concepts
Fatty acid metabolism wikipedia , lookup
Cryobiology wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Metalloprotein wikipedia , lookup
Magnesium in biology wikipedia , lookup
Nitrogen cycle wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Biochemistry wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Citric acid cycle wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Transcript
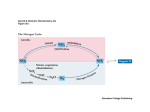
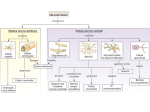
Ammonia toxicity Ammonia is highly toxic. Normally blood ammonium concentration is < 50 µmol /L, and an increase to only 100 µmol /L can lead to disturbance of consciousness. A blood ammonium concentration of 200 µmol /L is associated with coma and convulsions. 200 µmol /L is far too low a concentration of ammonium to affect plasma pH or the normal transport of sodium and potassium ions across nerve cell membranes. The explanation of the toxicity of such (relatively) low concentrations of ammonium lies with the enzyme glutamate dehydrogenase. This enzyme catalyses the oxidative deamination of glutamate to ammonium and ketoglutarate; the reaction is readily reversible, and the direction of reaction (towards deamination of glutamate or glutamate formation) depends on the relative concentrations of the various substrates. As the concentration of ammonium rises, so the reaction proceeds in the direction of formation of glutamate from ketoglutarate. The effect of forming glutamate from ketoglutarate is to deplete the mitochondrial pool of ketoglutarate, which is a key intermediate in the citric acid cycle. As a result, the rate of citric acid cycle activity falls, so reducing very considerably the rate of formation of ATP. It is this lack of ATP that affects ion transport across nerve cell membranes, so resulting in disturbance, then loss, of consciousness. Formation of glutamine Ammonium is produced in most cells of the body, as a result of deamination of amino acids and amines. It is exported into the bloodstream as glutamine, formed by the actions of glutamate dehydrogenase, to form glutamate from ketoglutarate and ammonium, then glutamine synthetase, forming glutamine from glutamate and ammonium.