* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Basil Fathalla presentation
Acute pancreatitis wikipedia , lookup
Transmission (medicine) wikipedia , lookup
Inflammation wikipedia , lookup
Periodontal disease wikipedia , lookup
Rheumatic fever wikipedia , lookup
Hygiene hypothesis wikipedia , lookup
Psychoneuroimmunology wikipedia , lookup
Anti-nuclear antibody wikipedia , lookup
Autoimmune encephalitis wikipedia , lookup
Behçet's disease wikipedia , lookup
African trypanosomiasis wikipedia , lookup
IgA nephropathy wikipedia , lookup
Systemic scleroderma wikipedia , lookup
Neuromyelitis optica wikipedia , lookup
Globalization and disease wikipedia , lookup
Kawasaki disease wikipedia , lookup
Germ theory of disease wikipedia , lookup
Multiple sclerosis research wikipedia , lookup
Ankylosing spondylitis wikipedia , lookup
Sjögren syndrome wikipedia , lookup
Autoimmunity wikipedia , lookup
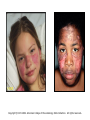
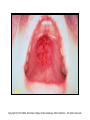
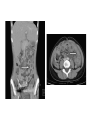
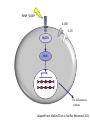
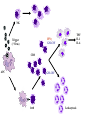
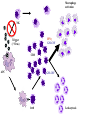
Pediatric Rheumatology Basil M Fathalla, MD, ABP, FAAP Consultant Pediatric Rheumatology Al Jalila Children’s Specialty Hospital Faculty Disclosure Statement Basil Fathalla, MD discloses that he nor any member of his immediate family does not have any relevant financial arrangements or affiliations with any corporate organization associated with the manufacture, license, sale, distribution or promotion of a drug or device. Objectives Review the spectrum of clinical manifestations for systemic autoimmune diseases Describe the diagnostic approach to children with autoinflammatory disorders Describe management of children with unclassifiable presentations of rheumatic entities Case 1 Transit in DXB • 9 years old Arabic boy at DXB coming from Vienna in transit to Kuwait. • Developed generalized seizures and sustained head trauma from falling on the ground in terminal 1 • Transfer to Latifa hospital • Blood work revealed severe anemia and head CT scan showed signs of old infarcts Transit in DXB • Systemic investigations for organ involvement • Rheumatologic work up for specific diagnosis • Spectrum of clinical manifestations of systemic autoimmune connective tissue diseases The lupus, Latin for Wolf, was used to describe recurrent, florid, facial Ulcerations since the 13th century. From "Diseases of the skin. An Outline of the principles and practice of Dermatology" Malcolm Morris, Cassel & Co., London, 1894 SLE: Historic review 13th -19th centuries: the term lupus appeared in the literature. 1872: acute and chronic types of skin disease described by Osler. 1895: systemic nature of disease and characteristic exacerbations / remissions described by Osler; the term “erythema exudativum” suggested. 1924: Libman and Sacks described detailed cardiac involvement. 1948: LE cell was described by Hargraves, Richmond and Morton. SLE : Definition / Classification An episodic, multi-system, autoimmune connective tissue disease characterized by widespread inflammation of blood vessels and connective tissues and by the presence of anti-nuclear antibodies especially to native DNA. ACR Classification of SLE ACR 1982 criteria: Malar rash Discoid-lupus rash Photosensitivity Mucocutaneous ulcers Nonerosive arthritis Nephritis Encephalopathy Serositis Cytopenia ANA Immunoserology: Anti-DsDNA + LE + ENA False + VDRL ACR 1997 criteria: Same as 1982 Immunoserology: Anti-DsDNA + ENA + APL: 1)IgG or IgM for ACL 2)Lupus anticoagulant 3)False + VDRL Epidemiology of Juvenile-SLE Estimated 5,000-10,000 U.S. children Incidence (early studies in USA): 0.53-0.6 per 100,000 per year SLE accounts for <1% of rheumatology clinics in UK, 1.5-3% in Canada, & 4.5% in USA. 15-17% of all SLE patients had onset in childhood Female to Male ratio < 12 yrs. of age: 3:1 > 12 yrs. of age: 10:1 All races affected Etiology & Pathogenesis Immune dysregulation: B cell function Immune complex mediated disease T cell function Apoptosis Hormones: May play a role in disease predisposition and severity Environmental factors: Ultraviolet light Viral infections Drugs / chemicals Clinical features of SLE: Constitutional: fever, malaise, weight loss Skin: malar rash, discoid lupus, photosensitivity, alopecia, ulcers MSK: arthritis, tenosynovitis, myositis, aseptic necrosis Vascular: Raynaud’s, acrocyanosis, thrombosis Cardiac: pericarditis, effusions, myocarditis, Libman-Sacks endocarditis Pulmonary: Pluritis, effusions, pneumonitis, hemorrhage GI: peritonitis, pancreatitis, mesenteric vasculitis Neuro: seizures, psychosis, CVA, neuropathy, pseudotumor cerebri, cranial nerve palsies Ocular: retinopathy, papilledema Renal: glomerulonephritis Copyright ©1972-2004. American College of Rheumatology Slide Collection. All rights reserved. Copyright ©1972-2004. American College of Rheumatology Slide Collection. All rights reserved. Copyright ©1972-2004. American College of Rheumatology Slide Collection. All rights reserved. Lupus Nephritis in Children Uncommon disorder of childhood; most important aspect of SLE. Typically presents after the age of 10 yrs; very rare less than 5 yrs of age. F:M 4.5:1 (more males compared to adult onset). Present at time of disease onset in a higher percentage than adults (82%). Usually develops in the first 2 years of disease onset. Lupus Nephritis in Children Most common initial presentation is microscopic hematuria (~ 79%) proteinuria including nephrotic syndrome (~ 55%), WHO classification is used to classify lupus nephritis. Indicators of active disease on kidney biopsy: cellular proliferation, necrosis, cellular crescents, hyaline thrombi, leukocytic glomerular infiltration & interstitial infiltration. Indicators of chronicity: glomerular sclerosis, interstitial fibrosis, fibrous crescents, tubular atrophy. Class Type of GN Description I Normal No detectable disease IIA IIB Minimal change Mesangial glomerulitis Normal LM, mesangial Ig & c by IFM, mesangial deposits by EM, IIB: mesangial hypercellularity. III Focal & segmental proliferation Focal cellular proliferation, necrosis, leukocyte infiltrates in < 50% of glomeruli. Subendothelial or mesangial deposits. Focal tubular & interstitial disease. IV Diffuse proliferative GN Class III in >50% of glomeruli. Abundant Subendothelial deposits. Marked interstitial disease. V Membranous GN No mesangial, endothelial or epithelial proliferation Diffusely uniformly thickened capillary walls. IFM & EM show mesangial & subepithelial deposits. VI Glomerular sclerosis Segmental or extensive sclerosis of glomeruli; fibrous crescent are common. SLE: Management General: team approach, counseling, education,, rest, nutrition, sunscreen, immunization, management of infection, growth monitoring. NSAIDs: for MSK problems, careful in presence of renal disease. Preventive medications: Vit D + Ca, baby aspirin, hydroxychloroquine. Immunosuppressives: Glucocorticosteroids, DMARDs, cytotoxic drugs SLE: system specific management Cutaneous disease: Topical Glucocorticosteroids, hydroxychloroquine, sunscreen. Hematologic manifestations: Glucocorticosteroids, IVIG. APL-syndrome: baby aspirin, warfarin, LMWH. CNS: Glucocorticosteroids, cyclophosphamide. Cardio-Pulmonary: Glucocorticosteroids, hydroxychloroquine, mycophenolate mofetil, cyclophosphamide. SLE nephritis Mesangial glomerulitis: symptomatic, prednisone. Focal nephritis: Glucocorticosteroids, consider mycophenolate mofetil. Diffuse nephritis: Glucocorticosteroids + cyclophosphamide. Membranous nephritis: Glucocorticosteroids, consider azathioprine, mycophenolate mofetil, cyclophosphamide. Case 2 Inflammation in the eye of the beholder • 7 years old Arabic girl • Recurrent episodes of fever, abdominal pain, and chest pain • 2 years later seen by GI and diagnosed with clinical entity classifiable as an autoinflammatory disease • Patient responsive to treatment • Routine referral to rheumatology proved beneficial Inflammation in the eye of the beholder • Differential diagnosis for periodic fever • Importance of systemic evaluation even when diagnosis is known • Practical approach to autoinflammatory diseases Case 3 The cytokine storm • 14 month old ill looking Arabic boy with high spikes of fever and rashes • Very high acute phase reactants • No arthritis • Systemic evaluation was unremarkable • Bone marrow ruled out leukemia The cytokine storm • Differential diagnosis for a prolong febrile illness • What to do if diagnosis is not conclusive • Combination of DMRARDs vs. biologic therapies: advantages and disadvantages SoJIA Unique clinical features with adult-onset equivalent Monocyclic, polycyclic, and persistent courses, sometimes without arthritis Marked role for innate immunity Low risk for uveitis Singh-Grewal D et al. Arthritis Rheum 2011 Maritini A. Ann Rheum Dis 2012 Distinctive Clinical Manifestations No gender bias or peak age at onset Geographic variation (North America 10% of JIA, India & Japan ≈ 30-50% of JIA) Extra-articular features, including quotidian fever, rash, lymphadenopathy, HSM, and polyserositis Elevated acute phase reactants reflecting systemic inflammation Fujikawa S et al. Acta Paediatr1997 Sawhney S et al. Best Pract Res Clin Rheumatol 2006 Singh-Grewal D et al. Arthritis Rheum 2011 Macrophage Activation Syndrome Clinically: febrile, ill looking, rapid hepatic failure with encephalopathy, renal failure with hematuria & proteinuria, DIC-like presentation; bruising, purpura, & mucosal bleeding ↑ Nodes, liver & spleen May occur in infectious & neoplastic disorders Unknown etiology / cytokine storm Sawhney S et al. Arch Dis Child. 2001 Stephan JL at al.Rheumatology (Oxford). 2001 Ravelli A et al. J Pediatr. 2005 Macrophage Activation Syndrome ↓ Blood cell count (Hgb. , WBC , Plts) ↓ ESR (hypofibrinogenemia: consumptive coagulopathy & DIC) Prolonged PT, PTT, fibrin-split products ↓ Fibrinogen, Vit K dependent clotting factors BM: Reactive hematophagocytic lymphohistiocytosis Increased morbidity & mortality Treatment: IV glucocorticoid & cyclosporine Sawhney S et al. Arch Dis Child. 2001 Stephan JL at al.Rheumatology (Oxford). 2001 Ravelli A et al. J Pediatr. 2005 PAMP / DAMP IL-18R IL-1R MyD88 NFκB Pro-inflammatory cytokine Adapted from: Mellins ED et al. Nat Rev Rheumatol 2011 IL-1b IL-18R IL-1R IL-1b ASC Casp-1 Casp-1 Pro-IL-1 Adapted from: Mellins ED et al. Nat Rev Rheumatol 2011 ? MyD8 8 NFK B ? IL-6 TNF M-CSF IL-1 IL-18 Adapted from: Mellins ED et al. Nat Rev Rheumatol 2011 MyD8 8 NFK B IL-6 Vascular endothelium: Coagulopathy TNF M-CSF IL-1 IL-18 Hypothalamus: Liver: Joints: Bone marrow: Fever Increased acute phase reactants Increased inflammation Platelets Monocyte/n eutrophils S 100 proteins Adapted from: Mellins ED et al. Nat Rev Rheumatol 2011 MyD8 8 NFK B IL-6 TNF M-CSF IL-1 Pro-inflammatory molecules with cytokinelike action secreted by activated neutrophils and monocytes. SoJIA have high levels of S100 proteins in contrast to other febrile illnesses Wittkowski H et al. Arthritis Rheum 2008 Frosch M et al. Arthritis Rheum 2009 IL-18 Bone marrow: Platelets Monocyte/n eutrophils S 100 proteins Adapted from: Mellins ED et al. Nat Rev Rheumatol 2011 MyD8 8 NFK B IL-6 TNF M-CSF IL-1 Forms a complex that triggers TLR4 that lead to pro-inflammatory cytokines, including IL-1β and increased S100 proteins Wittkowski H et al. Arthritis Rheum 2008 Frosch M et al. Arthritis Rheum 2009 IL-18 Bone marrow: Platelets Monocyte/n eutrophils S 100 proteins Adapted from: Mellins ED et al. Nat Rev Rheumatol 2011 SoJIA Arthritis Rashes Serositis Leukocytosis ↑Platelets ↑ESR ↑CRP ↑Fibrinogen MAS Encephalopathy Fever Liver failure HSM Leukopenia Lymphadenopathy↓Platelets Hepatitis ↓ESR Coagulopathy ↓Fibrinogen ↑Ferritin DIC ↓Albumin ↑↑↑Ferritin ↑ sIL2Rα NK TNF IL-1 IL-6 IFNγ GM-CSF Trigger (? Virus) CD8 APC GM-CSF CD8 Leukocytosis Macrophage activation NK IFNγ GM-CSF Trigger (? Virus) CD8 APC GM-CSF CD8 Leukocytosis Macrophage activation NK sIL2-R ↑ TNF ↑ IL-1 ↑ IL-6 IL-18 IFNγ GM-CSF Trigger (? Virus) CD8 ↑ Ferritin & sCD163 APC GM-CSF CD8 Hemostatic TF Leukopenia References • • • • • • • • • • • • L.B. Tucker, A.G. Uribe, M. Fernández, et al.: Adolescent onset of lupus results in more aggressive disease and worse outcomes: results of a nested matched case-control study within LUMINA, a multiethnic US cohort (LUMINA LVII). Lupus. 17:314-322 2008 E.M. Tan, A.S. Cohen, J.F. Fries, et al.: The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum.. 25:1271-1277 1982 M.C. Hochberg: Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum.. 40:1725 1997 I.E.A. Hoffman, B.R. Lauwerys, F. De Keyser, et al.: Juvenile-onset systemic lupus erythematosus: different clinical and serological pattern than adult-onset systemic lupus erythematosus. Ann. Rheum. Dis.. 68:412-415 2009 L.T. Hiraki, S.M. Benseler, P.N. Tyrrell, et al.: Clinical and laboratory characteristics and long-term outcome of pediatric systemic lupus erythematosus: a longitudinal study. J. Pediatr. 152:550-556 2008 J.L. Platt, B.A. Burke, A.J. Fish, et al.: Systemic lupus erythematosus in the first two decades of life. Am. J. Kidney Dis.. 2:212-222 1982 M.I. Steinlin, S.I. Blaser, D.L. Gilday, et al.: Neurologic manifestations of pediatric systemic lupus erythematosus. Pediatr. Neurol.. 13:191-197 1995 C.L. Yancey, R.A. Doughty, B.H. Athreya: Central nervous system involvement in childhood systemic lupus erythematosus. Arthritis Rheum.. 24:1389-1395 1981 W.F. Loh, I.M. Hussain, A. Soffiah, Y.N. Lim: Neurological manifestations of children with systemic lupus erythematosus. Med. J. Malaysia. 55:459-463 2000 R. Cervera, R.A. Asherson: Clinical and epidemiological aspects in the antiphospholipid syndrome. Immunobiology. 207:5-11 2003 R. Abdwani, R. Mani: Anti-CD20 monoclonal antibody in acute life threatening haemolytic anaemia complicating childhood onset SLE. Lupus. 18:460-464 2009 S. Kumar, S.M. Benseler, M. Kirby-Allen, E.D. Silverman: B-cell depletion for autoimmune thrombocytopenia and autoimmune hemolytic anemia in pediatric systemic lupus erythematosus. Pediatrics. 123:e159-e163 2009 References • • • • • • • • • • R.E. Petty, T.R. Southwood, P. Manners, et al.: International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J. Rheumatol.. 31 (2):390-392 2004 G.F. Still: On a form of chronic joint disease in children. Med. Chir. Trans.. 80:47-60.9 1897 R.A. Russo, M.M. Katsicas: Patients with very early-onset systemic juvenile idiopathic arthritis exhibit more inflammatory features and a worse outcome. J. Rheumatol. 40 (3):329-334 2013 P. Pignatti, M. Vivarelli, C. Meazza, et al.: Abnormal regulation of interleukin 6 in systemic juvenile idiopathic arthritis. J. Rheumatol.. 28 (7):1670-1676 2001 V. Pascual, F. Allantaz, E. Arce, et al.: Role of interleukin-1 (IL-1) in the pathogenesis of systemic onset juvenile idiopathic arthritis and clinical response to IL-1 blockade. J. Exp. Med.. 201 (9):1479-1486 2005 M. Gattorno, A. Piccini, D. Lasiglie, et al.: The pattern of response to anti-interleukin-1 treatment distinguishes two subsets of patients with systemic-onset juvenile idiopathic arthritis. Arthritis Rheum.. 58 (5):1505-1515 2008 N. Ruperto, H.I. Brunner, P. Quartier, et al.: Two randomized trials of canakinumab in systemic juvenile idiopathic arthritis. N. Engl. J. Med.. 367 (25):2396-2406 2012 F. De Benedetti, H.I. Brunner, N. Ruperto, et al.: Randomized trial of tocilizumab in systemic juvenile idiopathic arthritis. N. Engl. J. Med.. 367 (25):2385-2395 2012 F. Minoia, S. Davi, A. Horne, et al.: Clinical features, treatment and outcome of macrophage activation syndrome complicating systemic juvenile idiopathic arthritis A multinational, multicenter study of 362 patients. Arthritis Rheumatol.. 66:3160-3169 2014 S. Sawhney, P. Woo, K.J. Murray: Macrophage activation syndrome: a potentially fatal complication of rheumatic disorders. Arch. Dis. Child.. 85 (5):421-426 2001 Thank You [email protected] T: 0503989415