* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Sixth Grade Science v. 2016
Introduction to general relativity wikipedia , lookup
List of unusual units of measurement wikipedia , lookup
Electromagnetic mass wikipedia , lookup
Newton's theorem of revolving orbits wikipedia , lookup
Faster-than-light wikipedia , lookup
Internal energy wikipedia , lookup
Lorentz force wikipedia , lookup
Gibbs free energy wikipedia , lookup
Conservation of energy wikipedia , lookup
Standard Model wikipedia , lookup
Mass versus weight wikipedia , lookup
Weightlessness wikipedia , lookup
Stoic physics wikipedia , lookup
Electromagnetism wikipedia , lookup
Modified Newtonian dynamics wikipedia , lookup
Weakly-interacting massive particles wikipedia , lookup
Speed of gravity wikipedia , lookup
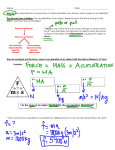
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
History of subatomic physics wikipedia , lookup
History of physics wikipedia , lookup
Negative mass wikipedia , lookup
Condensed matter physics wikipedia , lookup
Elementary particle wikipedia , lookup
Classical mechanics wikipedia , lookup
History of thermodynamics wikipedia , lookup
Fundamental interaction wikipedia , lookup
Equations of motion wikipedia , lookup
Time in physics wikipedia , lookup
Anti-gravity wikipedia , lookup
Classical central-force problem wikipedia , lookup
Work (physics) wikipedia , lookup
Aristotelian physics wikipedia , lookup
Newton's laws of motion wikipedia , lookup
Science: Sixth Grade In sixth grade physical science, students learn about the physical world around them including basic chemistry and physics concepts. Topics of study include elements, particles, types of energy, phase changes and reactions, forces and motion, and magnets. Course Information: Frequency & Duration: 43 minutes; 5 periods per week; full year Text: Physical Science 6th – 8th grade supplemental, Prentice Hall Physical Science Asset Modules: Chemical Interactions Forces and Motion Science: Sixth Grade 2016 - 2017 Content: Scientific Method Duration: August/September (3 weeks) How do people solve problems? Essential How do we find answers with scientifically reliable method? Question: What makes a question testable? Skill: Assessment: Identify the parts of the scientific methods and use a lab sheet. Infer and predict as part of completing a lab activity. Draw conclusions as part of completing a lab activity. Graph results of a lab activity. Students will be able to identify and apply the scientific method as part of a lab investigation. Students will identify key vocabulary as part of an assessment. Science Explorer Physical Science Student edition. (2006) Pearson Prentice Hall pages 1-28 Save Fred activity Resources: Swing Time Lab Graph sheets Standards: 3.2.6.B7.Science as Inquiry Chemistry- the study of properties of matter and how matter changes; Communicating- the process of sharing ideas with others through writing and speaking; Controlled Experimentan experiment in which only one variable is manipulated at a time; Data- facts, figures, and other evidence that scientists collect through observing; Dependent Variable or Responding Variable- the factor that changes as a result of changes to the manipulated, or independent, Vocabulary: variable in an experiment; Hypothesis- a possible explanation for a set of observations or answer to a scientific question; Inferring- the process of making an inference, an interpretation based on observations and prior knowledge; Independent Variable or Manipulated Variable- the one factor that a scientist changes during an experiment; Observing- the process of using one or more of your scenes to gather information; Physics- the study of 1 Science: Sixth Grade 2016 - 2017 matter and how they interact; Qualitative Observation-an observation that deals with characteristics that are not expressed numbers; Quantitative Observation-an observation that deals with a number or amount; Predicting- the process of forecasting what will happen in the future based on past experience or evidence; Science- a way of learning about the natural world through observations and logical reasoning leads to a body of knowledge; Scientific Inquiry or Scientific Method- the ongoing process of discovery in science; Scientific Law-a statement that describes what scientist expect to happen every time under a particular set of conditions; Scientific Theory- a single explanation that connects a large set of related observations or results from experiments; Variable- a factor that can change in an experiment Comments 2 Science: Sixth Grade 2016 - 2017 Content: Mystery Mixture Duration: September/October (3 weeks) How can mixtures be separated? Essential How do you know when a chemical reaction has occurred? Question: What makes up a good observation? Skill: Assessment: Differentiate between elements, compounds, and mixtures. Identify groups of elements that have similar properties. Compare and contrast a pure substance with a mixture. Identify elements are the basic building blocks of matter. Identify the characteristics of elements of the periodic table. Describe how reactants change into products. Given a set of matter the students will identify if the matter is a(n) element, compound, or mixtures. Using the periodic table the students will Identify the groups or families and their properties Students will list the differences of a pure substance and mixture given a set of matter. Students will display and list the elements that make up a given substance. Students will be able to identify the characteristics of an element by its location on the periodic table. Viewing a simple chemical reactions describe how a reactant changes to product. Asset Science Kit: Chemical Interactions Resources: Student notebook pages 1-11 Student resource book Chemical Interactions pages 87, 97-101 3.2.7.A1. Differentiate between elements, compounds, and mixtures. Identify groups of elements that have similar properties. Explain how materials are characterized by having a specific amount of mass in each unit of volume (density). 3.2.6.A2. Compare and contrast pure substances with mixtures. Standards: 3.2.7.A2. Identify atoms as the basic building blocks of matter and that elements are composed of one type of atom. 3.2.8.A2. Identify characteristics of elements derived from the periodic table. 3.2.7.A4. Describe how reactants change into products in simple chemical reactions. Chemical Reaction- a substance produced in a chemical reaction; Compressed- the physical appearance of a sample of matter based on the kinetic energy of its particles. Common phases include solid, liquid, and gas; Density- the smallest piece of a substance that is still that Vocabulary: substance; Dissolve- two or more substances together; Electron- a phase of matter that has definite volume but no definite shape. Particles of liquid are loosely bonded, but can flow over and around one another; Expansion- a phase of matter that has no definite shape or volume. 3 Science: Sixth Grade 2016 - 2017 Particles of gas fly independently through space; Gas- an increase of volume; Liquid- a subatomic particle with a negative charge; Mass- a subatomic particle with a positive charge; Matter- capable of being dissolved. Table salt is soluble in water; Mixture- to incorporate one substance uniformly into another substance at the particle level; Neutron- a way to organize the elements based on atomic number and chemical property; Particle- the ratio of mass and volume in a sample of matter; Periodic Table of Element- a characteristic of a substance that can be observed without changing it chemically, such as size, shape, density, and phase; Phasereduced in volume as a result of applied pressure; Physical Property- a type of matter defined by a unique particle; Product- a process during which starting substances (reactants) change into new substances (products) with different arrangements of atoms; Proton- a mixture formed when one substance dissolves in another; Reactant- a defined quantity of space; Solida subatomic particle with no charge; Soluble- a measure of the quantity of matter; Solution- a starting substance in a chemical reaction; Substance- anything that has mass and takes up space; Volume- a phase of matter that has definite volume and definite shape. The particles of a solid are tightly bonded and cannot move around Comments 4 Science: Sixth Grade 2016 - 2017 Content: Elements Duration: October (1 week) How do elements combine to make other stuff? Essential How much matter is in there? Question: How is an element’s identify determined? Skill: Assessment: Given a set of matter the students will identify if the matter is a(n) element, compound, or mixtures. Using the periodic table the students will Identify the groups or families and their properties Identify elements are the basic building blocks of matter. Identify the characteristics of elements of the periodic table. Explain the differences between and physical and chemical change. Compare and contrast atomic properties of conductors and insulators. The students will be able to identify if a change is a chemical or physical change and support with evidence. Given a set of compounds the students will be able to determine the ratio of elements. Given a set of activities the students will compare and contrast atomic properties of conductors and insulators. Asset Science Kit: Chemical Interactions Resources: Student notebook pages 13-21 Student resource book Chemical Interactions pages 3-13, 90 3.2.6.A4. Differentiate between physical changes and chemical changes. 3.2.6.A1. Distinguish the differences in properties of solids, liquids, and gases. Differentiate between volume and mass. Investigate that equal volumes of different substances usually have Standards: different masses. 3.2.8.B4. Compare and contrast atomic properties of conductors and insulators 3.2.7.A1. 3.2.8.A1. 3.2.7.A2. 3.2.8.A2. Chemical Reaction- a substance produced in a chemical reaction; Compressed- the physical appearance of a sample of matter based on the kinetic energy of its particles. Common phases include solid, liquid, and gas; Density- the smallest piece of a substance that is still that substance; Dissolve- two or more substances together; Electron- a phase of matter that has Vocabulary: definite volume but no definite shape. Particles of liquid are loosely bonded, but can flow over and around one another; Expansion- a phase of matter that has no definite shape or volume. Particles of gas fly independently through space; Gas- an increase of volume; Mass- a subatomic particle with a positive charge; Mixture- to incorporate one substance uniformly 5 Science: Sixth Grade 2016 - 2017 into another substance at the particle level; Neutron- a way to organize the elements based on atomic number and chemical property; Particle- the ratio of mass and volume in a sample of matter; Periodic Table of Element- a characteristic of a substance that can be observed without changing it chemically, such as size, shape, density, and phase; Phase-reduced in volume as a result of applied pressure; Physical Property- a type of matter defined by a unique particle; Proton- a mixture formed when one substance dissolves in another; Reactanta defined quantity of space; Solid-a subatomic particle with no charge; Soluble- a measure of the quantity of matter; Solution- a starting substance in a chemical reaction; Substanceanything that has mass and takes up space; Volume- a phase of matter that has definite volume and definite shape. The particles of a solid are tightly bonded and cannot move around Comments 6 Science: Sixth Grade 2016 - 2017 Content: Particles Duration: October (1 week) How is matter different and the same? Essential What is the smallest part? Question: How do you determine what makes up a compound? Skill: Assessment: Distinguish the differences in properties of a solid, liquid, and gasses. Identify the difference of volume and mass of different substances. The students will be able to identify if a change is a chemical or physical change and support with evidence. Identify how energy is transferred Explain the effects on the physical and chemical properties of matter during energy transfer Demonstrate that heat moves in predictable ways from warmer objects to cooler ones. SCALE Investigate that materials may be composed of parts too small to be seen without magnification. Students will explain how the properties of matter changed during energy transfer. Using lab resource the students will Demonstrate that heat moves in predictable ways from warmer objects to cooler ones. Using models the students will Investigate that materials may be composed of parts too small to be seen without magnification. Asset Science Kit: Chemical Interactions Resources: Student notebook pages 22-31 Student resource book Chemical Interactions pages 14-22 3.2.6.A1. Distinguish the differences in properties of solids, liquids, and gases. Differentiate between volume and mass. Investigate that equal volumes of different substances usually have different masses. 3.2.7.A3. Explain how energy transfer can affect the chemical and physical properties of Standards: matter. 3.2.6.B6. ENERGY Demonstrate that heat moves in predictable ways from warmer objects to cooler ones. SCALE Investigate that materials may be composed of parts too small to be seen without magnification. 3.2.6.A4. 3.2.8.B4. 7 Science: Sixth Grade 2016 - 2017 Chemical Reaction- a substance produced in a chemical reaction; Compressed- the physical appearance of a sample of matter based on the kinetic energy of its particles. Common phases include solid, liquid, and gas; Density- the smallest piece of a substance that is still that substance; Dissolve- two or more substances together; Electron- a phase of matter that has definite volume but no definite shape. Particles of liquid are loosely bonded, but can flow over and around one another; Expansion- a phase of matter that has no definite shape or volume. Particles of gas fly independently through space; Gas- an increase of volume; Mass- a subatomic particle with a positive charge; Mixture- to incorporate one substance uniformly into another substance at the particle level; Neutron- a way to organize the elements based on Vocabulary: atomic number and chemical property; Particle- the ratio of mass and volume in a sample of matter; Periodic Table of Element- a characteristic of a substance that can be observed without changing it chemically, such as size, shape, density, and phase; Phase-reduced in volume as a result of applied pressure; Physical Property- a type of matter defined by a unique particle; Proton- a mixture formed when one substance dissolves in another; Reactanta defined quantity of space; Solid-a subatomic particle with no charge; Soluble- a measure of the quantity of matter; Solution- a starting substance in a chemical reaction; Substanceanything that has mass and takes up space; Volume- a phase of matter that has definite volume and definite shape. The particles of a solid are tightly bonded and cannot move around Comments 8 Science: Sixth Grade 2016 - 2017 Content: Kinetic Energy Duration: November (1 week) What is energy? Essential How can energy be transferred from one object or system to another? Question: How does energy interact with matter to cause change and do work? Skill: Assessment: Distinguish the differences in properties of a solid, liquid, and gasses. Identify the difference of volume and mass of different substances. Identify how energy is transferred Explain the effects on the physical and chemical properties of matter during energy transfer Explain how a change in energy, changes matter. Explain the differences between and physical and chemical change. Demonstrate that heat moves in predictable ways from warmer objects to cooler ones. SCALE Investigate that materials may be composed of parts too small to be seen without magnification. Given a set of matter the students will be able to identify the state of matter and the properties The students will investigate different substances to compare the volumes and masses. Students will explain how the properties of matter changed during energy transfer. Asset Science Kit: Chemical Interactions Resources: Student notebook pages 32-41 Student resource book Chemical Interactions pages 23-31 Standards: 3.2.7.A3. Explain how energy transfer can affect the chemical and physical properties of matter. 3.2.6.A1. 3.2.8.A3. 3.2.6.A4. 3.2.8.A4. 3.2.6B6. 9 Science: Sixth Grade 2016 - 2017 Contract- to shorten or to become reduced in size; Contraction- the act of decreasing (something) in size or volume or quantity or scope; Kinetic Energy-the type of energy that an Vocabulary: object has because it is in motion; Temperature- the degree of hotness or coldness of an object or the environment corresponding to its molecular activity; Thermometer- an instrument used for measuring temperature Comments 10 Science: Sixth Grade 2016 - 2017 Content: Energy Transfer Duration: November (1.5 weeks) What are the different forms of energy? How is energy conserved as it transforms from one form to another/from one object to another? Essential How does energy affect the motion of an object? Question: How are temperature and energy related? How does energy get transferred from one object to another? What is a system? What is thermal energy? Skill: Assessment: Identify the difference of volume and mass of different substances. Distinguish the differences in properties of a solid, liquid, and gasses. Identify how energy is transferred. Explain the effects on the physical and chemical properties of matter during energy transfer. Explain how a change in energy, changes matter. Students will explain how the properties of matter changed during energy transfer. Students will explain how energy changes matter during a lab activity. Asset Science Kit: Chemical Interactions Resources: Student notebook pages 43-52 Student resource book Chemical Interactions pages 32-37 Standards: 3.2.6.A1. 3.2.7.A3. 3.2.8.B3. 3.2.6.B6. Calorie- the energy it takes to raise the temperature of 1 gram of water 1 degree Celsius; Conduction- when heat or electricity moves from one place to another; Conservation of Energy- a principle stating that energy cannot be created or destroyed, but can be altered from one form to another; Cooling- is a form of energy to become less hot, calm or less excited; Vocabulary: Energy Transfer- the conversion of one form of energy into another, or the movement of energy from one place to another; Equilibrium- a state in which opposing forces or influences are balanced; Heating- a form of energy associated with the movement of atoms and molecules in any material. The higher the temperature of a material, the faster the atoms are moving, and hence the greater the amount of energy present as heat Comments 11 Science: Sixth Grade 2016 - 2017 Content: Heat of Fusion, Phase Change and Reactions Duration: November/December (3 weeks) What happens to the particles when chemical changes occur? How do particles in various phases interact with each other.. (pushes and pulls between Essential particles) Question: What determines whether a reaction is exothermic or endothermic? What are the three methods of heat transfer and how do particles interact when transferring heat by these methods? Skill: Track the movement of heat between objects Explain how heat effects particle motions Explain what happens to particles during phase changes Differentiate among convection, conduction, and radiation Explain why heat energy consists of the random motion and vibrations Explain how changes in temperature are accompanied by changes in kinetic energy Compare and contrast atomic properties of conductors and insulators. Explain how electrical current is produced by the flow of electrons Explain and demonstrate how electric current produces magnetic forces and how moving magnets produce electric current. Provided examples the students Give examples of how heat moves in predictable ways, normally flowing from warmer objects to cooler ones until they reach the same temperature. Explain the effect of heat on particle motion by describing what happens to particles during a phase change. Given a set of heat transfers the students will be able to determine if is convection, conduction, or radiation Illustrate the motion of particles and explain during heat transfer. During a lab activity the students will explain how electrical current is produced by the flow of electrons Explain and demonstrate how electric current produces magnetic forces and how moving magnets produce electric current. Given a set of activities the students will compare and contrast atomic properties of conductors and insulators. Assessment: Asset Science Kit: Chemical Interactions Resources: Student notebook pages 54-73 Student resource book Chemical Interactions pages 38-48 3.2.6.B3. Give examples of how heat moves in predictable ways, normally flowing from warmer objects to cooler ones until they reach the same temperature. Explain the effect of heat on particle motion by describing what happens to particles during a phase change. Standards: 3.2.7.B3. Differentiate among convection, conduction, and radiation. Explain why heat energy consists of the random motion and vibrations of the particles of matter. 3.2.7.B4. Explain how electrical current is produced by the flow of electrons. Explain and demonstrate how electric current produces magnetic forces and how moving magnets produce 12 Science: Sixth Grade 2016 - 2017 electric current. 3.2.8.B3. 3.2.6.B6. Atom- the basic unit of a chemical element; Bond- A force of attraction that holds atoms or ions together in a molecule or crystal; Burning- a chemical reaction that involves the rapid combination of a fuel with oxygen; Compound- a thing that is composed of two or more separate elements; a mixture; Condensation- change of a gas or vapor to a liquid, either by cooling or by being subjected to increased pressure; Conservation of Matter- the principle that matter cannot be created or destroyed; Constraint- a limitation or restriction; Criterionsomething that is used as a reason for making a judgment or decision; Crystal- a piece of a homogeneous solid substance having a natural geometrically regular form with symmetrically arranged plane faces; Deposition- The accumulation or laying down of matter by a natural process; Dissolve- to make a solution of, as by mixing with a liquid; Engineering Probleminvolves stating the problem to be solved as clearly as possible in terms of criteria for success, and constraints or limits; Evaporation- the process of a liquid changing into a gas; Freeze- the process through which a substance changes from a liquid to a solid; Freezing Pointtemperature at which a liquid changes into a solid; the same temperature as the melting point; Insulation- material that is used to stop the passage of electricity, heat, or sound from one Vocabulary: conductor to another; Ionic Compound- a chemical compound comprising ions held together by electrostatic forces termed ionic bonding; Melt- make or become liquefied by heat; Mixture- a substance made by mixing other substances together; Melting Point- temperature at which a given material changes from a solid to a liquid, or melts; the same temperature as freezing point; Molecule- group of atoms bonded together, representing the smallest fundamental unit of a chemical compound that can take part in a chemical reaction; Phase Change- a change from one state (solid or liquid or gas) to another without a change in chemical composition; Solubility- a chemical property referring to the ability for a given substance, the solute, to dissolve in a solvent. It is measured in terms of the maximum amount of solute dissolved in a solvent at equilibrium. The resulting solution is called a saturated solution; Solute- the minor component in a solution, dissolved in the solvent; Solution- a liquid mixture in which the minor component (the solute) is uniformly distributed within the major component (the solvent); Solvent- able to dissolve other substances; State of Matterone of the four principal conditions in which matter exists—solid, liquid, gas, and plasma; Sublimation- process of changing from a solid to a gas without passing through an intermediate liquid phase Comments 13 Science: Sixth Grade 2016 - 2017 Content: Here to There Duration: January (1 week) Does something have to move to be in motion? How can motion be observed, described, measured and represented? Essential How do different speeds look on different graphs? Question: How do we represent direction? What is the difference between a positive and negative slope? Identify and use the following: • Skill: • • • • Assessment: Position is the location of an object at any given time. Motion is the act of changing position. Distance is the amount of change of position. A reference point is an arbitrary point on an object, used to establish its position. Calculate distance (d ) using the distance equation. Observe and describe an object’s motion in terms of change of position. Explain how to use a reference point to determine the distance moved by an object. Measure distance in standard metric units. Use tools to gather data and mathematics to organize data. Asset Science Kit: Forces and Motion Student notebook pages Lab notebook page Resources: Text book Forces and Motion page Student resource book Forces and Motion 3.2.6.B1. Explain how changes in motion require a force. 3.2.7.B1. Describe how unbalanced forces acting on an object change its velocity. Analyze how Standards: observations of displacement, velocity, and acceleration provide necessary and sufficient evidence for the existence of forces Displacement (∆x)- amount and direction of change of position between an object’s initial position and final position; Distance (d)- amount of change of position between an initial position and a final position; always a positive number; Final Position (xf)- position of an Vocabulary: object at the end of a motion; Initial Position (xi)- The position of an object at the start of a motion; Motion- act of changing position; Position (x)- object’s location at a given time; Reference Point- specific location used to monitor change of position. Reference points can be on a moving object or in an object’s environment Comments 14 Science: Sixth Grade 2016 - 2017 Content: Speed and Comparing Speed Duration: January (3 weeks) Essential In what ways does speed change? Question: How can we represent speed? Skill: Assessment: Identify speed is the rate of change of position of an object: v = d / Δt. To use the slope of the line on a speed graph represents speed; steeper slopes represent higher speeds. Find distance using equation for calculating distance when speed and time are known is d = v ✕ Δt. Find average speed is the total distance traveled by an object divided by the total time needed to go that distance. The slope of a line on a distance-versus-time graph represents speed; steeper slopes represent higher speeds. A distance-versus-time graph can be used to determine an object’s speed. Conduct experiments to acquire distance and time data and to determine speed. Use tools to gather data and mathematics to organize data. Use mathematics to solve problems involving unknown quantities. Explain speed in terms of distance and time. Conduct experiments to acquire time and distance data and to determine speed. Asset Science Kit: Forces and Motion Student notebook pages Lab notebook pages Resources: Text book Forces and Motion pages Student resource book Forces and Motion 3.2.8.B1. Explain how inertia is a measure of an object’s mass. Explain how momentum is Standards: related to the force 3.2.6.B1. 3.2.7.B1. Acceleration (a)- change of velocity per unit of time; Air Resistance- force exerted by air molecules on objects moving through air; Average Speed- theoretical constant speed at which an object would have to travel in order to go a given distance in a given period of time. Total distance divided by total time; Constant Speed- speed that does not vary over time; Data Vocabulary: Table- two-dimensional system for recording data that shows correlations between the data entries; Delta (∆)- fourth letter in the Greek alphabet; the symbol indicates change: final state minus initial state; Dotcar- rolling car that records time and distance data as it moves. Data can be recorded mechanically by making an ink dot on paper at regular intervals or electronically in 15 Science: Sixth Grade 2016 - 2017 a microprocessor carried by the car; Force (F)- interaction between masses. A push or pull; Inertia- property of mass that resists change of motion. Large masses have a lot of inertia; Interval- amount, such as the time or distance between two markers. Standard intervals, like seconds and meters, are used to measure time and distance. LED- short for light-emitting diode, an electronic device that makes light using solid-state technology instead of a hot filament. leg: A segment of a complex motion; a logical part of a trip; Equation- mathematical statement showing relationships between quantifiable variables; Graph- two-coordinate system for displaying and analyzing an independent variable (y); Speed (v)- distance traveled by an object in a unit of time. Speed is reported in standard units of distance per unit time, such as meters per second or kilometers per hour; Time- difference between then and now; often quantified in standard intervals such as seconds and hours Comments 16 Science: Sixth Grade 2016 - 2017 Content: Representing Motion Duration: February (1.5 weeks) How do we model acceleration motion? How do pushes (forces) affect motion? Balanced or unbalanced? Essential How do forces affect motion? Question: What is gravity (mass vs. weight)? What relationships exist amongst force, weight and acceleration? What is friction? How does it affect motion? Skill: Assessment: Identify the difference between an object’s initial and final positions is displacement. Find and use constant speed and average speed yield straight lines on distance-versus-time graphs. Complex motion events can be analyzed into coherent segments called legs. Use tools to gather and organize data. Transform narrative accounts of motion events into graphic representations. Generate motion scenarios from graphic representations of motion events. Explain the difference between displacement and distance. Explain what a horizontal line on a speed graph represents. Student notebook pages Lab notebook pages Resources: Text book Forces and Motion pages Student resource book Forces and Motion Standards: 3.2.6.B1. 3.2.7.B1. 3.2.8.B1. Acceleration (a)- change of velocity per unit of time; Average Speed- theoretical constant speed at which an object would have to travel in order to go a given distance in a given period of time. Total distance divided by total time; Constant Speed- speed that does not vary over time; Data Table- two-dimensional system for recording data that shows correlations Vocabulary: between the data entries; Delta (∆)- fourth letter in the Greek alphabet; the symbol indicates change: final state minus initial state; Equation- mathematical statement showing relationships between quantifiable variables; Force (F)- interaction between masses. A push or pull; Interval- amount, such as the time or distance between two markers. Standard intervals, like seconds and meters, are used to measure time and distance. Graph- two-coordinate system for 17 Science: Sixth Grade 2016 - 2017 displaying and analyzing an independent variable (y); Speed (v)- distance traveled by an object in a unit of time. Speed is reported in standard units of distance per unit time, such as meters per second or kilometers per hour; Time- difference between then and now; often quantified in standard intervals such as seconds and hours Comments 18 Science: Sixth Grade 2016 - 2017 Content: Acceleration Duration: February (2 weeks) How can you show speed in different ways? How do we model acceleration motion? Essential What information is given on a v-t graph? Question: What does the slope of a v-t graph tells us? What is the acceleration of gravity? Skill: Assessment: Acceleration is change of velocity (Δv–) per unit time, measured in units of change of position (Δx) per unit of time per unit of time. Objects rolling down slopes accelerate; acceleration is greater on steeper slopes. The mass of a rolling car has little effect on its acceleration. Use tools (mechanical and electronic Dotcars) to collect time and distance data and mathematics to organize and analyze the data. Use equations to calculate acceleration, displacement, and velocity of rolling objects. Identify and interpret graphs of accelerating motion and constant velocity. Asset Science Kit: Forces and Motion Student notebook pages Lab notebook pages Resources: Text book Forces and Motion pages Student resource book Forces and Motion 3.2.6.B2. Describe energy as a property of objects associated with heat, light, electricity, magnetism, mechanical motion, and sound. Differentiate between potential and kinetic energy. Standards: 3.2.7.B2. Describe how energy can be changed from one form to another (transformed) as it moves through a system or transferred from one system to another system. 3.2.7.B1. 3.2.8.B1. Acceleration (a)- change of velocity per unit of time; Average Speed- theoretical constant speed at which an object would have to travel in order to go a given distance in a given period of time. Total distance divided by total time; Constant Speed- speed that does not vary over time; Data Table- two-dimensional system for recording data that shows correlations between the data entries; Delta (∆)- fourth letter in the Greek alphabet; the symbol indicates change: final state minus initial state; Equation- mathematical statement showing relationships Vocabulary: between quantifiable variables; Force (F)- interaction between masses. A push or pull; Interval- amount, such as the time or distance between two markers. Standard intervals, like seconds and meters, are used to measure time and distance. Graph- two-coordinate system for displaying and analyzing an independent variable (y); Speed (v)- distance traveled by an object in a unit of time. Speed is reported in standard units of distance per unit time, such as meters per second or kilometers per hour; Time- difference between then and now; often quantified in standard intervals such as seconds and hours Comments 19 Science: Sixth Grade 2016 - 2017 Content: Force Duration: February/March (1.5 weeks) How do forces affect motion? Essential What relationships exist amongst force, weight and acceleration? Question: How does tension exhibit equal and opposite forces? Skill: Assessment: Identify and use the fact that a force is a push or pull. Identify a net force is the sum of all the forces acting on a mass. Explain that a net force applied to a mass produces acceleration. Identify that friction is a force that acts to resist movement. Use tools (pushers, spring scales, and multimedia simulations) to apply force and investigate friction and motion. Analyze illustrations of forces in motion. Use multimedia simulations to investigate force and motion. Describe change of motion as a result of net force. Student notebook pages Lab notebook pages Resources: Text book Forces and Motion pages Student resource book Forces and Motion Standards: 3.2.7.B1. 3.2.8.B1. 3.2.6.B2. 3.2.7.B2. Net Force- sum of all the forces acting on a mass; Newton (N)- unit used to measure force in the metric system. Newton’s laws of motion: 1. Objects remain in uniform motion (at rest or constant velocity) until acted on by a net force. 2. A net force exerted on an object results in acceleration proportional to the strength of the force and inversely proportional to the mass of Vocabulary: the object. 3. For every action there is an equal and opposite reaction; Friction- force acting between surfaces in contact. Friction acts to resist FOSS Force and Motion Course Glossary 2 motion; Slope- angle at which a surface or line is inclined. In graphs, slope is rise (∆y) over run (∆x); Rate- mathematical relationship between two factors, such as the relationship between distance and time in a motion event; Velocity (v)- change in position per unit of time: ∆x/∆t. Comments 20 Science: Sixth Grade 2016 - 2017 Content: Gravity Duration: March (1.5 weeks) Essential What is gravity (mass vs. weight)? Question: Is gravity a force and how can prove it? Skill: Assessment: Identify gravity is a force pulling masses toward each other; the strength of the force depends on the objects’ masses. Identify the force of gravity accelerates objects in free fall and objects rolling downhill. Identify acceleration produced by the force of gravity is about 10 m/s2 toward Earth. Determine the relationship between mass and the force of gravity, using spring scales. Gather time and displacement data electronically to investigate the acceleration of gravity. Explain gravity as a universal force. Discuss Galileo’s discovery of acceleration due to gravity. Student notebook pages Lab notebook pages Resources: Text book Forces and Motion pages Student resource book Forces and Motion Standards: M3.2.7.B1. 3.2.8.B1. 3.2.6.B2. 3.2.7.B2. Gravity (g)- force due to gravitational attraction between Earth and other masses; Impulseinteraction between masses; a force exerted for a period of time: F X ∆t; Interaction- action between two (or more) objects that affects both objects; Mass (m)- measure of the quantity of matter in an object; Theoretical- based on a mathematically determined or reasoned Vocabulary: explanation of some natural phenomenon; Physicist- scientist who studies matter, energy, force, and motion; Terminal Speed- maximum speed a particular object can obtain during free fall through air; Momentum (p)- measure of the motion of an object in terms of its mass and its velocity. The greater the mass and/or velocity, the greater the momentum. Comments 21 Science: Sixth Grade 2016 - 2017 Content: Momentum Duration: April/May (2 weeks) Does all matter have the same momentum? What happens to the momentum of an object when interacts with other matter? Essential What does it mean when we say that momentum is conserved, and how does this apply to Question: collisions? How are Newton’s Laws connected to the concepts of momentum and impulse? Skill: Assessment: Inertia is the property of matter that tends to keep masses in uniform motion; it resists change of motion. Inertia is proportional to mass; large masses have a lot of inertia. Momentum is inertia in motion; it is the product of an object’s velocity and mass. Identify a net force applied to an object can change its momentum. Identify an impulse is a force applied for a period of time. Conduct simple investigations to demonstrate inertia of both stationary and moving masses. Use a force scale to determine the force needed to stop cars traveling at different speeds. Use electronic Dotcar data to calculate velocity and momentum. Explain how inertia and momentum affect passenger safety in car crashes. Explain and apply the interplay of force and time (impulse) and momentum in crashes. Create a working Rube Goldberg Machine. Student notebook pages Lab notebook pages Resources: Text book Forces and Motion pages Student resource book Forces and Motion Standards: M3.2.7.B1. 3.2.8.B1. 3.2.6.B2. 3.2.7.B2. Acceleration (a)- change of velocity per unit of time; Average Speed- theoretical constant speed at which an object would have to travel in order to go a given distance in a given period of time. Total distance divided by total time; Constant Speed- speed that does not vary over time; Data Table- two-dimensional system for recording data that shows correlations between the data entries; Delta (∆)- fourth letter in the Greek alphabet; the symbol indicates change: final state minus initial state; Equation- mathematical statement showing relationships Vocabulary: between quantifiable variables; Force (F)- interaction between masses. A push or pull; Interval- amount, such as the time or distance between two markers. Standard intervals, like seconds and meters, are used to measure time and distance. Graph- two-coordinate system for displaying and analyzing an independent variable (y); Speed (v)- distance traveled by an object in a unit of time. Speed is reported in standard units of distance per unit time, such as meters per second or kilometers per hour; Time- difference between then and now; often quantified in standard intervals such as seconds and hours Comments 22 Science: Sixth Grade 2016 - 2017 Content: Electric and Magnets Duration: May (2 weeks) What are magnetic fields? Essential What materials or objects can attract or repel a magnet? Question: How is magnetism transformed to electricity? How do circuits complete a pathway to produce light, heat or sound? Skill: Assessment: Describe how electric current produces magnetic forces and how moving magnets produce electric current Describe how electric current produces magnetic forces and how moving magnets produce electric current Explain how electrical current is produced by the flow of electrons Explain and demonstrate how electric current produces magnetic forces and how moving magnets produce electric current. Compare and contrast atomic properties of conductors and insulators. During investigations students will describe how electric current produces magnetic forces and how moving magnets produce electric current. Derive Ohm’s Law through investigation of voltage, current, and resistance. During a lab activity the students will explain how electrical current is produced by the flow of electrons Explain and demonstrate how electric current produces magnetic forces and how moving magnets produce electric current Given a set of activities the students will compare and contrast atomic properties of conductors and insulators. Student notebook pages Lab notebook pages Resources: Text book Forces and Motion pages Student resource book Forces and Motion 3.2.6.B4. Describe how electric current produces magnetic forces and how moving magnets produce electric current. Derive Ohm’s Law through investigation of voltage, current, and resistance. 3.2.7.B4. Explain how electrical current is produced by the flow of electrons. Explain and demonstrate how electric current produces magnetic forces and how moving magnets produce Standards: electric current. 3.2.8.B4. Compare and contrast atomic properties of conductors and insulators. 3.2.7.B5. Demonstrate that visible light is a mixture of many different colors. Explain the construct of the electromagnetic spectrum. Describe how sound and light energy are transmitted by waves 23 Science: Sixth Grade 2016 - 2017 Electromagnetic Wave- a transverse wave that transfers electrical and magnetic energy; Electromagnetic Radiation- energy that is transferred through space by electromagnetic waves; Polarized Light- light that passes through polarized filter; Photon- stream of tiny Vocabulary: packets of light; Infrared Rays- are electromagnetic waves with wavelengths shorter than those of radio waves; Gamma Rays-are electromagnetic waves with the shortest wave lengths and highest frequencies Comments 24