* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Heme- Fe 2+ (ferrous) - LSU School of Medicine
Survey
Document related concepts
Genetic code wikipedia , lookup
Human digestive system wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Citric acid cycle wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Oligonucleotide synthesis wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Gaseous signaling molecules wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Peptide synthesis wikipedia , lookup
Biochemistry wikipedia , lookup
Metalloprotein wikipedia , lookup
Transcript
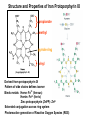
Porphyrin Metabolism Structure and Properties of Iron Protoporphyrin IX propionate methyl pyrrole ring vinyl Derived from protoporphyrin IX Pattern of side chains defines isomer Binds metals: Heme- Fe2+ (ferrous) Hemin- Fe3+ (ferric) Zinc protoporphyrin (ZnPP)- Zn2+ Extended conjugation across ring system Photoreactive generation of Reactive Oxygen Species (ROS) Amino Acid Pools are in Steady State Majority of amino acids used for de novo protein synthesis (80%) derives from the degradation of existing proteins Only 30 g (6%) used for synthesis of specialized products Only 70 g (14%) of total amino acid utilization is used for energy or stored as glycogen/fatty acids in the well-fed state (nitrogen balance) Overview of Heme Synthesis Heme Succinyl CoA + Glycine Protoporphyrin IX ALA synthase -aminolevulinic acid Protoporphyrinogen IX Coproporphyrinogen III mitochondrial matrix cytoplasm -aminolevulinic acid Porphobilinogen Uroporphyrinogen III Coproporphyrinogen III Uroporphyrinogen I Coproporphyrinogen I Heme synthesis occurs in all cells due to the requirement for heme as a prosthetic group on enzymes and electron transport chain. By weight, the major locations of heme synthesis are the liver and the erythroid progenitor cells of the bone marrow. -Aminolevulinate (ALA) Synthase is the Committed Step for Heme Biosynthesis •Rate limiting committed step; requires pyridoxal-5’-phosphate as coenzyme •Transcriptional regulation is the principal form of control since the enzyme has a short half life (t1/2 = 1 hr). Heme and hemin repress transcription •In erythrocytes heme synthesis is coordinated with that of the globin chains, all of which are stimulated by erythropoietin (Epogen©, Procrit©, and congeners) •Heme and hemin allosterically inhibit ALA synthase •Aromatic drugs, xenobiotics, and steroids induce synthesis of ALA synthase and can exacerbating certain porphyrias (later) -Aminolevulinate (ALA) Dehydratase •Asymmetry of the reaction results in acetate and propionate side chains •The enzyme active site contains a required cysteine, making the enzyme sensitive to inactivation by lead (Pb2+) and other heavy metals •Increased urinary excretion of -aminolevulinate is a leading indicator of heavy metal poisoning Formation of the Final Ring Requires a Bi-functional Enzyme This step occurs by elimination of the primary amines as the methylene adds across the double bond of the pyrrole ring. Note: Uroporphyrinogen I synthase is alternate name for Hydroxymethylbilane synthase Uroporphyrinogen Decarboxylase Remodels the Acetate Side Chains Spontaneously oxidizes to the biologically inactive Coproporphyrin I and III which are subsequently excreted in the urine Coproporphyrinogen III Oxidase Catalyzes the Oxidative Decarboxylation of Specific Propionate Side Chains Protoporphyrinogen IX Oxidase This reaction oxidizes the methylene bridge carbons between the pyrrole rings to methenyl bridge carbons, allowing extended conjugation through the entire tetrapyrrole ring system for the first time. Ferrochelatase •Inserts Fe2+ into Protoporphyrin IX to yield heme •The reaction also requires ascorbic acid and cysteine as reducing agents •Lead (Pb2+) acts as a competitive inhibitor of Fe2+ but does not insert into protoporphyrin IX •Iron deficiency leads to insertion of Zn2+ to yield zinc protoporphyrin (ZnPP), an important clinical indicator of iron deficiency Porphyrias Caused by hereditary or acquired defects in heme synthesis - Accumulation and increased excretion of metabolic precursors (each unique) - Most porphyrias show a prevalent autosomal dominant pattern, except congenital eythropoietic porphyria, which is recessive Can be hepatic or erythropoietic, reflecting the two major locations of heme synthesis - hepatic can be acute or chronic Those with tetrapyrrole intermediates show photosensitivity due to extended conjugated double bonds - Formation of superoxide radicals - Skin blisters, itches (pruritis) - Skin may darken, grow hair (hypertrichosis) Acquired Porphyrias Lead poisoning - inhibition of ferrochelatase and ALA dehydratase - displaces Zn+2 at enzyme active site Children - developmental defects - drop in IQ - hyperactivity - insomnia - many other health problems Adults - severe abdominal pain - mental confusion - many other symptoms Most heme from RBCs (85%) - rest from turnover of cytochromes, p450s, immature erythrocytes. RBCs last 120 days, degraded by reticuloendothelial (RE) system [liver and spleen]. Microsomal heme oxygenase hydroxylates methenyl bridge carbon and oxidizes Fe2+ to Fe3+. Second reaction open ring and release methenyl carbon as CO. The resulting biliverdin is poorly soluble due to ring stacking and aggregation. Serum albumin carries bilirubin in circulation, ligandin in hepatocytes. Types of Jaundice Hemolytic jaundice - Liver can handle 3000 mg bilirubin/day - normal is 300 - Massive hemolysis causes more than can be processed - cannot be conjugated - increased bilirubin excreted into bile, urobilinogen is increased in blood, urine - unconjugated bilirubin in blood increases = jaundice Obstructive jaundice - Obstruction of the bile duct - tumor or bile stones - gastrointestinal pain - nausea - pale, clay-colored stools - can lead to liver damage and increased unconjugated bilirubin Hepatocellular jaundice - Liver damage (cirrhosis or hepatitis) cause increased bilirubin levels in blood due to decreased conjugation - Conjugated bilirubin not efficiently exported to bile so diffuses into blood - Decreased urobilinogen in enterohepatic circulation so urine is darker and stool is pale, clay-colored - AST and ALT levels are elevated due to hepatic damage - Nausea and anorexia Jaundice in Newborns Premature babies often accumulate bilirubin due to late onset of expression of bilirubin glucuronyltransferase - Maximum expression (adult level) at ~ 4 weeks - Excess bilirubin can cause toxic encephalopathy (kernicterus) - Treated with blue fluorescent light - converts bilirubin to more polar compound - can be excreted in bile without conjugation - Crigler-Najjar syndrome is deficiency in bilirubin glucuronyltransferase