* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Pharmacogenomics and nutrigenomics
Survey
Document related concepts
Psychedelic therapy wikipedia , lookup
Compounding wikipedia , lookup
Orphan drug wikipedia , lookup
Polysubstance dependence wikipedia , lookup
Drug design wikipedia , lookup
Psychopharmacology wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Drug discovery wikipedia , lookup
Pharmacognosy wikipedia , lookup
Theralizumab wikipedia , lookup
Neuropharmacology wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Prescription drug prices in the United States wikipedia , lookup
Prescription costs wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Transcript
Envirenmental
factors
+
-
Biological
response
Genetic
factor
Pharmacogenomics and nutrigenomics;
Gene-environment interactions
Orsolya Lang MD. PhD.
ED 2017
To treat or not to treat?
Drug
+G
Effect
Drug
+ G’
Adverse effect
Adverse effect:
'A response to a drug which is noxious and unintended, and
which occurs at doses normally used in man for the
prophylaxis, diagnosis, or therapy of disease, or for the
modifications of physiological function’ (WHO 1972)
Adverse drug reactions (ADR) are responsible for 5-10% of hospital admissions
in the US and Europe.
Traditional Medical Treatment
Individual response:
Variation in therapeutic response
Different side effects, adverse reactions
Mainly (60- 80%) caused by genetic variation
Major Drugs Ineffective for Many …
… and harmful to some.
What is worst?
Adverse drug responses are included
In USA: >100,000 death/year
EU: 10% of hospital admission
To treat or not to treat?
To Treat but Apply Personalized or Tailored treatment
Fit the dose to the patient
Or consider the alternative treatments
Many factors influence the effective dose
Genes
Intrauterine environment
Age
Epigenetic factors
Physical activity
Disease
Drug interaction
Liver/Kidney function
Intestinal flora
Cyrcadian rhythm etc. …
The therapy is optimal when all are considered
Something new or something old?
‘right treatment for the right patient at the right
time' - has been practiced for millennia.
5th century BCE
Pythagoras - excitement about his mathematical
pursuits overcome by a terrible depression.
Hipoccrates’ treatment doled out is precise to
Pythagoras, including changing his dietary
habits, a temporary spate of abstinence, and a
harsh purging of his bowels. Following
treatment, Pythagoras is better.
Only phrase to capture what is happening in
our time
http://www.medicinenet.com/script/main/art.asp?articlekey=15313
Sykiotis et al. 2005
Pharmacogenomics
Smaller effect;
multiple variant
Pharmacogenomics
Pharmacogenetics
Large single
variant effect
Single gene
Small number
of genes
The study of genetic basis
for variability in drug
response
Complex
biological
pathway
Whole genome
Use of genetic information to
guide the choice of drug and
dose on an individual bases
Pharmacogenetics versus pharmacogenomics
• Pharmacogenomics: The study of how variations in
the human genome affect the response to
medications. Pharmacogenomics combines
traditional pharmaceutical sciences such as
biochemistry with annotated knowledge of genes,
proteins, and single nucleotide polymorphisms.
http://www.medicinenet.com/script/main/art.asp?articlekey=15313
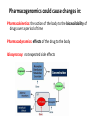
Pharmacogenomics could cause changes in:
Pharmacokinetics: the action of the body to the bioavailability of
drugs over a period of time
Pharmacodynamics: effects of the drug to the body
Idiosyncrasy: not expected side effects
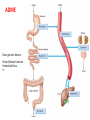
How does genetic variation affect drug effect?
Genetic polymorphisms or variants
Pharmacokinetic (PK)
Pharmacodynamic (PD)
Receptors
Absorption
Distribution
Excretion
Metabolism
ADME
Immune
Ion channels
Enzymes
Pharmacokinetics and Pharmacodynamics
Effect vs concentration
Concentration vs Time
Effect vs Time
http://www.nature.com/ijir/journal/v19/n3/fig_tab/3901522f2.html
ADME
Over genetic factors:
Liver/Kidney function
Intestinal flora
…
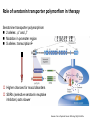
Drug Absorption
Multidrug resistance protein1 (MDR1, P-gp)
•
Genotype
Digoxin plasma
level
G/G2677
C/C3435
67.6% +/4.3%,
G/T2677
C/T3435
80.9% +/8.9%,
T/T2677
T/T3435
87.1% +/8.4%,
Digoxin-cardiotonic, smal therapeutical window
Exon26 mutation->lower MDR1
expression-> greater absorption> higher plasma digoxin level
Pharmaceutics 2010, 2(1), 61-77
Kazuya et al. Drug Metab Pharmacokinet 23(4) 2008
Kurrata et al. Clin Pharmacol Ther. 2002 Aug;72(2):209-19.
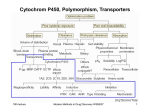
Drug Metabolism
Detoxification: chemical modifications, which increase the speed of excretion of a drug
Phase I enzymes
e.g. Cytochrome P450
- chemical modifications, which change the
polarity of the molecule
Phase II enzymes
e.g. N-acetyl-Transferase,
Glucuronyltransferase,
Glutatione-transferase
Conjugation:
- binding a very polar molecule to the drug
Phase I:
Cytochrome P450 enzymes (CYP)
Flavin monooxygenases (FMO)
Monoamine oxidases(MAO)
Esterases, including carboxylesterases
(CEs), cholinesterases
(acetylcholinesterase (AChE) and
butyrylcholinesterase (BChE)) and
paraoxonase (PON)
Aldehyde oxidase (AO)
Aldehyde dehydrogenase (ALDH1A1)
Aldo-keto reductases (AKR)
Phase II:
UDP-glucuronyltransferases (UGT)
Sulphotransferases (SULT)
N-Acetyltransferases (NAT)
Glutathione S-transferases (GST)
Evans and Relling (1999). Science, 286, 487-491
Phase I metabolism (oxidation by CYP450s)
Over 1000 CYP450 enzymes identified (50 active enzymes in human).
Expressed mainly in liver.
• Multiple alleles with different frequencies in different ethnic groups
• P450 enzymes oxidize drugs or other xenobiotics in order to:
1. increase polarity, and enhance excretion
(decrease resorption in distal nephron)
2. convert to substrate for phase2 metabolism
• RH + O2 + NADPH + H+
•
CYP450
ROH +H2O +NADP+
CYPs are involved 92% of drug metabolism
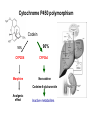
Cytochrome P450 polymorphism
CYP 1A2 individual: fast, medium, and slow turnover of caffeine
CYP 2B6 missing in 3-4 % of the caucasian population
CYP 2C9 deficit in 1-3 % of the caucasian population
CYP 2C19 individuals with inactive enzyme (3-6 % of the caucasian and 1520 % of the asian population)
CYP 2D6 poor metabolizers in 5-8 % of the european,
10 % of the caucasian, and <1% of the japanese population. Over
expression (gene duplication) among parts of the african and oriental
population.
CYP 3A4 only few mutations
UM. Zanger, M Schwab Pharmacology & Therapeutics 2013
Drug Metabolism
A. Poor metabolizer
B. Intermedier metabolizer
C. Extenzive metabolizer
D. Ultrarapid metabolizer
___
Normal
Plazma szint
…… Polimorfic
Plasma level (c) /time (t)
Plasma concentration depending on genetic variants
in the population
Plasma concentration
Genetic Polymorphism Based on Drug Metabolizing Ability
Cytochrome P450 polymorphism
• 62 year old patient with
bilateral pneumonia,
fiver, cough
• Creatinine: 2,0 mg/dl
• Part of therapy:
Clarithromycin, Codein
Cytochrome P450 polymorphism
After four days patient is in coma, symptoms show morphine toxification
He is receiving Naloxon (antimorphine) – he feels better
Metabolite (ug/L) in the
urine
Patient (ug/L)
Normally after codein
therapy (ug/L)
Codein
114
<75
Morphine
80
<10
Morphine-6 glucuronide
136
<13
Morphine-3 glucuronide
580
<70
Cytochrome P450 polymorphism
Codein
10%
CYP2D6
Morphine
90%
CYP3A4
Norcodeine
Codeine-6-glukuronide
Analgesic
effect
Inactive metabolites
Cytochrome P450 polymorphism
Genotypization: 4 copies of CYP2D6 gene
Codeine
10%
CYP2D6
CYP2D6
CYP2D6
CYP2D6
90%
CYP3A4
Clarithromycine
Morphine
toxification
Norcodeine
Codeine-6-glucuronid
Drug metabolism-phase II enzymes
Tuberculosis treatment -> e.g. Izoniasid
Inactivated by NAT1 and 2 (N-acetyl-transferase)
NAT2
variants
Acetylator
phenotype
NAT2*5A/N
AT2*5B
Slow
NAT2*5A/N
AT2*6A
Slow
Hepatotoxicity,
hepatitis
NAT2*5B/N
AT2*5B
Slow
Hepatotoxicity,
hepatitis
NAT2*5B/N
AT2*6A
Slow
Hepatotoxicity,
hepatitis
NAT2*6A/N
AT2*6A
Slow
Hepatotoxicity,
hepatitis
NAT2*4/NA
T2*5B
Intermedier
-
NAT2*4/NA
T2*6A
Intermedier
-
NAT2*4/NA
T2*4
Fast
Drug resistance
Side effect
Frequency of fast acetylators in the
population
Hepatotoxicity,
hepatitis
Population
Canadian eskimos
Frekvency %
95-100
Polinesian
93
Japanese
90
German
43
Czechs
40
Egyptian
18
Hungarian
43
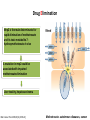
Drug Elimination
Mrp2 is the main determinants for
rapid elimination of methotrexate
and its toxic metabolite 7hydroxymethotrexate in vivo
A mutation in mrp2 could be
associated with impaired
methotrexate elimination
Liver toxicity, hepatocarcinoma
Mol Cancer Ther 2009;8(12):3350–9]
Methotrexate: autoimmun diseases, cancer
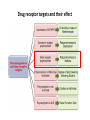
Drug receptor targets and their effect
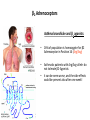
β2-Adrenoceptors
Asthma bronchiale and β2 agonists
• 16% of population is homozygote for β2
Adrenozeptor in Position 16 (Arg/Arg)
• Asthmatic patients with Arg/Arg-allele do
not tolerate β2-Agonists
• it can be even worse, and the side effects
could be present also after one week!
The morning peak flow increased in salmeterol-treated patients with the glycine/glycine genotype, but declined in the
arginine/arginine patients compared with placebo, suggesting that the arginine/arginine patients taking a LABA (long-acting beta
agonists) would experience an adverse effect in their morning peak flow.
Asthma Guidelines and Effective Utilization of Long-Acting Beta-Agonists
Role of serotonin transporter polymorfism in therapy
Serotonine transporter polymorphism
2 alleles: „s” and „l”
Mutation in promoter region
S alleles: transcription
Higher chances for mood disorders
SSRIs (selective serotonin reuptake
inhibitors) acts slower
.
Gressier F et al..Psychiatr Genet. 2009 Aug;19(4):195-200
Warfarin: Significant Problems for Rats!
Warfarin: Significant Problems for Humans!
• Ranks #1 in total mentions of deaths for drugs causing
adverse events (from death certificates)
• Ranks among the top drugs associated hospital
emergency room visits for bleeding
• Overall frequency of major bleeding range from 2% to
16% (versus 0.1% for most drugs)
• Minor bleeding event rates in randomized control trials of
new anticoagulants has been as high as 29% per year.
Pharmacokinetic and Pharmacodynamic changes Warfarin pharmacogenetics
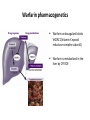
Warfarin pharmacogenetics
Warfarin anticoagulant blocks
VKORC1(Vitamin K epoxid
reductase complex subunit1)
Warfarin is metabolized in the
liver by CYP2C9
How Does the Warfarin Levels Depend on the
Two Enzymes – CYP2C9 & VKORC1?
PK
PD
How we can determine the optimal dose of warfarin?
How the therapeutic effect can be measured?
Why Maintaining Warfarin
Therapeutic Range is Critical
Optimal dose provide an INR (International Normalised Ratio )2.0-3.0
European Atrial Fibrillation Trial Study Group, N Engl J Med 1995;333:5-10.
Several common CYP2D6 alleles are comprised of
combinations of 3 or more polymorphisms
gene is deleted (3–5% allelic frequency worldwide)
gene duplications with from 2–
12 tandem copies, (allele
frequencies range from about 1 to about
30%, depending on the population)
More than 80 polymorphisms within the coding and promoter regions
are known: SNPs, In/Dels, repeats, and gene conversion events
41
Nature Reviews Drug Discovery 3, 749-761 (September 2004)
Affects of different genetic variations of CYP2D6
Poor metabolizers: Drugs are metabolized very slowly, so they
may accumulate to toxic concentrations
Prevalence of poor metabolizers
Europian poor metabolizers 20-30 million
Ultrarapid metabolizer : (1% to 2%) Unusually
high rate of drug metabolism. Drugs may not
reach therapeutic concentrations, (drugs may
be ineffective at standard dosages).
Amplification
Europian ultra rapid metabolizers 15-20 million
42
http://www.avma.org/onlnews/javma/oct08/081015q.asp
Estimated Warfarin Dose (mg/day) Based on Genotypes
Frequency of VKORC1 Alleles
in Various Populations
Sconce et al. Blood 2005, Yuan et al. Human Mol Genetics 2005, Schelleman et al. Clin Pharmacol Ther 2007, Montes et al Br J Haemat 2006
Warfarin pharmacogenetics
INFINITI™ 2C9 &
VKORC1 Multiplex
Assay for Warfarin
Ha az INR 2,0-nél kisebb, nem elégséges a trombózissal szembeni
védő hatás, míg az INR 3,0 fölött fokozott a vérzésveszély
http://www.warfarindosing.org/Source/Home.aspx
INR international normalized ratio; protrombin idő PTtest/PTnormal
Idiosyncrasy:
not expected side effect
Drug intake causes side effects which can not be explained with the
pharmacodinamic effects of the drug or with the dose
Genetic variations in genes coding for proteins, which are not in the
drug target or pharmacokinetic pathways, but could cause side
effects
Affected organs are usually liver (liver toxicity) or skin
(pseudoallergy)
e.g. Chloramphenycol (antibiotics); aplastic anemia ( in case of oral
administration affecting 1 in 24,000–40,000)
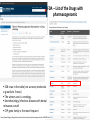
FDA – List of the Drugs with
pharmacogenomic
Biomarkers
238 rows in the table (not as many molecules
e.g warfarin 3 rows)
The winner area is oncology,
Anesthesiology, infectious dieases with dental
relevance as well
CYP gene family is the most frequent
https://www.fda.gov/drugs/scienceresearch/researchareas/pharmacogenetics/ucm083378.htm
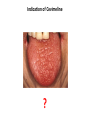
Indication of Cevimeline
?
Cevimeline
EVOXAC™ Capsules (cevimeline hydrochloride)
Cevimeline is indicated for the treatment of symptoms of dry
mouth in patients with Sjögren’s Syndrome.
PD: It is a cholinergic agonist which binds to muscarinic receptors. Muscarinic agonists in
sufficient dosage can increase secretion of exocrine glands, such as salivary and sweat glands
and increase tone of the smooth muscle in the gastrointestinal and urinary tracts.
PK:
…
Metabolism: Isozymes CYP2D6 and CYP3A3/4 are responsible for the metabolism of
cevimeline. After 24 hours 86.7% of the dose was recovered (16.0% Unchanged, 44.5% as cis
and trans-sulfoxide, 22.3% of the dose as glucuronic acid conjugate and 4% of the dose as Noxide of cevimeline). Approximately 8% of the trans-sulfoxide metabolite is then converted
into the corresponding glucuronic acid conjugate and eliminated. Cevimeline did not inhibit
cytochrome P450 isozymes 1A2, 2A6, 2C9, 2C19, 2D6, 2E1, and 3A4.
How does PG change the medicine?
Pharmacogenomics in drug development I.
Current existing therapies only hit about 400 different drug targets
There are at least 10 times more drug targets
The identification of NEW DRUG TARGETS can significantly be
accelerated by the new high throughput genomic methods
Genomic methods can be hypothesis-free
51
Pharmacogenomics in drug development II.
Adverse effects
Although drugs generally are safe and effective therapies for numerous
diseases, adverse drug reactions do occur and may even be fatal.
The incidence of fatal adverse drug reactions in hospitalized patients has been
estimated to be approximately 5%. Fatal adverse drug reactions account for
approximately 3% of all deaths in the general population. (Wester et al. 2008)
Adverse drug reaction (ADR) are responsible for 5-10% of hospital admissions
in the US and Europe,
Lead to the withdrawal of 4% of new medicines. (Ingelman-Sundberg 2005)
52
Br J Clin Pharmacol. 2008 April; 65(4): 573–579.
Effects of about 20% of the drugs on the market
are influenced by polymorphisms in genes coding
for enzymes responsible for the degradation of
the drugs.
53
54
Drug development – Preclinical phase
Pharmacogenomics
Pharmacogenomics useful in target indentification
Allen D. Roses Nature Reviews Genetics 5, 645-656 (September 2004)
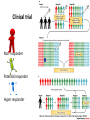
Drug development - Clinical trial
How are doses are determined?
Clinical development
Clinical trial
Non- responder
Potential responder
Hyper responder
Allen D. Roses Nature Reviews Genetics 5, 645-656 (September 2004)
59
24th of December 2004
…what many thought would not happen has already
happened
To detect CYP 2D6 and 2C19 variability
How does it work?
PCR
Detection
1.DNA Isolation
2.PCR amplification of the genes.
3.Fragmentation and labeling of the PCR productc
4.Hybridization and staining on the AmpliChip DNA microarray.
5.Scanning the chip.
6.Data analysis.
Advantage of pharmacogenomics
To predict a patient’s response to drug
To develope customized prescriptions
To minimize or eliminate adverse evenets
To improve efficacy and patient compliance
To improve rational drug development
Pharmacogenetic test need only be conducted once during
the life time
To improve the accuracy of determining appropiate dosage
of drugs
To screen and monitor certain diseases
To develop more powerful, safer vaccines
To allow improvements in drug discovery and development
Nutrigenomics
Nutrigenomics, nutrigenetics
„Let food be thy medicine and
let thy medicine be food.”
Hippocrates
Genes
Nutrigenomics
Nutrigenetics
Investigates complex gene-nutrient
interaction on genome level
Investigates interaction with genetic
background
Nutrients
Both healthy and
unhealthy subjects
belongs to target
population
66
The Pharmacogenomics Journal (2003) 3, 191–193
Importance of nutrigenomics
1. Malnutrition could cause health problems or disease
2. Symptomps of many genetic diseases could be minimized by proper diet
3. Food and food additives have direct effect on gene expression
4. The effect of food depends on the genetic background
5. Healthy nutrition is part of prevention in many diseases
6. Part of the approach of nutrigenomics involves finding markers of the
early phase of diet related diseases; this is the phase at which
intervention with nutrition can return the patient to health.
Transcription factors involved in nutrient –gene
interactions
68
Soya-derived phytoestrogens
•
•
•
•
•
Genistein, daidzein, coumesterol and equol binds to ER-and ER-
Modulates cell proliferation, growth, differentiation
Antioxidant, antiangiogenic molecule
Reduce risk for hormone-related cancers such as breast cancer, uterine cancer and
prostate cancer
Reduce the symptoms of menopause by mimicking estrogen
69
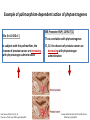
Example of polimorphism-dependent action of phytoestrogenes
ERß Promoter SNP (-13950 T/C)
ERα (Ex1-192G>C )
In subjects with this polimorfism, the
chances of prostate cancer are increasing
with phytoestrogen administration
Nutr Cancer 2006, 56 (1):31-39
.
Chae
et al. PLoS One. 2009 Aug 5;4(8):e6523
TT-no correlation with phytoestrogenes
TC, CC: the chances of prostate cancer are
decreasing with phytoestrogen
administration
70
Prostate. 2006 Oct1;66(14):1512-20 PLoS One.
2009 Aug 5;4(8):e6523.
Metabolic-diseases and nutrigenetics
Disease
Symptoms
Genetic
polymorphism
Diet
Lactase persistance
Diarochea, vomitus
MCM6
Lactose-free diet
Phenylketonuria
Mental problems
Phenilalaninehidroxilase (PAH)
Phenilalanine-free diet
Galactosemia
Letargia, yellow
skin, sepsis
Galactose metabolism
(GALE, GALK1 and
GALT)
Galactose-free diet
Alkaptonuria
Arthritis,
Dark spots on the
sclera
Homogentisinaciddioxigenase (HGD)
Vitamin C rich diet
Coeliakia
Ulcus, abdominal
pain
HLA genes
Gluten-free diet
Bassen-Kornzweg Syndrome
Off-balance, muscle
weakness, problems
with vision
Mikrosomal triglicerid
transfer protein (MTP)
Vitamin (A, D, E) and
non-saturated fatty
acids from diet
71
Lactase persistence
At birth lactase enzyme activity is high and with age it is reduced.
The genetically programmed hypolactasia is physiological and too much
lactose in adulthood could cause symptoms of lactose intolerance .
Lactase persistence is the continued activity of the enzyme lactase in adulthood.
Lactase activity with age
birth
suckling
childhood
adulthood
72
Global-spread of lactase persistence
• In Europeans the percentage of lactose persistence is high
• It could be explained with the fact that lactose persistence is typical to those nations
where milk is used frequently in diet
Milk:
1/ Source of nutrients
2/ source of water (for e.g. in the desert ->Tuaregs)
3/ Source of Ca +2 (In Scandinavia ->Vitamin D )
4/ Milk is rich in riboflavine (flavin deficiency gives protection against
Malaria-> in Malaria affected regions the % of lactose persistence is very low
(South-East Asia)
LCT*P (lactase
persistence)
73
Lactase persistence
The level of lactase enzyme is regulated by MCM6 gene which is upstream
from LCT promoter
C/T(-13910) substitution (autosomal dominant) shows correlation with
lactose persistence
MCM6-minichromosome maintenance complex component 6
n. b. Lactose intolerance is not the same as lactose persistence. It
is an autosomal recessive enzimophaty. These patients can not
process lactose at all.
Enattah et al. (2002) Nature Genet. 30, 233-237
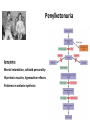
Phenylketonuria (PKU)
Inherited (AR) disorder, that increases the level of
phenylalanine in the blood
Penylketonuria
Symptoms:
Mental retardation, schizoid personality
Hipertonic muscles, hyperactive reflexes
Problems in melanin synthesis
Phenylketonuria (PKU)
PKU is not curable.
However, if PKU is diagnosed early enough, an affected newborn can grow up with normal
brain development by managing and controlling phenylalanine ("Phe") levels through diet,
or a combination of diet and medication.
PKU-test: 3-4 days after birth
Guthrie test - B. Subtilis growing capacity is higher in
the presence of blood full of PHE
Vitamin D receptor (VDR) – osteoporosis-coffein
RFLP of VDR (TT, Tt, tt)
• Association: osteoporosis (tt: spinal bone weight )
• Depends from diet
– Ca2+ (decreases the risk)
– Coffein (increases the risk)
>300mg coffein/day
<300mg coffein/day
Rapuri et al. Am J Clin Nutr, 2001
78
~ 2 cup of coffee
Interactions of genes and environmental factors
G
Ph
G’
Ph
G’+ E + E
Ph’
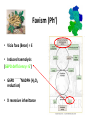
Favism (Ph’)
• Vicia fava (Bean) = E
• Induces haemolysis
(G6PD defficiency= G’)
• G6PD
NADPH (H2O2
reduction)
• X recessive inheritance
Favism
• More frequent in territories smitted with malaria
• Inducer drugs are (E):
- anti-malaria drugs (1950),
- sulfonamides
- chloramphenicol
- aspirin
• Allels (376A
(202G
(563C
G) normal enzyme
A) activity enzyme
T) activity
Fatal
Smoking-genome interactions
82
Genomic background of
smoking
• Heritability: 60%.
• D2 dopamine receptor gene
(DRD2,11q23), A1 allele is
associated with stressinduced cigarette craving.
83
Arg16 of the adrenergic β2 receptor gene (ADRB2), had a
significantly increased the risk of asthma in smokers
84