* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Document
Quantum vacuum thruster wikipedia , lookup
Probability amplitude wikipedia , lookup
Elementary particle wikipedia , lookup
History of quantum field theory wikipedia , lookup
Uncertainty principle wikipedia , lookup
ATLAS experiment wikipedia , lookup
Wave packet wikipedia , lookup
Renormalization wikipedia , lookup
Renormalization group wikipedia , lookup
Relational approach to quantum physics wikipedia , lookup
Eigenstate thermalization hypothesis wikipedia , lookup
Canonical quantization wikipedia , lookup
Future Circular Collider wikipedia , lookup
Quantum chaos wikipedia , lookup
Quantum electrodynamics wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
Quantum tunnelling wikipedia , lookup
Nuclear structure wikipedia , lookup
Photon polarization wikipedia , lookup
Compact Muon Solenoid wikipedia , lookup
Atomic nucleus wikipedia , lookup
Photoelectric effect wikipedia , lookup
Old quantum theory wikipedia , lookup
Electron scattering wikipedia , lookup
Double-slit experiment wikipedia , lookup
Introduction to quantum mechanics wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
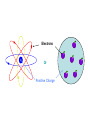
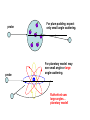
A BRIEF STORY OF QUANTUM THEORY PETER BALLO END OF 19th CENTURY Classical Mechanics Electromagnetism and Optics Heat State of Physics at 1895 •Lord Kelvin—arguably the greatest physicist of his day: • Strong opponent of existence of atoms •“There is nothing new to be discovered in physics now. All that remains is more and more precise measurement." •Albert A Michleson—the first US Physics Nobel laureate •“The grand underlying principles have been firmly established...further truths of physics are to be looked for in the sixth place of decimals" (Science, 1892) Hydrogen Spectrum: Balmer series Balmer Formula: IT WORKS! • “X-rays” discovered in 1895 by Roentgen - World Wide sensation! – Unknown ray produced from electric discharge that penetrates matter! • J.J. Thomson discovers the electron in 1897. • Henri Becquerel (1896) tries to produce X-rays from natural sources. – Finds radiation (less penetrating than X-rays) given off from solid containing Uranium. • Marie Curie (1897) discovers immense radiation energy from element she named Radium. – Surprising? Yes! Black body radiation Otto Lummer Ernst Pringsheim Blackbody Radiation Spectra • The true beginnings of the quantum theory lie in a strange place: the frequency spectrum emitted by a solid when it is heated (“blackbody” radiation). • Experimental measurements: the frequency spectrum was well determined.. a continuous spectrum with a shape that depended only on the temperature (light bulb, … ) • Theoretical prediction: Classical kinetic theory predicts the energy radiated to increase as the square of the frequency (Completely Wrong! - “ultraviolet catastrophe”). Planck’s Solution • Max Planck (1900): In order to describe the data Planck made the bold assumption that light is emitted in packets or quanta, each with energy E = h f, where f is the frequency of the light. – Some texts use the notation n for frequency. • The factor h is now called Planck’s constant, h = 6.626 (10-27) erg-sec. o Data Theory Atomic Models • Conclusion: Atoms contain electrons. Questions: How are they arranged? Since atoms are neutral, where is the positive charge? • Two models: –“Plum pudding”: Electrons are embedded in continuum of positive electricity like plums in a pudding. –“Planetary model”: Electrons orbit a small nucleus of positive charge like planets orbit the Sun. Electrons Or Positive Charge probe probe For plum pudding: expect only small angle scattering. For planetary model: may see small angle or large angle scattering. Rutherford saw large angles… planetary model! The Problem of the atom • Experiments supported the picture that an atom is composed of light electrons around a heavy nucleus • Problem: if the electrons orbit the nucleus, classical physics predicts they should emit electromagnetic waves and loose energy. If this happens, the electrons will spiral into the nucleus! • The atom would not be stable! • What is the solution to this problem? E=hf Bohr’s Revolutionary Idea • Can the new quantum theory explain the stability of the atom? • If the energies can take on only certain discrete values, i.e., it is quantized, there would be a lowest energy orbit, and the electron is not allowed to fall to a lower energy! • What is the role of Planck’s Constant h? The Bohr Atom (NOT Correct in detail!) 4 • The allowed orbits are labeled by the integers: n = 1, 2, 3, 4. • The radii of these orbits can be determined from the quantization condition: radius = n2 a0 = n2 (h/2p)2/ me2 • The energy can only have the values En= E1 /n2, E1 = - (1/2)(e2 / a0)/n2 • The spectra are the result of transitions between these orbits, with a single photon (f = E/h) carrying off the difference in energy E between the two orbits. 3 2 1 The Schrodinger Equation • In 1926 Erwin Schrodinger proposed an equation which describes completely the time evolution of the matter wave where m = characteristic mass of “particle” V = potential energy function to describe the forces Newton: Given the force, find motion F = ma = m (d2x/dt2) solution: x = f(t) Schrodinger Given potential, find wave (- (h2 / 2m) 2 + V) i h (d /dt) solution: = f(x,t) Note: Schrodinger’s equation is more difficult to solve, but it is just as well-defined as Newton’s. If you know the forces acting, you can calculate the potential energy V and solve the Schrodinger equation to find . Key Results of Schrodinger Eq. • The energy is quantized –Only certain energies are allowed –Agrees with Bohr’s Idea in general –Predicts the spectral lines of Hydrogen exactly –Applies to many different problems - still one of the key equations of physics! • The wavefunction is spread out –Very different from Bohr’s idea –The electron wavefunction is not at a given radius but is spread over a a range of radii. Probability interpretation for 2 • The location of an electron is not determined by . The probability of finding it is high where 2 is large, and small where 2 is small. • Example: A hydrogen atom is one electron around a nucleus. Positions where one might find the electron doing repeated experiments: Lower probability to find electron far from nucleus Higher probability to find electron near nucleus Nucleus The Two-Slit Experiment • We will first examine an experiment which Richard Feynman says contains “all of the mystery of quantum mechanics”. • The general layout of the experiment consists of a source, two-slits, and a detector as shown x below; source detector slits The idea is to investigate three different sources (a classical particle (bullets), a classical wave (water), and a quantum object (electron or photon)). We will study the spatial distribution (x) of the objects which arrive at the detector after passing through the slits. Classical Particles • Classical particles are emitted at the source and arrive at the detector only if they pass through one of the slits. • Key features: – particles arrive “in lumps”. ie the energy deposited at the detector is not continuous, but discrete. The number of particles arriving per second can be counted. – The number which arrive per second at a particular point (x) with both slits open (N12) is just the sum of the number which arrive per second when only the top slit is opened (N1) and the number which arrive per second when only the bottom slit is opened (N2). only bottom slit open only top slit open Both slits open N N x x • Classical waves are emitted at the source and arrive at the detector only if they pass through the slits. • Key features: – detector measures the energy carried by the waves. eg for water waves, the energy at the detector is proportional to the square of the height of the wave there. The energy is measured continuously. – The energy of the wave at a particular point (x) with both slits open (I12) is NOT just the sum of the energy of the wave when only the top slit is opened (I1) and the energy of the wave when only the bottom slit is opened (I2). An interference pattern is seen, formed by the superposition of the piece of the wave which passes through the top slit with the piece of the wave which passes through the bottom slit. only bottom slit open only top slit open Both slits open I I x x Timeline - Modern Physics Einstein Bohr Michelson Rutherford Thomson De Broglie Planck Schrodinger Curie Heisenberg 1900 Special Relativity Start of Quantum General Mechanics Relativity Nuclear Energy Neutron Stars Released discovered 1950 Expansion Laser of Universe Invented discovered Quantum Mechanics Transistor Invented 2000 All the Quarks discovered • “Modern Physics” was a sudden revolution starting around 1900, and ending ???? “Thirty Years That Shook Physics” • • • • • • • • • • • • • • • • • 1887 Michelson-Morley exp. debunks “ether” 1895 Rontgen discovers x rays 1897 Becquerel discovers radioactivity 1897 Thomson discovers the electron 1900 Planck proposes energy quantization 1905 Einstein proposes special relativity 1915 Einstein proposes general relativity 1911 Rutherford discovers the nucleus 1911 Braggs and von Laue use x rays to determine crystal structures 1911 Ones finds superconductors 1913 Bohr uses QM to explain hydrogen spectrum 1923 Compton demonstrates particle nature of light 1923 de Broglie proposes matter waves 1925 Davisson & Germer prove matter is wavelike 1925 Heisenberg states uncertainty principle 1926 Schrodinger develops wave equation 1924-6 Boson and Fermion distributions developed • 1949 Murphy's Law stated • The two most important formulas in modern physics • E = mc2 (Einstein – special relativity - 1905) E = h f (Planck – quantum mechanics - 1900) • Planck initially called his theory “an act of desperation”. – “I knew that the problem is of fundamental significance for physics; I knew the formula that reproduces the energy distribution in the normal spectrum; a theoretical interpretation had to be found , no matter how high.” • Leads to the consequence that light comes only in certain packets or “quanta” • A complete break with classical physics where all physical quantities are always continuous