* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download CBS Reduction
Fischer–Tropsch process wikipedia , lookup
Asymmetric hydrogenation wikipedia , lookup
Polythiophene wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
Bottromycin wikipedia , lookup
Asymmetric induction wikipedia , lookup
Diels–Alder reaction wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Kinetic resolution wikipedia , lookup
Vinylcyclopropane rearrangement wikipedia , lookup
Stille reaction wikipedia , lookup
Hydroformylation wikipedia , lookup
Petasis reaction wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Elias James Corey wikipedia , lookup
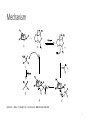
CBS Reduction Christine Beshay CHE 676 Dr.Totah 12/1/2016 Overview • History of COREY-BAKSHI-SHIBATA reduction and selectivity • General features • Mechanism • Organic synthesis applications 2 History of CBS reduction and selectivity • In 1981 Itsuno et al reduced achiral ketones to chiral alcohols using alkoxy-amine-borane complexes in enantioselectivity and in high yield. • In 1987 E.J. Corey, with his co-workers, used oxazaborolidines to rapidlly reduce ketones in the presence of BH3-THF to give 2o alcohol in enantioselectivity and in high yield. • The enantioselective reduction of ketones using oxazaborolidine is called the CBS reduction. 3 General features Corey, E. J.; Bakshi, R. K.; Shibata, S. J. Am. Chem. Soc. 1987, 109 (18), 5551–5553. 4 Mechanism Quallich, G. J.; Blake, J. F.; Woodall, T. M. J. Am. Chem. Soc. 1994, 116 (19), 8516–8525. 5 Organic synthesis application B.W.Spur synthesis of prostaglandin E1 Rodríguez, A.; Nomen, M.; Spur, B. W.; Godfroid, J.-J. European J. Org. Chem. 1999, 1999 (10), 2655–2662. 6 Organic synthesis application E.J. Corey and co-workers synthesis protein phosphatase(Dysidiolide) Corey, E. J.; B. E.; Roberts, J. Am. Chem. Soc. 1997, 119, 12425-12431. 7 Organic synthesis application C.J. Forsyth et al. synthesis of okadic acid Sabes, S. F.; Urbanek, R. A.; Forsyth, C. J. J. Am. Chem. Soc. 1998, 120, 2534-2542. 8 Optical yield • In a chemical reaction, if the reaction is stereospecific and no racemization occurs, the optical yield is 100%. % Optical yield = (ee product / ee starting material) x 100% Seeman, J. I.; Eliel, E. L. 1921. 9 Recent organic synthesis Synthesis of (+)-pseudohygroline and (+)-hygroline Bhoite, S. P.; Kamble, R. B.; Suryavanshi, G. M. Tetrahedron Lett. 2015, 56 (32), 4704–47 10 Recent paper 2016 • Sanderson and coworkers developed flow process for CBS asymmetric reduction of ketones , using chip-microreactors. • They used BH3 , (85% 2-MeTHF, 15% THF) and oxazaborolidine for reduction. • Under such reaction conditions, the reaction was complete in 10 minutes and alcohol was produced with 95% yield and a 91 : 9 enantiomeric ratio (highly enantioselectivety). 11 CBS reduction using chip-microreactor De Angelis, S.; De Renzo, M.; Carlucci, C.; Degennaro, L.; Luisi, R. Org. Biomol. Chem. 2016, 14 (18), 4304–4311. 12 Conclusion • This method of reduction using CBS catalyst achieved higher enantioselectivity and yield. • CBS reduction used not only in natural product synthesis, but also in industry. • This method is more effective to reduce ketones in stereoselective and chemoselective way depending on the substituents, catalyst type, and temperature. 13 Refrences 1- Corey, E. J.; Bakshi, R. K.; Shibata, S. J. Am. Chem. Soc. 1987, 109 (18), 5551–5553. 2- Quallich, G. J.; Blake, J. F.; Woodall, T. M. J. Am. Chem. Soc. 1994, 116 (19), 8516– 8525. 3- Rodríguez, A.; Nomen, M.; Spur, B. W.; Godfroid, J.-J. European J. Org. Chem. 1999, 1999 (10), 2655–2662. 4- Corey, E. J.; B. E.; Roberts, J. Am. Chem. Soc. 1997, 119, 12425-12431. 5- Sabes, S. F.; Urbanek, R. A.; Forsyth, C. J. J. Am. Chem. Soc. 1998, 120, 2534-2542. 6- Seeman, J. I.; Eliel, E. L. 1921. 7- Bhoite, S. P.; Kamble, R. B.; Suryavanshi, G. M. Tetrahedron Lett. 2015, 56 (32), 4704–47 8- De Angelis, S.; De Renzo, M.; Carlucci, C.; Degennaro, L.; Luisi, R. Org. Biomol. Chem. 2016, 14 (18), 4304–4311. 14