* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Diagnosis and ablation of atypical atrial tachycardia and flutter
Survey
Document related concepts
Heart failure wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Coronary artery disease wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
History of invasive and interventional cardiology wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Cardiac surgery wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Electrocardiography wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Ventricular fibrillation wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Atrial septal defect wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
Transcript
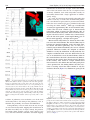
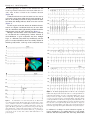
Diagnosis and ablation of atypical atrial tachycardia and flutter complicating atrial fibrillation ablation Fred Morady, MD, Hakan Oral, MD, Aman Chugh, MD From the Division of Cardiovascular Medicine, University of Michigan Health System, Ann Arbor, Michigan. Depending on the ablation strategy, up to 30% to 50% of patients will develop an atrial tachycardia after undergoing radiofrequency catheter ablation of atrial fibrillation. This review discusses the mechanisms, mapping techniques, and catheter ablation of atrial tachycardias that occur after radiofrequency ablation of atrial fibrillation. KEYWORDS Atrial tachycardia; Entrainment mapping; Postpacing interval The incidence of atrial tachycardia after radiofrequency catheter ablation of atrial fibrillation varies depending on the extent of ablation in the left atrium. When the ablation strategy is limited to ostial ablation to isolate the pulmonary veins, atrial tachycardias are unusual, occurring in only 1% to 2% of patients.1– 4 In contrast, when pulmonary vein isolation is achieved by a wide area ablation or when the left atrium is targeted directly with ablation lines or by ablation of complex, fractionated atrial electrograms, left atrial tachycardias are common, with a reported incidence ranging between 10% and 30%.3– 6 Furthermore, when a concerted effort is made to terminate persistent atrial fibrillation using an extensive stepwise approach of catheter ablation in the left and right atria, atrial tachycardias are observed in ⬎50% of patients.7 Of note is that the incidence of atrial tachycardia after radiofrequency catheter ablation of atrial fibrillation can be lowered by creating a complete line of block in the mitral isthmus and across the left atrial roof during the first catheter ablation procedure.8,9 Atrial tachycardias may occur acutely during an atrial fibrillation ablation procedure and are often the arrhythmia to which persistent atrial fibrillation converts during the course of radiofrequency ablation.7 Atrial tachycardias also commonly occur several days to weeks or months after radiofrequency catheter ablation of atrial fibrillation. The mechanism of the atrial tachycardia likely varies depending on whether it is an acute or a late phenomenon after ablation of atrial fibrillation. Recent evidence based on spectral analysis suggests that atrial tachycardias observed acutely during radiofrequency ablation of atrial fibrillation often may be due to preexisting drivers of atrial fibrillation that become manifest after elimination of higher-frequency sources and fibrillatory conduction (Yoshida et al, unpublished data). In contrast, atrial tachycardias that occur late after radiofrequency ablation of atrial fibrillation probably most often are a manifestation of gap-related proarrhythmia.10 Atrial tachycardia may be macroreentrant, microreentrant, or focal. In reentrant atrial tachycardias, endocardial activation spans all of diastole. The diameter of the reentrant circuit is ⬎3 cm in macroreentry and ⬍3 cm in microreentry. Focal atrial tachycardias are caused by triggered activity or abnormal automaticity, and endocardial activation is presystolic, generally limited to the second half of diastole. In microreentrant and focal atrial tachycardias, there is centrifugal spread away from the site of origin of the arrhythmia. Approximately 75% of atrial tachycardias that occur after wide-area radiofrequency ablation to isolate the pulmonary veins is caused by macroreentry.10 The other 25% is evenly split between microreentry and focal atrial tachycardia.10 If a sampling of local electrograms in the left and right atria demonstrates that the electrograms at all sites are limited to the second half of diastole, a focal mechanism is likely. In contrast, if the electrograms span all or most of diastole, this finding indicates that the atrial tachycardia is due to reentry. If the atrial tachycardia is paroxysmal as opposed to persistent, or if more than 20 to 30 ms of variability is seen in the tachycardia cycle length, then a focal mechanism is favored over reentry. Atrial tachycardias can be mapped by endocardial activation mapping and/or entrainment mapping. With either approach, use of a three-dimensional nonfluoroscopic mapping system is helpful in identifying and tagging appropriate target sites and in creating contiguous ablation lesions when an ablation line is needed to traverse a critical isthmus. The majority of focal atrial tachycardias that occur after radiofrequency catheter ablation of atrial fibrillation originate in a pulmonary vein or in the antrum of a pulmonary vein.10 Therefore, mapping of a focal atrial tachycardia should first be focused on these sites. A ring catheter is helpful in identifying a focal pulmonary vein tachycardia that may not be identified with a standard ablation catheter. Pulmonary vein isolation usually is appropriate if there is Drs Morady, Oral and Chugh have no conflicts of interest to report. Address reprint requests and correspondence: Dr. Fred Morady, Cardiovascular Center, SPC 5853, 1500 East Medical Center Drive, Ann Arbor, Michigan 48109-5853. E-mail address: [email protected]. (Heart Rhythm 2009;6:S29 –S32) © 2009 Heart Rhythm Society. All rights reserved. 1547-5271/$ -see front matter © 2009 Heart Rhythm Society. All rights reserved. doi:10.1016/j.hrthm.2009.02.011 S30 Heart Rhythm, Vol 6, No 8S, August Supplement 2009 demonstrates an unusual site of origin, such as the anterior wall of the left atrium. Once the site of origin has been accurately identified, focal atrial tachycardias usually are successfully ablated with a single application of radiofrequency energy. In a series of 116 macroreentrant atrial tachycardias that started several days to weeks after circumferential pulmonary vein ablation for paroxysmal or persistent atrial fibrillation, the most common type was a perimitral tachycardia traversing the mitral isthmus.10 This type of tachycardia accounted for approximately 40% of macroreentrant tachycardias. The next most common type of macroreentrant tachycardia, which accounted for approximately 20% of macroreentrant atrial tachycardias, had a wavefront traversing the left atrial roof. Less common sites of macroreentry were the left atrial septum, cavotricuspid isthmus, base of the left atrial appendage, and right atrial septum.10 Macroreentrant tachycardias are ablated by linear ablation across the critical isthmus. The critical isthmus may lie between two anatomic landmarks, such as the mitral annulus and the left inferior pulmonary vein, or it may be a relatively narrow channel bounded by sites of scar or double potentials. Whenever feasible, the endpoint of ablation should be not only the termination of tachycardia but also complete conduction block across the ablation line. In the case of an ablation line across the mitral isthmus, block in the counterclockwise direction is demonstrated by differential pacing in the coronary sinus. If pacing from a distal site in the coronary sinus results in a longer stimulus–atrial interval anterior to the ablation line than when pacing from a more proximal site, this finding indicates that the wavefront is traveling around the mitral annulus in only the clockwise direction (Figure 1A). Furthermore, pacing in the Figure 1 A: Counterclockwise block across an ablation line in the mitral isthmus. The ablation catheter is positioned in the left atrial appendage. Pacing was performed in the coronary sinus, and as the pacing site was switched from the distal electrodes (CS1-2) to a more proximal pair of electrodes (CS3-4), the interval between the pacing stimulus and the ablation catheter shortened from 230 to 210 ms. B: Clockwise block at the mitral isthmus. Note that atrial activation progresses from the proximal (CS9-10) to distal (CS1-2) electrodes during pacing with the ablation catheter in the left atrial appendage. Abld and Ablp ⫽ distal and proximal bipolar recordings from the ablation catheter, respectively; Stim ⫽ stimulus. evidence of pulmonary vein conduction, even if the focal atrial tachycardia is not arising in the pulmonary vein, to minimize the possibility of recurrent atrial fibrillation. If the pulmonary veins are ruled out as the site of origin of a focal atrial tachycardia, mapping then should focus on the other most likely sites of origin, namely, the posterior left atrium, mitral annulus, coronary sinus, superior vena cava, and crista terminalis. Sometimes activation mapping Figure 2 Block across a roof line. During left atrial appendage (LAA) pacing, the interval between the stimulus and the electrogram recorded by the ablation catheter was 140 ms at an inferior posterior left atrial site (top left panel) and 160 ms at a more superior site (bottom left panel). This finding indicates an ascending wavefront on the posterior wall of the left atrium, consistent with block at the roof. In the absence of roof block, there would be a descending wavefront on the posterior left atrium during LAA pacing. Right: Posterior views of electroanatomic left atrial maps with the ablation catheter icon at the two positions on the posterior wall. Abbreviations as in Figure 1. Morady et al Atrial Tachycardias S31 left atrial appendage or anterior to the ablation line will result in proximal-to-distal atrial activation within the coronary sinus if there is block in the clockwise direction (Figure 1B). Conduction block across the left atrial roof also can be assessed by pacing from within the left atrial appendage. If there is an ascending wavefront on the posterior wall of the left atrium, this finding indicates block across the roof line (Figure 2). In other regions of the left atrium, the definitive demonstration of complete conduction block may be more difficult. At a minimum, widely split double potentials should be demonstrated along the entire ablation line (Figure 3). Multiple macroreentrant atrial tachycardias commonly are encountered after radiofrequency catheter ablation of atrial fibrillation. A given tachycardia may have multiple loops, or a different tachycardia may immediately take the place of a tachycardia that has been successfully ablated. In multiloop tachycardias, each loop of the tachycardia must Figure 3 A: Termination of an atrial tachycardia by an ablation line across the left atrial septum. The final application of radiofrequency energy (arrow) resulted in conversion to sinus rhythm. Red tags on the electroanatomic map indicate the sites at which radiofrequency energy was applied. B: Widely split double potential (180 ms) was present along the entire ablation line, consistent with complete block across the line. CSd ⫽ distal pair of electrodes of the coronary sinus catheter; CSp ⫽ proximal pair of electrodes of the coronary sinus catheter. Other abbreviations as in previous figures. Figure 4 A: Perfect postpacing interval of 260 ms at the cavotricuspid isthmus, indicating that this atrial tachycardia was cavotricuspid isthmus dependent. B: After ablation across the cavotricuspid isthmus, there was no change in atrial cycle length or P-wave morphology, but double potentials (circled) were present along the cavotricuspid isthmus, suggesting that the cavotricuspid isthmus was blocked. C: Postpacing interval just lateral to the ablation line in the cavotricuspid isthmus was found to be 60 ms longer than the tachycardia cycle length, indicating that the tachycardia no longer was using the cavotricuspid isthmus. A site with a perfect postpacing interval was found at the upper right atrial septum. Abbreviations as in previous figures. be addressed. A change in atrial activation sequence or P-wave morphology, or a sudden change in a cycle length, indicates that the tachycardia that was being ablated has S32 changed to a different loop or to a different tachycardia. However, the transition to a different loop of a tachycardia or to a different tachycardia may occur with no discernable change in the activation sequence among the atrial electrograms that are being recorded, P-wave morphology, or cycle length. It is important to keep this in mind when ablation across an isthmus appears to not be affecting the tachycardia (Figure 4A). The isthmus already may have been blocked and may no longer be participating in the tachycardia. This should be suspected if double potentials are present along the ablation line and is easily confirmed by determining the postpacing interval near the ablation line (Figure 4B). If a previously perfect postpacing interval now is ⬎30 ms longer than the tachycardia cycle length, this finding indicates that the tachycardia circuit has changed (Figure 4C). In a series of 21 microreentrant atrial tachycardias that occurred after circumferential pulmonary vein ablation, the most common sites of origin were the coronary sinus, the antrum of a pulmonary vein, and the anterior wall of the left atrium or base of the left atrial appendage.10 Entrainment mapping usually is the most efficient method for localizing an effective target site for ablation. A single application of radiofrequency energy often is sufficient for eliminating an atrial tachycardia caused by microreentry. Two situations may necessitate radiofrequency ablation within the coronary sinus. The first is a perimitral flutter that has persisted despite thorough endocardial ablation at the mitral isthmus such that double potentials are present along the line. In approximately 70% of cases, epicardial fibers continue to conduct despite extensive endocardial ablation and can be ablated only from within the coronary sinus. The second situation that necessitates ablation within the coronary sinus is a microreentrant or macroreentrant tachycardia involving the coronary sinus, independent of the mitral isthmus.11 When radiofrequency catheter ablation is performed in the coronary sinus, the catheter of choice is an irrigated-tip catheter. Power should be limited to 20 to 25 W, and the application of energy should be discontinued if there is a rapid rise or fall in impedance or a rapid rise in temperature. Sometimes a power setting of 30 to 35 W is required for successful ablation. A higher power setting should be considered when a tachycardia fails to respond to ablation with 20 to 25 W and when entrainment mapping or activation mapping indicates that the coronary sinus is still an appropriate target site. In a series of 78 patients who underwent radiofrequency catheter ablation of 155 atrial tachycardias that occurred Heart Rhythm, Vol 6, No 8S, August Supplement 2009 after circumferential pulmonary vein ablation, 86% of the tachycardias were successfully ablated and became noninducible, and the ablation procedure was deemed successful in 85% of the patients.10 When the pulmonary veins that demonstrated recovery of conduction were reisolated during the same ablation session, only a small proportion of patients had recurrent atrial fibrillation or atrial tachycardia during long-term follow-up. Because atrial tachycardias that occur after radiofrequency ablation of atrial fibrillation often are persistent, are difficult to suppress with rhythm control medications, and often are associated with rapid ventricular rates, these arrhythmias may cause more severe symptoms than the atrial fibrillation for which the patient originally underwent catheter ablation. However, although catheter ablation of atrial tachycardias that occur after radiofrequency ablation of atrial fibrillation may be a time-consuming process that requires mental and physical fortitude, the tachycardias usually can be successfully eliminated. References 1. Oral H, Knight BP, Tada H, et al. Pulmonary vein isolation for paroxysmal and persistent atrial fibrillation. Circulation 2002;105:1077–1081. 2. Oral H, Knight BP, Morady F. Left atrial flutter after segmental ostial radiofrequency catheter ablation for pulmonary vein isolation. Pacing Clin Electrophysiol 2003;26:1417–1419. 3. Karch MR, Zrenner B, Deisenhofer I, et al. Freedom from atrial tachyarrhythmias after catheter ablation of atrial fibrillation: a randomized comparison between 2 current ablation strategies. Circulation 2005;111:2875–2880. 4. Oral H, Ozaydin M, Tada H, et al. Mechanistic significance of intermittent pulmonary vein tachycardia in patients with atrial fibrillation. J Cardiovasc Electrophysiol 2002;13:645– 650. 5. Deisenhofer I, Estner H, Zrenner B, et al. Left atrial tachycardia after circumferential pulmonary vein ablation for atrial fibrillation: incidence, electrophysiological characteristics, and results of radiofrequency ablation. Europace 2006; 8:573–582. 6. Oral H, Chugh A, Good E, et al. Radiofrequency catheter ablation of chronic atrial fibrillation guided by complex electrograms. Circulation 2007;115:2606 – 2612. 7. Matsuo S, Lim KT, Haissaguerre M. Ablation of chronic atrial fibrillation. Heart Rhythm 2007;4:1461–1463. 8. Pappone C, Manguso F, Vicedomini G, et al. Prevention of iatrogenic atrial tachycardia after ablation of atrial fibrillation: a prospective randomized study comparing circumferential pulmonary vein ablation with a modified approach. Circulation 2004;110:3036 –3042. 9. Knecht S, Hocini M, Wright M, et al. Left atrial linear lesions are required for successful treatment of persistent atrial fibrillation. Eur Heart J 2008;29:2359 – 2366. 10. Chae S, Oral H, Good E, et al. Atrial tachycardia after circumferential pulmonary vein ablation of atrial fibrillation: mechanistic insights, results of catheter ablation, and risk factors for recurrence. J Am Coll Cardiol 2007;50:1781–1787. 11. Chugh A, Oral H, Good E, et al. Catheter ablation of atypical atrial flutter and atrial tachycardia within the coronary sinus after left atrial ablation for atrial fibrillation. J Am Coll Cardiol 2005;46:83–91.