* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Atomic Structure Atomic Structure
Bremsstrahlung wikipedia , lookup
Matter wave wikipedia , lookup
Particle in a box wikipedia , lookup
Nitrogen-vacancy center wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
Molecular Hamiltonian wikipedia , lookup
Quantum electrodynamics wikipedia , lookup
Ferromagnetism wikipedia , lookup
Wave–particle duality wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Electron scattering wikipedia , lookup
Molecular orbital wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Auger electron spectroscopy wikipedia , lookup
Chemical bond wikipedia , lookup
Hydrogen atom wikipedia , lookup
Tight binding wikipedia , lookup
Atomic theory wikipedia , lookup
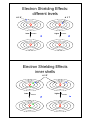
Atomic Structure Schrödinger equation has approximate solutions for multielectron atoms, which indicate that all atoms are like hydrogen Atomic Structure 3s 3p 2s 2p 3d Energy Energy Schrödinger equation has approximate solutions for multielectron atoms, which indicate that all atoms are like hydrogen 3s 3p 2s 2p 3d 1s 1s hydrogen multi-electron Atomic Structure • orbitals are populated by electrons according to certain rules Additional quantum number! ! spin quantum number (ms) spin -1/2 spin +1/2 Atomic Structure • orbitals are populated by electrons according to certain rules Name Symbol Permitted Values Property Principal n 1, 2, 3, etc orbital size (energy) Angular Momentum l 0 to n-1 orbital shape Magnetic ml -l to 0 to +l orbital orientation Spin ms +1/2 or –1/2 direction of e- spin Atomic Structure • orbitals are populated by electrons according to certain rules exclusion principle: no two electrons in the same atom can have the same four quantum numbers Atomic Structure exclusion principle: no two electrons in the same atom can have the same four quantum numbers an orbital electrons Atomic Structure exclusion principle: no two electrons in the same atom can have the same four quantum numbers empty an orbital electrons Atomic Structure exclusion principle: no two electrons in the same atom can have the same four quantum numbers empty an orbital electrons one electron Atomic Structure exclusion principle: no two electrons in the same atom can have the same four quantum numbers empty an orbital one electron two electron electrons only three options! Atomic Structure • orbitals are populated by electrons according to certain rules exclusion principle: no two electrons in the same atom can have the same four quantum numbers n=4 Electron Shielding Effects: different levels n=1 lower Eionization higher Eionization Electron Shielding Effects inner shells n=2 lower Eionization higher Eionization Electron Shielding Effects: same orbital 2+ 2+ He+ He lower Eionization higher Eionization 2+ 2+ He+ He2+ Energy Profile of Sublevels >f>d>p>s l=3 l=2 l=1 l=0 Energy Profile of Sublevels E N E R G Y 1s Energy Profile of Sublevels E N E R G Y 2p 2s Hund’s rule: maximum number of unpaired electrons for the same sublevel (n, l) 1s Energy Profile of Sublevels E N E R G Y 3s 3p 2p 2s 1s Energy Profile of Sublevels E N E R G Y 3d 3s 2p 2s 1s 3p 4s 4p Types of Electrons ! inner (core) electrons: those in the previous noble gas ! outer electrons: after that highest energy levels ! valence electrons: same as outer, involved in forming chemical bonds Practice Problems 8.44. One reason spectroscopists study excited states is to gain information about the energies of orbitals that are unoccupied in an atom’s ground state. Each of the following electron configurations represent an atom in an excited state. Identify the element and write its condensed ground-state configuration. (a) 1s22s22p63s1 (b) 1s22s22p63s23p64s23d44p1 (c) 1s22s22p63s23p44s1 (d) 1s22s22p53s1 Trends in Periodic Table metallic behavior increases ionization energy decreases Trends in Periodic Table Non-metallic behavior increases electron affinity increases Practice Problems 8.68. Which element would you expect to be more metallic? (a) Ca or Rb (b) Mg or Ra (c) Br or I (d) Si or P I or Se? Practice Problems Sample 8.6. Using condensed electron configurations, write reactions for the formation of the following elements: (a) Iodine (Z=53) (b) Potassium (Z=19) (c) Indium (Z=49)