* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Thermal Energy from Chemical Reactions
Survey
Document related concepts
X-ray photoelectron spectroscopy wikipedia , lookup
Electrolysis of water wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Heat transfer wikipedia , lookup
Marcus theory wikipedia , lookup
Solar air conditioning wikipedia , lookup
Stoichiometry wikipedia , lookup
Energy harvesting wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Internal energy wikipedia , lookup
Transition state theory wikipedia , lookup
Heat transfer physics wikipedia , lookup
Transcript
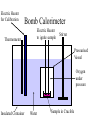
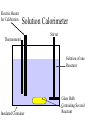
Thermal Energy from Chemical Reactions Thermochemical Equations • An equation with the amount of energy produced or absorbed – C8H18(l) + 12½O2(g) 8CO2(g) + 9H2O (g) ΔH = – 5054kJ mol –1 • If you burnt twice as much octane, twice the amount of energy is produced – 2C8H18(l) + 25O2(g) 16CO2(g) + 18H2O(g) ΔH = – 10108kJ mol –1 Thermochemical Equations • The coefficients of the reactants indicates the number of moles • The equation states that 2 moles of C8H18 reacting with 25 moles of O2 produces 10108 kJ • States must be specified since change of state can produce or needs energy Thermochemical Equations • The combustion of Octane can also produce liquid water – C8H18(l) + 12½O2(g) 8CO2(g) + 9H2O (l) ΔH = – 5450kJ mol –1 • This is because the evaporation of water absorbs energy • H2O (l) H2O (g) ΔH = + 44kJ mol–1 Thermochemical Equations • A Reaction that occurs in reverse has the same size ΔH but reversed sign • H2O (l) H2O (g) ΔH = + 44kJ mol–1 • H2O (g) H2O (l) ΔH = – 44kJ mol–1 Calculations Involving Thermal Equations • Involves Stoichiometry to determine how many moles are reacting • multiplying the ΔH by the number of moles and dividing by the coefficient from equation Calculations Involving Thermal Equations • Example, how much energy would 11g of C3H8 generated if burnt in O2 – C3H8(l) + 10O2(g) 3CO2(g) + 4H2O (l) ΔH = – 2220kJ mol –1 11 n(C3H8) 0.25mol 44 0.25 energy x 2220 555kJ 1 Connection Between Energy and Temperature Change • Objects heat up at different rates • This is expressed by the Specific Heat Content • Values are given in a table of values Specific Heat Content • The amount of energy needed to raise the temperature of 1g of a substance by 1°C • The higher the specific heat, the more effectively the substance will store heat • Has the unit Jg–1°C –1 Temperature Energy needed Specific X mass (g) X = to heat Heat Rise (°C) Specific Heat Content • For Water this would become • 4.184 X mass of water X temperature rise • 1 g = 1mL for water density = 1 g mL-1 Enthalpy • The energy in a chemical bond • During a chemical reaction where chemical bonds are broken then formed there will be a change in enthalpy • If energy (products) < energy (reactants) – Energy change is negative – Energy will be released – Exothermic Reaction Measuring Heat Released During a Reaction (Enthalpy Change) • Measured by a Calorimeter • When a reaction takes place, the heat change causes a rise or fall in temperature in the contents of the calorimeter. • Before use calorimeter must be calibrated • Find out how much energy is needed to change the temperature by 1°C (Calibration Factor) Electric Heater for Calibration Bomb Calorimeter Thermometer Electric Heater to ignite sample Stirrer Pressurised Vessel Oxygen under pressure Insulated Container Water Sample in Crucible Electric Heater for Calibration Solution Calorimeter Stirrer Thermometer Solution of one Reactant Insulated Container Glass Bulb Containing Second Reactant Calibration Factor • Energy = Voltage X Current X Time • E = VIt – Voltage is measured in Volts – Current is measured in Amps – Time is measured in seconds Calculate Energy Change During Reaction Energy Calibration X = Change Factor Temperature Change Calculate ΔH • This is the change of energy for 1 mole • If reaction produced heat ΔH = negative • If reaction absorbed heat ΔH = positive If change in heat = 30kJ for 0.25mol ΔH = 30 X 0.25 = 120 kJ mol-1 Heat of Combustion of a Substance • The energy released when a specified amount (1g, 1L or 1 mol) of the substance burns completely in Oxygen. • Substances that are mixtures of chemical are measured in terms of grams or litres Which Fuel is Best • • • • • • Factors to be considered Energy released per unit of mass Availability Cost Ease of transport Hazards associated with waste products






























![Second review [Compatibility Mode]](http://s1.studyres.com/store/data/003692853_1-a578e4717b0c8365c11d7e7f576654ae-150x150.png)







