* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download X-Ray Production
Survey
Document related concepts
Alternative energy wikipedia , lookup
International Energy Agency wikipedia , lookup
Internal energy wikipedia , lookup
Energy returned on energy invested wikipedia , lookup
Energy efficiency in transport wikipedia , lookup
Negawatt power wikipedia , lookup
Directed-energy weapon wikipedia , lookup
Conservation of energy wikipedia , lookup
Energy policy of the European Union wikipedia , lookup
Energy Independence and Security Act of 2007 wikipedia , lookup
Shockley–Queisser limit wikipedia , lookup
Microbial fuel cell wikipedia , lookup
Transcript
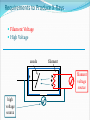
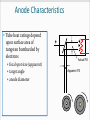
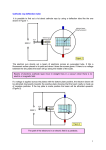
CT X-Ray Production George David Associate Professor The Atomic Nucleus Protons + Charges # protons = atomic # (Z) + + + Neutrons ~ No charge ~ ~ Mass about the same as proton Atomic Weight(mass)= # protons + # neutrons Orbital Electrons Electrons - charges very small mass compared with protons / neutrons Electrons reside only at certain energy levels or Shells Designations start at K shell K shell closest to nucleus L shell next closest Shells proceed up from K, L, M, N, etc. Except for K shell, all shells contain sub-shells L K - ~ + ~ + + ~ - Binding Energy energy required to remove orbital electron from atom Negative electrons attracted to positive nucleus more binding energy for shells closer to nucleus K shell has highest binding force higher atomic # materials (higher Z) result in more binding energy more positive charge in nucleus L K ~ + ~ + + ~ - - Electron Shells (cont.) Electrons can only reside in a shell electron has exactly the energy associated with its shell electrons attempt to reside in lowest available energy shell L K ~ + ~ + + ~ - - The Shell Game * Electrons can move from shell to shell to move to higher energy shell requires energy input equal to difference between shells L K ~ + ~ + + ~ - - - Requires energy input! The Shell Game (cont.) to move to a lower energy shell requires the release of energy equal to the difference between shells characteristic x-rays L K ~ + ~ + + ~ Energy released - - Output X-Ray Beam Producing X-Rays Electrons emitted by filament Electrons slam into target Reminder Electrons carry their energy as kinetic energy Energy of motion + Requirements to Produce X-Rays Filament Voltage High Voltage anode + high voltage source filament filament voltage source X-Ray Production(cont.) X-Rays are produced in the x-ray tube by two distinct processes Characteristic radiation Bremsstrahlung Output Beam Spectrum Output photon beam made up of Characteristic Radiation characteristic of target material several discrete energies # Energy Bremsstrahlung continuous range of energies 0 - kVp setting most photons have low energy Spectrum # depicts fraction of beam at each energy value combination of Bremsstrahlung and characteristic radiation Energy Characteristic Radiation Interaction of high speed incident electron with orbital electron of target #1: orbital electron removed from atom #2: electrons from higher energy shells cascade down to fill vacancies L K - #3: characteristic x-ray emitted + ~ + ~ #1 + - #2 ~ - #3 Characteristic Radiation Consists only of discrete x-ray energies corresponding to energy difference between electron shells of target Specific energies are characteristic of target material for tungsten 59 keV corresponds to the difference in energy between K and L shells # Energy L K ~ + ~ + + ~ - - Bremsstrahlung interaction of moving electron with nucleus of target atoms Positive nucleus causes moving electron to change speed / direction Kinetic energy lost Emitted in form of Bremsstrahlung x-ray L K ~ + ~ + + ~ - - Bremsstrahlung (cont.) Bremsstrahlung means braking radiation Moving electrons have many Bremsstrahlung reactions small amount of energy lost with each L K ~ + ~ + + ~ - - Bremsstrahlung (cont.) Energy lost by moving electron is random & depends on distance from nucleus charge (Z) of nucleus Bremsstrahlung Energy Spectrum 0 - peak kilovoltage (kVp) applied to x-ray tube most x-ray photons low energy lowest energy photons don’t escape tube easily filtered by tube enclosures or added filtration # Energy Beam Intensity Product of # photons in beam energy per photon Units Roentgens (R) per unit time Measure of ionization rate of air Depends on kVp mA target material filtration Intensity & Technique beam intensity proportional to mA beam Intensity ~ proportional to kVp2 + high voltage source filament voltage source keV = kilo-electron volt energy of an electron Kinetic energy Higher energy electron moves faster Electrons can be manipulated by electric fields Accelerated Steered + * kVp = kilovolts peak peak kilovoltage applied across x- ray tube kVp Time kVp kVp corresponds to maximum photon energy in beam spectrum kVp affects quality (spectrum) & quantity of x-rays produced energy spectrum changed subject contrast changes higher kVp reduces subject contrast # -------- Higher kVp Energy mA mA affects only quantity of x-rays does not affect quality spectrum # -------- Higher mA Energy X-Ray Technique Kilovoltage [peak] (kV or [kVp]) maximum high voltage applied between cathode & anode Exposure time Length of time high voltage is applied Milliamps (mA) see following slides kVp 77 mA 200 time .040 Tube Current (mA) rate of electron flow from filament to target Measured in milliamperes (mA) mA controlled primarily by filament voltage increasing filament voltage / current results in increased filament temperature emission of electrons + Tube Rating Chart Indicates load limit for tube Maximum time for given kVp & mA Maximum time depends upon Rate at which heat generated kVp mA Speed of rotating anode Anode characteristics See next slide Anode Characteristics Tube heat ratings depend upon surface area of tungsten bombarded by electrons focal spot size (apparent) target angle anode diameter + Actual FS Apparent FS Typical Single-Exposure Tube Rating Chart • shows maximum exposure time for single exposure at given kV & mA Example • What is the maximum exposure time at 90 kVp & 300 mA? Example • What is the maximum exposure time at 120 kVp & 400 mA? Can’t do 120 kVp at 400 mA for any exposure time. ? Tube Anode Damage Single exposure heat capacity exceeded melted spots on anode Anode Thermal Characteristics Chart 2 charts in one cooling curve in absence of heating anode heating for continuous heat input (Spiral CT) Cooling Start on cooling curve with current heat units 100,000 heat units in this example Cool for 2 minutes x x 2 minutes The Many Ways Tubes Die Warning Tube Anode Damage thermal shock (high mA on cold anode) can cause in cracks in anode (tube death) Tube warm-up eliminates thermal shock from high mA exposures on cold anode warm-up needed whenever tube cold once in the morning not sufficient if tube not used for several hours High Voltage Arcs electrons move from filament to tube housing instead of to anode can be caused by filament evaporation deposition of filament on glass envelope as result of high filament currents or filament boost time very short exposure with instantaneously very high mA Generator often drops off line + arcing Tube Insert Damage Bearing Damage prevents proper rotation of anode Anode can run too slow stop results in thermal damage to anode (melted spots) Anode not running at design speed Filament breaks renders one focal spot completely inoperative Oil Leaks May be accompanied by air bubble in housing Eventually causes high voltage arcing Requires immediate service attention