* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Section 2.6
Survey
Document related concepts
Fluorescence correlation spectroscopy wikipedia , lookup
Cluster chemistry wikipedia , lookup
State of matter wikipedia , lookup
Homoaromaticity wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Molecular orbital wikipedia , lookup
Host–guest chemistry wikipedia , lookup
Coupled cluster wikipedia , lookup
Rigid rotor wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Aromaticity wikipedia , lookup
Rotational spectroscopy wikipedia , lookup
Rotational–vibrational spectroscopy wikipedia , lookup
Transcript
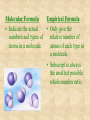
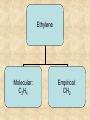
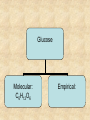
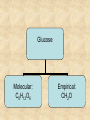
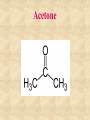
Section 2.6 Molecules and Molecular Compounds Remember… • Atoms are the smallest representative sample of an element Building Blocks of Matter! • BUT- only the noble gases are found as isolated atoms • The rest exist as molecules or ions Molecules • Assembly of two or more atoms tightly bound together, behaving as a single object Chemical Formula • Notation that uses chemical symbols and subscript notation to convey relative proportions of atoms of elements in a molecule Diatomic Molecule • Molecule composed of two atoms • Elements normally occurring as diatomic: –Hydrogen (H2) –Oxygen (O2) –Nitrogen (N2) –The halogens (F2, Cl2, Br 2, I2) Molecular Compounds • Compounds composed of molecules • Contain more than one type of atom • Examples: CH4, H2O, H2O2 Most molecular compounds contain only nonmetals!! Chemical Formulas Molecular Formula Empirical Formula Molecular Formula Empirical Formula • Indicate the actual • Only give the numbers and types of relative number of atoms in a molecule. atoms of each type in a molecule. • Subscript is always the smallest possible whole-number ratio Examples Hydrogen Peroxide Molecular: H 2 O2 Empirical: HO Ethylene Molecular: C 2H 4 Empirical: CH2 Now You Try… Glucose Molecular: Empirical: Glucose Molecular: C6H12O6 Empirical: Glucose Molecular: C6H12O6 Empirical: CH2O If we know the molecular formula, we can determine the empirical formula! Does the converse hold true? Structural Formula • Shows which atoms are attached to each other within a molecule • Atoms are represented by their chemical symbol • Lines represent bonds • Do not depict the geometry of the molecule (the angles of the bonds) Acetone Perspective Drawings • Structural formula that gives a sense of the three-dimensional shape of a molecule Methane Ball-and-stick models • Shows atoms as spheres and bonds as sticks • Accurately represents angles of the bonds • Also demonstrates relative size of atoms Water Space-filling models • Depict a molecule scaled up to size • Show relative size of atoms • Difficult to see the angles of the bonds