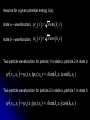* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Document
Bell's theorem wikipedia , lookup
EPR paradox wikipedia , lookup
Renormalization wikipedia , lookup
Fundamental interaction wikipedia , lookup
Old quantum theory wikipedia , lookup
Condensed matter physics wikipedia , lookup
Density of states wikipedia , lookup
Photon polarization wikipedia , lookup
Standard Model wikipedia , lookup
Introduction to gauge theory wikipedia , lookup
Quantum electrodynamics wikipedia , lookup
Spin (physics) wikipedia , lookup
Atomic nucleus wikipedia , lookup
Nuclear structure wikipedia , lookup
Elementary particle wikipedia , lookup
Nuclear physics wikipedia , lookup
Introduction to quantum mechanics wikipedia , lookup
Hydrogen atom wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
History of subatomic physics wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
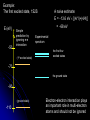
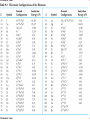
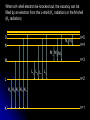
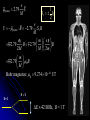
自旋—軌道作用 internal magnetic field The spin-orbit interaction In the absence of an external magnetic field, internal field generated by electron motion (proportional to orbital angular momentum) will interact with spin dipole moment Frame of electron LB S when L is parallel to S Frame of nucleus S e L Ze Ze e s Nucleus circulates around electron a B-field due to the nuclear motion Orbital dipole moment is anti-parallel to spin dipole moment higher energy U s B 0 state (spin up) Frame of electron when L is anti-parallel to S s Frame of nucleus Ze e Ze S 2p3/2 2p1/2 1s (l = 0, |L| = 0) S Nucleus circulates around electron a B-field due to the nuclear motion Orbital dipole moment is parallel to spin dipole moment (l = 1, |L| > 0) 2p e LB L S s S s U s B 0 lower energy state (spin down) The spin-orbit interaction causes fine structure doubling of atomic spectral lines (3/3/2010) Total angular momentum (as a result of spin-orbit interaction) J LS magnitude: J j j 1 z-component: J z m j m j j, j 1, , j 1, j Neither L nor S is conserved separately! Permissible values for the total angular momentum quantum number j j l s, l s 1, , | l s | (maximum value) For an atomic electron s = 1/2 1 1 jl , l 2 2 (minimum value) Example, for the 2p state l = 1, j = 3/2 or 1/2 for the 3d state l = 2, j = 5/2 or 3/2 for the s state l = 0, j = 1/2 l=1 j = 1/2 3 | J | 2 1 1 mj , 2 2 j = 3/2 | J | 15 2 3 1 1 3 mj , , , 2 2 2 2 mj has an even number of values (a) A vector model for determining the total angular momentum J = L + S of a single electron (b) The allowed orientations of the total angular momentum J for the states j = 3/2 and j = 1/2. Notice that there are now an even number of orientations possible, not the odd number familiar from the space quantization of L alone Energy shift due to spin-orbit interaction Square of total angular momentum J LS 2 L S L S U LS s Binternal Binternal ? 2 L2 S 2 2 L S J 2 L2 S 2 LS 2 Spin-orbit interaction energy (a relativistic effect): U LS Ze2 ge 1 LS 2 2 3 4 o 2 4me c r U LS L S 2 j j 1 2 U U Write LS o j j 1 r: radius of the orbiting electron c: speed of light 1 s( s 1) 3 1 4 Spin-orbit energy shift 2P3/2 3 1 j , l 1, s 2 2 2P1/2 1 1 j , l 1, s 2 2 2S1/2 1 1 j , l 0, s 2 2 e.g., For a hydrogen atom Uo = 1.510-5 eV U LS U o j j 1 ms=1/2 (n = 2) 3 1 1 E 2P1/ 2 E2 U o 1 11 1 E2 2U 0 4 2 2 3 1 1 E 2S1/ 2 E2 U o 1 0 0 1 E2 4 2 2 B=0 (2) 2S1/2 (8) n1 2 2 1 0 3 1 4 3 3 3 E 2P3/ 2 E2 U o 1 11 1 E2 U 0 4 2 2 (4) 2P3/2 2S, 2P (2) 2P1/2 B0 (but small) mj 3/2 1/2 1/2 3/2 1/2 1/2 1/2 1/2 Remarks: In a small external B field, the spin-orbit interaction is dominant and the total angular momentum J as a whole precesses around the B field. The no. of split lines = the no. of mj values In a large external B field, both the orbital angular momentum L and the spin angular momentum S precess independently around the B field. The no. of splitting lines = 2 (2l + 1) Quantum numbers – in the absence of spin-orbit effect, a state of an atomic electron is specified by (n, l, ml, ms). If the spin-orbit interaction is taken into account, the state may be specified by (n, l, j, mj) 2P3/2, 2P1/2, 2S1/2 Example: electronic states associated with the principle quantum number n = 2 (n, l , ml , ms ) ( n, l , j , m j ) 1 2,0,0, , 2 1 2,1,0, , 2 1 1 2,0, , , 2 2 3 3 2,1, , , 2 2 1 2,0,0, 2 1 2,1,0, 2 1 1 2,0, , 2 2 3 1 2,1, , 2 2 1 1 2,1,1, , 2,1,1, 2 2 1 1 2,1, 1, , 2,1, 1, 2 2 3 1 3 3 2,1, , , 2,1, , 2 2 2 2 1 1 1 1 2,1, , , 2,1, , 2 2 2 2 (in the absence of spin-orbit interaction) (in the presence of spin-orbit interaction) Weak B field Strong B field The angular momentum vectors L, S, and J for a typical case of a state with l = 2, j = 5/2, mj = 3/2. The vectors L and S precess uniformly about their sum J, as J precesses randomly about the z axis In a strong B field, the orbital angular momentum L precesses about the z axis. (Similarly for the spin angular momentum S ) (3/8/2010) Pauli Exclusion Principle Pauli (1900–1958) From spectra of complex atoms, Pauli (1925) deduced a new rule: In a given atom, no two electrons can be in the same quantum state, i.e. they cannot have the same set of quantum numbers n,,m,ms Every “atomic orbital with n,,m” can hold two electrons: (n,,m,) and (n,,m,) Thus, electrons do not pile up in the lowest energy state, i.e, the (1,0,0) orbital They are distributed among the higher energy levels according to the Pauli Principle Particles that obey Pauli exclusion principle are called “fermions” More generally, no two identical fermions (any particle with spin of ħ/2, 3ħ/2, etc.) can be in the same quantum state Two-particle problems – Consider two particles in a one-dimensional potential well U = U(x) There exist a series of eigenstates Quantum state labels: a, b, c, …… Eigenfunctions: a(x), b(x), c(x), …… Eigenvalues: Ea, Eb, Ec, …… e.g., one-particle problem – if there is only one particle in the potential well and the particle is in the state a 2 d2 a x U ( x ) a x Ea a x 2 2m dx Write: H a x Ea a x H: Hamiltonian Now consider two particles, 1 and 2, in the potential well. Assume particle 1 be in the state a and particle 2 be in the state b 2 d2 a x1 U ( x1 ) a x1 Ea a x1 2 2m dx1 2 d2 b x2 U ( x2 ) b x2 Eb b x2 2 2m dx2 H ( x1 ) a x1 Ea a x1 H ( x2 ) b x2 Eb b x2 Guess: the total wavefunction for this two-particle system 2 d2 2 d2 2m dx 2 U ( x1 ) 2m dx 2 U ( x2 ) x1 , x2 1 2 H ( x1 ) H ( x2 ) x1 , x2 H ( x1 ) H ( x2 ) a x1 b x2 Ea a x1 b x2 a x1 Eb b x2 ( Ea Eb ) a x1 b x2 ( Ea Eb ) x1 , x2 The wavefunction for particle 1 in state a and particle 2 in state b (x1,x2) = a(x1)b(x2) is a solution, with the total energy E = Ea + Eb Now let the positions of the two particles be exchanged. Let particle 2 be in the state a and particle 1 be in the state b 2 d2 a x2 U ( x2 ) a x2 Ea a x2 2 2m dx2 H ( x2 ) a x2 Ea a x2 2 H ( x1 ) b x1 Eb b x1 d2 b x1 U ( x1 ) b x1 Eb b x1 2 2m dx1 Guess: the total wavefunction for this two-particle system The wavefunction for particle 2 in H ( x2 ) H ( x1 ) x2 , x1 state a and particle 1 in state b H ( x2 ) H ( x1 ) a x2 b x1 Ea a x2 b x1 a x2 Eb b x1 (x ,x ) = (x ) (x ) 2 1 a 2 b 1 ( Ea Eb ) a x2 b x1 is a solution, with the total energy E = Ea + Eb ( Ea Eb ) x2 , x1 Assume for a given potential energy U(x), state a – wavefunction, a x A sin ka x state b – wavefunction, b x A cos kb x Two-particle wavefunction for particle 1 in state a, particle 2 in state b x1, x2 a ( x1 ) b ( x2 ) Asin(ka x1 )cos(kb x2 ) Two-particle wavefunction for particle 2 in state a, particle 1 in state b x2 , x1 a ( x2 ) b ( x1 ) Asin(ka x2 )cos(kb x1 ) “Probability density” for particle 1 in state a and particle 2 in state b P x1, x2 | x1, x2 | | A | sin (ka x1 )cos (kb x2 ) 2 2 2 2 “Probability density” for particle 2 in state a and particle 1 in state b P x2 , x1 | x2 , x1 | | A | sin (ka x2 )cos (kb x1 ) 2 2 2 2 The guess solutions (x1,x2) = a(x1)b(x2) and (x2,x1) = a(x2)b(x1) imply that the “probability density” for finding the two particles is not the same under exchange of the two particle coordinates P x2 , x1 P x1, x2 Can measurable physical quantities be differing under particle exchange ? Quantum particles are “indistinguishable” – a purely quantum-mechanical concept; no classical analogy ! The scattering of two electrons as a result of their mutual repulsion. The events depicted in (a) and (b) produce the same outcome for identical electrons but are nonetheless distinguishable classically because the path taken by each electron is different in the two cases. In this way, the electrons retain their separate identities during collision. (c) According to quantum mechanics, the paths taken by the electrons are blurred by the wave properties of matter. In consequence, once they have interacted, the electrons cannot be told apart in any way! Electrons are identical particles. It is impossible to distinguish one electron from another ! Also, wavefunctions can overlap !! a(x1)b(x2) and a(x2)b(x1) are not the correct wavefunctions Need to search for the right wavefunctions ?! Exchange Symmetry The simplest case: Two identical (“indistinguishable”) particle system Consider two identical particles satisfying 3D Schrödinger equations with coordinates r1 and r2. Assume no interaction between them 2 2m 2 2m 12 a r1 U r1 a r1 Ea a r1 H r1 a r1 22 b r2 U r2 b r2 Eb b r2 H r2 b r2 H r1 H r2 r1 , r2 E r1 , r2 r1, r2 a r1 b r2 Particle 1 has energy Ea and particle 2 has energy Eb. E = Ea + Eb exchange particles r1 r2 r2 , r1 a r2 b r1 A distinguishable solution ! Particle 1 has energy Eb and particle 2 has energy Ea Try linear combinations of the two wavefunctions: 1 S r1 , r2 a r1 b r2 a r2 b r1 2 S is a solutions of the (linear) Schrödinger eq. with E = Ea + Eb H r1 H r2 S r1 , r2 1 H r1 H r2 a r1 b r2 a r2 b r1 2 1 H r1 a r1 b r2 H r1 a r2 b r1 2 1 H r2 a r1 b r2 H r2 a r2 b r1 2 1 Ea a r1 b r2 Eb a r2 b r1 2 1 Eb a r1 b r2 Ea a r2 b r1 2 1 Ea Eb a r1 b r2 a r2 b r1 ( Ea Eb ) S (r1 , r2 ) 2 Try linear combinations of the two wavefunctions: 1 A r1, r2 a r1 b r2 a r2 b r1 2 A is a solutions of the (linear) Schrödinger eq. with E = Ea + Eb H r1 H r2 A r1 , r2 1 H r1 H r2 a r1 b r2 a r2 b r1 2 1 Ea a r1 b r2 Eb a r2 b r1 2 1 Eb a r1 b r2 Ea a r2 b r1 2 1 Ea Eb a r1 b r2 a r2 b r1 2 ( Ea Eb ) A ( r1, r2 ) Under exchange of particle coordinates r1 r2 1 symmetric S r1,r2 a r1 b r2 a r2 b r1 2 1 S r2 , r1 a r2 b r1 a r1 b r2 S r1 , r2 2 1 anti-symmetric A r1 , r2 a r1 b r2 a r2 b r1 2 1 A r2 , r1 a r2 b r1 a r1 b r2 A r1 , r2 2 Both satisfy the Exchange Symmetry Principles S r1, r2 S r2 , r1 , A r1, r2 A r2 , r1 2 2 2 2 No observable difference !! Exchange symmetry: For both S and A, all the probability densities (observable effects) are unaffected by the interchange of particles A two-electron system: the helium atom He a r1 2 2me 4 o r1 a r1 Ea a r1 H r1 a r1 Ea a r1 2e+ b r2 12 a r1 2e 2 2 2 2 e 22 b r2 b r2 Eb b r2 2m 4 o r2 H r2 b r2 Eb b r2 S r1 , r2 1 a r1 b r2 a r2 b r1 2 1 A r1 , r2 a r1 b r2 a r2 b r1 2 symmetry space wavefunction anti-symmetric space wavefunction Total two-particle wavefunction: (r1 , r2 ) space r1, r2 spin (, ) A describes a class of particles called “fermions” A (r1 , r2 ) A ( r2 , r1 ) S describes a class of particles called “bosons” S ( r1 , r2 ) S ( r2 , r1 ) (Both the spatial coordinates and the spin orientations of the two particles are to be simultaneously interchanged) Pauli exclusion principle states that no two electrons in an atom can have the same set of quantum numbers (no two electrons can occupy the same quantum state). The exclusion principle applies only to fermions It is an experimental fact that integer spin particles are bosons, but half-integer spin particles are fermions The symmetry character of various particles particle symmetry Spin (s) Electron antisymmetric 1/2 Fermions(費米子) Positron antisymmetric 1/2 Proton antisymmetric 1/2 half-integer spin particles Neutron antisymmetric 1/2 Muon antisymmetric 1/2 particle symmetric 0 Bosons(玻思子) He atom (G) symmetric 0 integer spin particles meson symmetric 0 Photon symmetric 1 Deuteron symmetric 1 e.g., Helium isotope 3He (2 protons + 1 neutron) – a fermion Example 9.5 Ground state of the helium atom Construct explicitly the two-electron ground state wavefunction for the helium atom in the independent particle approximation (Assume each helium electron sees only the doubly charged helium nucleus) Hydrogen-like atom with the lowest energy, Z = 2 1 2 100 r R10 ( r )Y00 ( , ) ao 3/ 2 e 2 r ao E1 = 2213.6 = 54.4 eV 1 S r1 , r2 100 ( r1 ) 100 ( r2 ) 100 ( r2 ) 100 ( r1 ) 2 3 2 2 2( r1 r2 ) / a0 e a0 symmetric spatial wavefunction Spin eigenfunctions for two-electron systems Singlet state (單重態), S = 0, ms = 0 1 1 A | | | , | , 2 2 Linear combinations of |, | |, | anti-symmetric spin wavefunction Triplet state (三重態), S = 1, ms = 0, 1 | | , 1 1 S | | | , | , 2 2 | | , ms=1 ms=0 symmetric spin wavefunction ms=1 Total two-electron wavefunction for a helium atom The ground state – only once choice: 1S2, spin singlet (total spin = 0) A ( r1 , r2 ) S ( r1 , r2 ) A 3 1 2 2( r1 r2 ) / a0 e | , | , a0 The equal admixture of the spin states |+ and |+ means the spin of the first helium electrons is just as likely to be up as it is to be down The spin of the second electron must always be opposite the first If both electrons had the same spin orientations A = 0. The theory allows no solution no two electrons can occupy the same quantum state Energy for the two electrons in a helium atom A naïve estimate: E = 2 ( Z213.6 eV) = 108.8 eV, with Z = 2, n = 1 Remarks: In practice, it requires only 24.6 eV to remove the first electron. To remove the second electron requires 54.4 eV. The electron left behind screens the nuclear charge and make the ionization of the first electron easier The other possible choice of wavefunction A(r1,r2) = A(r1,r2)triplet must comprise a higher energy eigenstate symmetric under spin exchange Example: The first excited state, 1S2S A naive estimate: E = 13.6 eV [(4/1)+(4/4)] E (eV) -50 = 68 eV Simple prediction by ignoring e-e interaction (1st excited state) Experimental spectrum the first four excited states -70 the ground state -90 (ground state) -110 Electron-electron interaction plays an important role in multi-electron atoms and should not be ignored Hydrogen molecular ion: H2+ (Serway, Fig. 11.7) The wavefunction (a) and probability density (b) for the approximate molecular wave + formed from the symmetric combination of atomic orbitals centered at r = 0 and r = R. (c) and (d): Wavefunction and probability density for the approximate molecular wave - formed from the anti-symmetric combination of these same orbitals The bonding state (a), a spin singlet state, possesses a lower energy than the anti-bonding state (c), a spin triplet state 1921 – Stern-Gerlach experiment of anomalous Zeeman effect 1925 – Pauli exclusion principle (the fourth degree of freedom) 1925 – Goudsmit-Uhlenbeck theory of electron spin 當泡利處心積慮的鑽研反常塞曼效應時,當時在慕尼黑的朋友 問他:「你為何總是愁眉苦臉的?」泡利馬上反問道:「要是 你陷入了反常塞曼效應的思慮,難道還快樂的起來嗎?」根據 當時的條件,泡利是無法圓滿的解釋這個效應的。而沿著這條 路走下去,卻使他得以發現不相容原理。 《微觀絕唱》,p.143 Ehrenfest on Goudsmit-Uhlenbeck theory: 「你們還很年輕,做點蠢事也沒有什麼關係!」 Wolfgang Pauli Otto Stern Lorentz Kondo effect – a singlet ground state formed by the localized moment and the screening conduction electron spins Electron-electron interactions and Screening effects Helium atom: ( e)( e) H r1 , r2 H r1 H r2 4 0 | r1 r2 | (??) Each electron sees not only the attractive helium nucleus but also the other electron Electrons confined to the small space of an atom exert strong repulsive electrical forces on each other (especially in multi-electron atoms) Is the charge of nucleus = +2e from electron’s viewpoint ? Inner electrons screen the positive charge of nucleus, and hence reduce the effective Z and the attractive potential smaller negative potential energy (especially for multi-electron atoms) The resulting effective potential energy seen by an electron in the atom can be notably smaller than the bare potential energy The Periodic Table Mendeleev’s table as published in 1869 – 63 elements Mendeleev 1834~1907 Ga Ge Sc “All good theories should be able to make predictions and this was no exception” Build a multiple electron atom Chemical properties of an atom are determined by the least tightly bound electrons Occupancy of subshell Energy separation between the subshell and the next higher subshell n 1 2 3 s shell =0 Helium and Neon and Argon are inert. Their outer subshell is closed p shell =1 Hydrogen: (n, , mℓ, ms) = (1, 0, 0, ±½) in ground state In the absence of a magnetic field, the state ms = ½ is degenerate with the ms = −½ state Helium: (1, 0, 0, ½) for the first electron; (1, 0, 0, −½) for the second electron. The two electrons have anti-aligned spins (ms = +½ and ms = −½) The principle quantum number has letter codes. n= 1 2 3 4... n = shells (e.g., K shell, L shell, …) Letter = K L M N… How many electrons may be placed in each subshell? Total For each mℓ: two values of ms 2 For each : (2 + 1) values of mℓ = 0 1 2 3 4 5 … Letter = s p d f g h … 2(2 + 1) n = subshells (e.g., 1s, 2p, 3d, …) 1s 1s2 1s22s The filling of electronic states must obey the Pauli exclusion principle and Hund’s rule (spinspin correlation) 1s22s2 1s22s22p1 1s22s22p2 1s22s22p3 1s22s22p4 1s22s22p5 1s22s22p6 1s22s22p63s 1s22s22p63s2 Energies of orbital subshells increase with increasing Energies of orbital subshells are lower for lower . Lower angular momentum implies more eccentric classical orbits, with greater penetration into the nuclear regime Fill electrons to states according to the lowest value of n+, with “preference” given to n n+ The lower values have more eccentric classical orbits than the higher values Electrons with higher values are more shielded from the nuclear charge Electrons lie higher in energy than those with lower values 4s fills before 3d 7s 6s 5s 4s 3s 2s 1s 7p 6p 5p 4p 3p 2p 7d 6d 5d 4d 3d 7f 7g 7h 7i 6f 6g 6h 5f 5g 4f 8 8 7 7 6 6 6 5 5 5 4 4 3 3 2 1 Groups: Vertical columns Same number of electrons in an orbit Can form similar chemical bonds Periods: Horizontal rows Correspond to filling of the subshells Inert Gases: Last group of the periodic table Closed p subshell except helium Zero net spin and large ionization energy Their atoms interact weakly with each other (van der Waals forces) Alkalis: Single s electron outside an inner core Easily form positive ions with a charge +1e Lowest ionization energies Electrical conductivity is relatively good Alkaline Earths: Two s electrons in outer subshell Largest atomic radii High electrical conductivity Halogens: Need one more electron to fill outermost subshell Form strong ionic bonds with the alkalis More stable configurations occur as the p subshell is filled Transition Metals: Three rows of elements in which the 3d, 4d, and 5d are being filled Properties primarily determined by the s electrons, rather than by the d subshell being filled Have d-shell electrons with unpaired spins As the d subshell is filled, the magnetic moments, and the tendency for neighboring atoms to align spins are reduced Lanthanides (rare earths): Z = 57 ~ Z = 70 Have the outside 6s2 subshell completed As occurs in the 3d subshell, the electrons in the 4f subshell have unpaired electrons that align themselves The large orbital angular momentum contributes to the large ferromagnetic effects Actinides: Z = 89 ~ Z = 102 Inner subshells are being filled while the 7s2 subshell is complete Difficult to obtain chemical data because they are all radioactive Have longer half-lives X-ray spectra and Moseley’s law Target :W Electronic transitions within the inner shells of heavy atoms are accompanied by emission of highenergy photons (x rays) series of sharp lines: “characteristic spectrum” of the target material 1913–4, Moseley measured characteristic x-ray spectra of 40 elements, and observed “series” of x-ray energy levels, called K, L, M, … etc. Analogous to optical series for hydrogen (e.g. Lyman, Balmer, Paschen…) The characteristic spectrum hole in K-shell (n = 1) discovered by Bragg systematized by Moseley ejected K-shell electron incident electron scattered incident electron hole in L-shell (n = 2) The characteristic peak is created when a hole in the inner shell created by a collision event is filled by an electron from a higher energy shell x-ray When a K-shell electron be knocked out, the vacancy can be filled by an electron from the L-shell (K radiation) or the M-shell (K radiation) O N N N n=5 n=4 M M M n=3 M L L L L n=2 L K K K K K K n=1 K series: n = 2, 3, … to n = 1 Consider the K line. An electron in the L shell is partially screened from the nucleus by the one remaining K shell electron ( Z 1)2 EL 13.6 eV 2 n ( Z 1)2 EK 13.6 eV 2 1 1 2 EL EK 13.6 1 2 Z 1 eV n hc e.g., (K) = 0.723 Å for molybdenum (Z = 42) Atomic number versus square root of frequency (Moseley, 1914) L series: n = 3, 4, … to n = 2 2 1 1 EL 13.6 2 2 Z 7.4 eV 2 n hc Moseley’s law: the square root of photon frequency should vary linearly with the atomic number Z (A direct way to measure Z ) Moseley established the fact that the correct sequence of elements in the periodic table is based on atomic number rather than atomic mass Nuclear Magnetic Resonance Imaging (NMRI or MRI) MRI is a medical imaging technique used in radiology to visualize the internal structure and function of the body. It provides great contrast between the different soft tissues of the body, making it very useful in brain and cancer imaging. It uses no ionizing radiation, but uses a powerful magnetic field to align the nuclear magnetization of hydrogen atoms in water in the body Proton magnetic moment: (very small compared with electron magnetic moment) proton 2.79 e M proton 1 Sproton , s = 2 U proton B 10 102 MHz Resolution: 1 – 0.1 mm An expensive, large-volume superconducting magnet of several Tesla proton e 2.79 S M 1 Sz 2 e U proton B 2.79 S z B M e m e 2.79 B 2.79 B 2M M 2m m 2.79 B B M Bohr magneton: B 9.274 10 24 J/T B0 B=0 E 42 MHz, B = 1 T Summary: Orbital magnetism and the normal Zeeman effect Electron spin The spin-orbit interaction Exchange symmetry and the Exclusion principle The periodic table X-ray spectra and Moseley’s law