* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Catalytic hydrogenation
Survey
Document related concepts
Strychnine total synthesis wikipedia , lookup
Aromaticity wikipedia , lookup
Marcus theory wikipedia , lookup
Petasis reaction wikipedia , lookup
Cracking (chemistry) wikipedia , lookup
Ene reaction wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Polythiophene wikipedia , lookup
Stille reaction wikipedia , lookup
Asymmetric induction wikipedia , lookup
Kinetic resolution wikipedia , lookup
Fischer–Tropsch process wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Asymmetric hydrogenation wikipedia , lookup
Transcript
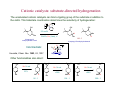
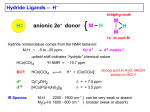
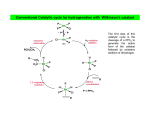
Catalytic hydrogenation 1 Wilkinson’s catalyst The complex RhCl(PPh3)3 (also known as Wilkinson’s catalyst) became the first highly active homogeneous hydrogenation catalyst that compared in rates with heterogeneous counterparts. Wilkinson, J. Chem. Soc. (A) 1966, 1711. Ph3P PPh3 Rh Cl Ph3P 1-octene H2 (1 atm), RT Benzene octane 2 Wilkinson’s catalyst Wilkinson’s catalyst is compatible with a range of functional groups because the mechanism does not involve hydride ion transfer. O C N O O OR OH NO2 OR But ethylene is not hydrogenated due to formation of a strongly bonded ethylene complex. + H2C=CH2 Cl PPh3 Rh PPh3 Ph3P -PPh3 Cl PPh3 Rh Ph3P However, ethylene reacts with the preformed dihydride complex. This implies that the dihydride formation precedes olefin complexation in the catalytic cycle. 2 H2C=CH2 + H Cl PPh3 Rh Ph3P H PPh3 -PPh3 Cl PPh3 Rh Ph3P + H3C-CH3 3 Hydrogenation mechanism Wilkinson’s catalyst, RhCl(PPh3)3 is used in benzene/ethanol solution in which it dissociates to some extent; a solvent molecule (Solv) fills the vacant site: RhCl(PPh3)3 + Solv ' RhCl(Solv)(PPh3)2 + PPh3 PPh3 HH H C C R HH Cl Rh Solv PPh3 16-e (4) H2 (1) PPh3 H H2C R Rh PPh3 H Cl H PPh3 CH H 16-e (3) (2) H H PPh3 Cl Rh Rh Cl PPh3 16-e R PPh3 18-e R Steps: (1) H2 addition, (2) alkene addition, (3) migratory insertion, (4) reductive elimination of the alkane, regeneration of the catalyst. Halpern, Chem. Com. 1973, 629; J. Mol. Cat. 1976, 2, 65; Inorg. Chim. Acta. 1981, 50, 11.4 Wilkinson’s catalyst selectivity Increasing rate The rate of hydrogenation depends on (a) presence of a functional group in the vicinity of the C=C bond and (b) degree of substitution of the C=C fragment. A polar functional group may accelerate catalysis by assisting olefin coordination to Ru Terminal C6-C12 alkenes are hydrogenated at the same rate Conjugated dienes react slower Hydrogenation of internal and branched alkenes is the slowest (note: cis is faster than trans!) 5 Wilkinson’s catalyst selectivity Hydrogenation is stereoselective: H HO2C H CO2H Cl PPh3 Rh PPh3 Ph3P D2 benzene, rt D H HO2C Cl PPh3 Rh Ph3P PPh3 D H CO2H C3H7 D D + hexane CH3 C3H7 D2 benzene, rt meso compound, major product CH3 cis: trans > 20:1 Rh preferentially binds to the least sterically hindered face of the olefin: less hindered Cl PPh3 Rh PPh3 Ph3P R H2 benzene/EtOH, rt H Ph3P Cl Rh H PPh3 R H Ph3P Cl Rh H PPh3 + R more hindered CH2H H CH2H Wilkinson, J. Chem. Soc. (A) 1966, 1711 Rousseau, J. Mol. Cat. 1979, 5, 163. Jardine, Prog. Inorg. Chem. 1981, 28, 63. R endo R=H : 73% endo R=Me : 92% endo H R exo 6 Wilkinson’s catalyst selectivity Site selectivity: Preferential hydrogenation of the least sterically hindered C=C bonds (note that heterogeneous hydrogenation catalysts are often not selective): O O O O Pd/C cis-disubstituted acetone, H2 (1 atm) rt, 75% O O H Cl PPh3 Rh Ph3P PPh3 tetrasubstituted C6H6/EtOH, H2 (1 atm) rt, 95% O O O Pedro, JOC 1996, 61, 3815. Cis-disubstituted C=C react faster than trans-disubstituted C=C: cis-disubstituted O CO2Me HO OAc Cl PPh3 Rh PPh3 Ph3P H2 (1atm), benzene/EtOH, rt, 80% O CO2Me HO OAc trans-disubstituted Schneider, JOC 1973, 38, 951. 7 Wilkinson’s catalyst selectivity Site selectivity – Directing group effects: OH OH Cl PPh3 Rh Ph3P PPh3 H KOR, H2 (6.8 atm), benzene, 50 °C, 68% MeO OK MeO MeO PPh3 O PPh3 Rh H H cis-isomer (exclusive) note: a mixture of cis and trans isomers resulted with Pd/C MeO Base-assisted formation of the alkoxide resulted in displacement of the chloride ligand and directed olefin complexation. Thompson, JACS 1974, 96, 6232. Jardine, Prog. Inorg. Chem. 1981, 28, 63 8 Cationic catalysts Cationic catalysts are the most active homogeneous hydrogenation catalysts developed so far: PPh3 Rh PPh3 Ir Schrock-Osborn catalyst PPh3 Cl PPh3 Rh Ph3P PPh3 N Crabtree’s catalyst Substrates Wilkinson’s catalyst TOF 4000 6400 700 10 4500 650 3800 13 4000 9 Catalytically active species With bidentate ligands, olefin coordination can precede oxidative addition of H2 (S = methanol, ethanol, acetone). Rh Ph2 P P Ph2 H2 solvent = S Ph2 P S Rh S P Ph2 H Ph2 P S Rh H P S Ph2 only species observed by NMR in the absence of olefin unobservable With monodenate ligands, the hydrogenation may involve formation of a dihydride intermediate: Rh PPh3 PPh3 Catalyst precursor H2 solvent = S PPh3 S Rh S Ph3P unobservable intermediate H2 H PPh3 S Rh Ph3P H S Only species observable by NMR The difference is due to the strong trans-influence of hydride and phosphine ligands, which make unfavorable a trans H-M-PR3 structural arrangement. 10 Halpern, JACS 1977, 99, 8055; Schrock & Osborn, JACS 1976, 98, 2134. Halpern’s mechanism of hydrogenation for cationic Rh catalysts with bidentate phosphines Ph2 P Ph S Rh S P Ph2 R R = CO2Me Ph NHAc (E)-methyl 2-acetamido3-phenylacrylate NHAc Ph HN R Ph R H Ph 2 P Rh P O S Ph2 Ph2 RP NH Rh P O Ph2 observed by NMR HN observed by NMR Ph H Ph2 P R Rh P H O Ph2 H2 rate-detrmining step Steps: (1) alkene addition, (2) H2 addition, (3) migratory insertion, (4) reductive elimination of the alkane, regeneration of the catalyst. Halpern, Science 1982, 217, 401. 11 Cationic catalysts: substrate-directed hydrogenation The unsaturated cationic catalysts can bind a ligating group of the substrate in addition to the olefin. This bidentate coordination determines the selectivity of hydrogenation: Ir OH PCy3 N OH OH 2.5 mol% Me H Me Me CH2Cl2, H2 (1atm), rt H 64 : 1 6-isopropyl-3methylcyclohex-2-enol 2-isopropyl-5-methylcyclohexanol H Intermediate: Cy3P Py Ir OH H Hoveida, Chem. Rev. 1993, 93, 1307. i Me Pr Other functionalities also direct: OH OH O Me H2 / Ir cat. 97% Me Me H 56 : 1 Me O Me O Me O H2 / Ir cat. H2 / Ir cat. >99% >99% Me H 124 : 1 Me Me Me H 999 : 1 12 Asymmetric hydrogenation A bidentate, C2 symmetric version of the cationic Schrock-Osborn catalyst affords high levels of enantioselectivity in the hydrogenation of achiral enamides. This was the first demonstration that a chiral metal complex could effectively transfer chirality to a non-chiral DIPAMP - chiral (C2) substrate. Knowles, JACS 1975, 97, 2567. Ph diphosphine MeO P Rh P (S)-2-acetamido-3-phenylpropanoic acid OMe CO2H NHAc (E)-2-acetamido-3phenylacrylic acid A variety of bidentate chiral diphosphines have been synthesized and used to make amino acids by hydrogenation of enamides: NHAc H2 (1 atm), rt i-PrOH, >99% yield 93 % ee PPh2 PPh2 PPh2 O PPh2 PPh2 PPh2 PPh2 O PPh2 Chiraphos NORPHOS SKEWPHOS DIOP R PPh2 PPh2 For review on DuPhos: Burk, Acc. Chem. Res 2000, 33, 363. CO2H Ph P R P R PPh2 H H PPh2 Fe NMe2 PPh2 PPh2 R BINAP DuPHOS BICP JOSIPHOS 13 H2-hydrogenation and transfer hydrogenation of C=O (ketones, aldehydes) and C=N (imines) bond The catalytic hydrogenation of polar C=O and C=N bonds are key reactions in fine chemical and pharmaceutical synthesis. A very important group of catalysts operate by hydride transfer to the substrate in the outer coordination sphere of the complex. Hydrogen can come from H2 or from an organic donor, such as 2-propanol. H2 hydrogenation: R1R2C=Q + H2 → R1R2CH-QH Transfer hydrogenation: R1R2C=Q + DH2 → R1R2CH-QH + D e.g. DH2 = (CH3)2CH-OH and D = (CH3)2C=O 14 Metal-ligand bifunctional catalysts. Noyori has coined the term “metal-ligand bifunctional catalysts, describing systems containing an ancillary ligand cis to the hydride that assists in the hydride transfer step and this ligand must have an NH or OH (protic) group. Steps: (I) substrate addition (outer sphere), (II) simultaneous hydride and proton transfer, (III) H2 addition, (IV) regeneration of the catalyst. Morris, Coord. Chem. Rev. 2004, 248, 2201-2237. 15 Enantioselective hydrogenation of polar bonds Ruthenium complexes containing chiral diphosphine (e.g. (R)-binap) and diamine (e.g. (R,R)-diamine) ligands are very efficient enantioselective hydrogenation catalysts: Only the S-form of the alcohol is produced Note: Only trans-RuH2 are active catalysts, because of the strongly hydridic nature of transdihydrides. Morris, Coord. Chem. Rev. 2004, 248, 2201-2237. 16 Structures of the intermediate species 18-e trans-dihydride 16-e amido-hydride 17 Noyori’s transfer hydrogenation catalysts Very efficient for enantioselective transfer hydrogenation. Noyori, Acc. Chem. Res. 1997, 30, 97; JACS 2000, 122, 1466; JOC 2001, 66, 7931 18 Intermediates in Noyori’s transfer hydrogenation 18-e hydride 16-e amido complex 19