* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Atomic Structure and Periodicity
Bremsstrahlung wikipedia , lookup
Ferromagnetism wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
Bohr–Einstein debates wikipedia , lookup
Molecular orbital wikipedia , lookup
Particle in a box wikipedia , lookup
Double-slit experiment wikipedia , lookup
Quantum electrodynamics wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Auger electron spectroscopy wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Tight binding wikipedia , lookup
Matter wave wikipedia , lookup
Hydrogen atom wikipedia , lookup
Atomic theory wikipedia , lookup
Wave–particle duality wikipedia , lookup
Atomic orbital wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
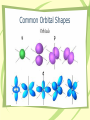
Atomic Structure and Periodicity Chapter 7 Section 7.1 Electromagnetic Radiation • The electromagnetic spectrum organizes waves by wavelength, frequency, and energy l – wavelength- the length from a point on a wave to a corresponding point later in the wave. n – frequency- the number of times a full wave cycle passes by a reference point in one second. The electromagnetic spectrum Relationships between l, n, and E • Wavelength and frequency are inversely proportional (a long wave will take longer to pass by a reference point, thus making its frequency lower). • Energy is directly related to frequency. Higher frequency waves have more energy than lower frequency waves. • The speed of a wave is constant in a vacuum. (3.0 x 108 m/s = c, speed of light) l*n=c Show Me Problem • A red light emits light of about 650 nm wavelength. What is the frequency of the red light? l*n=c 650 nm 6.5 x 10-7m (6.5x10-7)(n) = (3.0 x 108) n = 4.61 x 1014 Hz Section 7.2 The Nature Of Matter • Max Plank – Quantizes energy • Matter can absorb energy, but only in whole number ratios of the term hn. EQUATION: DE = (h*n) Plank’s constant 6.626 x 10-34 J*s Einstein’s Contribution • Albert Einstein – Quantizes Radiation • E = mc2 … Energy has mass and velocity. Electromagnetic Radiation must be made up of particles called photons. Duality of Light: Electromagnetic radiation has the capacity to behave both as a wave and a particle. DeBroglie’s Contribution • Louis DeBroglie – Determines the Duality of Matter • If waves act as particles, do particles act as waves? • Set the Einstein and the Plank equations equal to each other. mc2 = E = hc/l m = h/cl l = h/mn Duality of Matter: Electrons have the capacity to behave both as a particle and a wave. How can we be certain? • X Ray Crystallography Different color and shading patters appear as the electron “waves” cause diffraction: constructive and destructive interference with the x-rays that are exposed to the crystal. X Ray Diffraction Pattern of 2-terphenyl-4-yl-5-phenyl thiophene (PPPTP) X-Ray Diffraction Pattern of Beryl. Atomic Spectrum of Hydrogen • Extensively studied by atomic theorists such as Bohr. • High energy sparks cause hydrogen gas molecules (H-H) to break apart suddenly, with some electrons in higher energy levels than would be expected normally. • As the electrons fall back to their ground states, energy is released. • Each color in the spectrum relates to an electron in a different energy level. • Plank’s equation can be used to determine the color of the light produced or the energy of the electron that is being observed. More on the atomic spectrum of hydrogen Section 7.4 The Bohr Model • Based upon the study of the Hydrogen Spectrum, Bohr designs paths for electrons to travel while orbiting the nucleus. [ORBITS] • Each orbit corresponded to a different energy level. E = 2.178 x10 energy of e- Dr Quantum Video 18 Z 2 n2 energy level nuclear charge (protons) Show Me • An electron in a hydrogen atom moves from energy level one to energy level 2. What is the change in energy the electron experiences? E1 = -2.178 x 10-18 (12/12)= -2.178 x 10-18 E2 = -2.178 x 10-18 (12/22)= -5.445 x 10-19 DE = E2-E1 = -5.445 x 10-19-(-2.178 x 10-18) DE = 1.634 x 10-18 Joules Endo or Exo? Does this make sense? Section 7.5 The Quantum Mechanical Model of the Atom Heisenberg De Broglie Scrhödinger • Determine that if the electron acts as a standing wave (a wave that is fixed in place), then there are only certain orientations for it to exist without causing destructive interference with itself. Heisenberg Uncertainty Principle • There is a fundamental limitation to just how precisely we can know both the position and momentum of a particle. Dx * Dmn = h/4p • The more certain you are of the location of an electron, the less certain you can be of its momentum • The more certain you are of the momentum of an electron, the less certain you can be of its position. The Wave Equation • Schrodinger’s wave equation is used to define the location of electrons as waves. • A wave function is called an orbital. – ORBITALS = ORBITS • Wave functions are impossible to visualize. We picture the electron density map (aka: electron probability diagram) HY = EY Section 7.6: Quantum Numbers • Quantum numbers describe the properties of orbitals. symbol name n l ml Values Principal quantum 1, 2, etc number Angular 0 to (n-1) momentum l to -l Magnetic quantum number meaning Energy level Orbital shape Orientation of orbital n l orbital ml # of orbitals 1 0 1s 0 1 2 0 2s 0 1 2 1 2p -1, 0, 1 3 3 0 3s 0 1 3 1 3p -1, 0, 1 3 3 2 3d -2, -1, 0, 1, 2 5 4 0 4s 0 1 4 1 4p -1, 0, 1 3 4 2 4d -2, -1, 0, 1, 2 5 4 3 4f -3, -2, -1, 0, 1, 2, 3 7 Common Orbital Shapes Section 7.7 Orbital Shapes • Areas of high probability are separate by areas of low probability. (NODES) • Degenerate orbitals have different orientation or shape but the same ENERGY. • Lowest available energy level for an electron = ground state • Higher energy levels than expected = excited states Section 7.8 Electron Spin and Pauli Principle • Electrons exhibit a fourth quantum number. • Electron spin quantum number (ms) • Values of ½ and -½. Indicates magnetic moment of electron. Electrons can only spin in one of 2 opposite directions. Pauli exclusion Principle: In a given atom, no two electrons Can have the same set of Quantum numbers. Electron Configurations “Diagonal Rule” 5s 4s 3s 2s 1s 5p 4p 3p 2p 5d 4d 3d 5f 4f 3 Ways for Electron Configurations Electron Configuration Diagrams Nobel Gas Configurations Long-Hand Configurations Electron Configuration Rule Summary • Electrons enter lowest energy orbitals first. • Only two electrons per degenerate orbital. • Electrons spread out among degenerate orbitals before pairing. Exceptions to the Configuration Rules • A fully filled orbital is more stable than a partially filled orbital. • half-filled orbital is more stable than a more/less partially filled orbital. Cr Cu Eu Am Mo Ag Copper and Chromium Cr expected configuration: 1s22s22p63s23p64s23d4 Cu expected configuration: 1s22s22p63s23p64s23d9 Cr actual configuration: 1s22s22p63s23p64s13d5 Cu actual configuration: 1s22s22p63s23p64s13d10 Molybdenum and Silver Mo expected configuration: 1s22s22p63s23p64s23d104p65s24d4 Mo actual configuration: 1s22s22p63s23p64s23d104p65s14d5 Ag expected configuration: 1s22s22p63s23p64s23d104p65s24d9 Ag actual configuration: 1s22s22p63s23p64s23d104p65s14d10 Europium and Americium Eu expected configuration: Am expected configuration: 1s22s22p63s23p64s23d104p65s24d10 5p66s24f6 1s22s22p63s23p64s23d104p65s24d10 5p66s24f145d107s25f6 Eu actual configuration: Am actual configuration: 1s22s22p63s23p64s23d104p65s24d10 5p66s14f7 1s22s22p63s23p64s23d104p65s24d10 5p66s24f145d107s15f7 Electron Configuration Diagrams Noble Gas Notation Expanded Titanium : 1s22s22p63s23p64s23d2 Noble Gas Titanium: [Ar] 4s23d2 Use the noble gas that comes before the element as a benchmark, then tack on the extra occupied orbitals. Periodic Trends: Electron Configurations • Counting down tells what energy orbital. • Counting over tells how many electrons. Periodic Trends Activity • http://academic.pgcc.edu/~ssinex/excele ts/PT_interactive.xls Go to this excel sheet and click on the bottom tab labeled “atom properties” Periodic Trends: Atomic Size Increasing Atomic Size Periodic Trends: Ionization Energy Increasing 1st Ionization Energy Periodic Trends: Electron Affinity Increasing electron affinity Ion Size • Negative ions indicate a gain of electrons. • They are larger than the atom from whence they are formed. • Positive ions indicate a loss of electrons. • They are smaller than the atom from whence they are formed.