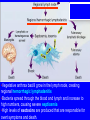
* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Brucella
Chagas disease wikipedia , lookup
West Nile fever wikipedia , lookup
Sexually transmitted infection wikipedia , lookup
Clostridium difficile infection wikipedia , lookup
Gastroenteritis wikipedia , lookup
Onchocerciasis wikipedia , lookup
Hepatitis C wikipedia , lookup
Middle East respiratory syndrome wikipedia , lookup
Rocky Mountain spotted fever wikipedia , lookup
Marburg virus disease wikipedia , lookup
Neonatal infection wikipedia , lookup
Biological warfare wikipedia , lookup
African trypanosomiasis wikipedia , lookup
Dirofilaria immitis wikipedia , lookup
Leishmaniasis wikipedia , lookup
Bioterrorism wikipedia , lookup
History of biological warfare wikipedia , lookup
Hepatitis B wikipedia , lookup
Yersinia pestis wikipedia , lookup
Sarcocystis wikipedia , lookup
Oesophagostomum wikipedia , lookup
Hospital-acquired infection wikipedia , lookup
Schistosomiasis wikipedia , lookup
Anthrax vaccine adsorbed wikipedia , lookup
Trichinosis wikipedia , lookup
Coccidioidomycosis wikipedia , lookup
Fasciolosis wikipedia , lookup
Leptospirosis wikipedia , lookup
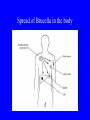
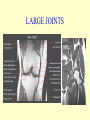
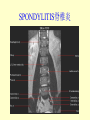
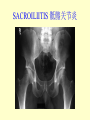
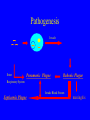
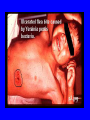
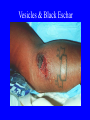
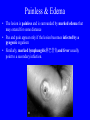
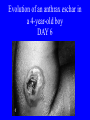
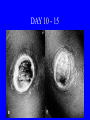
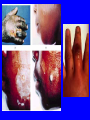
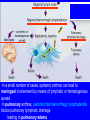
Brucella • The genus Brucella consists of six species, four of which cause human brucellosis 布 鲁菌病: Brucella melitensis 羊布鲁菌, Brucella suis 猪布鲁菌, Brucella abortus 牛布鲁菌, and Brucella canis 犬布鲁菌 • Are all intracellular organisms • B. neotomae; B. ovis • Brucella are small (0.4~0.8 ×0.5~1.5μm), non-motile, non-capasulate, gramnegative coccobacilli. • The organism is aerobic, and their nutritional requirements are complex. • All strains grow best in a medium enrich with animal serum and glucose • 5-10% carbon dioxide Antigenic Structure and classification • Two main antigen: A and M • The three main Brucella differ from one another in the amount or the two main antigen they have in common : B.abortus : A:M=20:1 B.melitensis: A:M=1:20 B.suis: A:M=2:1 B. abortus • Bacteria is excreted in genital secretions (including semen), milk, colostrum. • Survival time: Cheese at 4oC: 180 days !!! Water at 25oC: 50 days Meat and salted meat: 65 days Manure at 12oC: 250 days !!!! • Widespread: Cattle, Bison, Elk, Deer, Moose, Horse, Sheep, Goat, Swine, Donkey, Dogs, Birds, Hares, Fox, Rats, mice, Camels and Human. B. abortus • Bacteria is excreted in genital secretions (including semen), milk, colostrum. • Survival time: Cheese at 4oC: 180 days !!! Water at 25oC: 50 days Meat and salted meat: 65 days Manure at 12oC: 250 days !!!! • Widespread: Cattle, Bison, Elk, Deer, Moose, Horse, Sheep, Goat, Swine, Donkey, Dogs, Birds, Hares, Fox, Rats, mice, Camels and Human. B. abortus • Sources of Human Infection: Raw milk and products /Direct contact • Portal of entry: oral mucosa, nasopharynx and conjunctivae, genital then X in regional lymph node and spread to RES (nodes of udder, uterus, erythritol...). Placentitis with endometritis. Fetus die with edema /congestion of lung, dissimenated hemorrhages of epicardium and splenic capsule. Bacteria in lung and digestive tract of the fetus. B. suis B. melitensis • Goat (1886), Sheep, Cow (1905 in Malta), Swine, Hares, Camels, Buffalo, Impala. • Wild pigs, Rats, Swine. • Abortion,metritis, bursitis, spondylitis (Lumbar and sacral), arthritis, orchitis, paralysis. Brucella canis • Brucella canis was first described as a cause of abortion in beagles in the USA • It was subsequently shown to infect dogs in many other countries, irrespective of breed • An occasional cause of brucellosis in humans Spread of Brucella in the body Incubation period • Acute or subacute disease follows an incubation period which can vary from 1 week to 6 or more months. • In most patients for whom the time of exposure can be identified, the incubation period is between 2 and 6 weeks • The length of the incubation period may be influenced by many factors – virulence of the infecting strain – size of the inoculum – route of infection – resistance of the host Portals of entry • Oral entry - most common route – Ingestion of contaminated animal products (often raw milk or its derivatives) – contact with contaminated fingers • Aerosols – Inhalation of bacteria – Contamination of the conjunctivae • Percutaneous infection through skin abrasions or by accidental inoculation Clinical Manifestations • The presentation of brucellosis is characteristically variable • The onset may be insidious or abrupt • Influenza-like with fever reaching 38 to 40oC – Limb and back pains are unusually severe, night sweating and fatigue are marked. – Anorexia, weakness, severe fatigue and loss of weight, depression – Headache • The leukocyte count tends to be normal or reduced, with a relative lymphocytosis – Relative leukopenia • On physical examination, splenomegaly may be the only finding. COURSE OF BRUCELLOSIS • If the disease is not treated, the symptoms may continue for 2 to 4 weeks – Many patients will then recover spontaneously – Others may suffer a series of exacerbations • May produce an undulant fever in which the intensity of fever and symptoms recur and recede at about 10 day intervals. Brucellosis • Cyprus fever/Gibraltar fever/Malta fever/Rock fever/Undulant fever • Most affected persons recover entirely within 3 to 12 months • Some will develop complications – involvement of various organs, – a few may enter an ill-defined chronic syndrome. Undulant fever 39.5 37.0 COMPLICATIONS • Arthritis, often sacroiliitis, and spondylitis (in about 10 percent of cases) • central nervous system involvement including meningitis (in about 5%) • Uveitis, epididymo-orchitis • Endocarditis very rare • In contrast to animals, abortion is not a feature of brucellosis in pregnant women. LARGE JOINTS SPONDYLITIS脊椎炎 SACROILIITIS 骶髂关节炎 Chronic Brucellosis- Depression Population risk • The main source of infection for the general population is dairy produce prepared from infected milk. • B. melitensis presents the greatest hazard. • The milk of infected sheep and goats may contain large numbers of viable organisms, which become concentrated in products such as soft cheeses. • Indeed, soft cheese has been recognized as a major vehicle of infection in the Mediterranean region, the Middle East and Latin America Occupational hazard • Infection arises from occupational or domestic contact with infected animals or with an environment contaminated by their discharges • Farmers and their families, abattoir workers, butchers and veterinarians are particularly at risk • Infected animals that have recently aborted or given birth present the greatest hazard Extending spectrum of zoonosis • The recent isolation of distinctive Brucella strains, tentatively named Brucella maris, from marine animals in the United Kingdom and the United States extends the ecologic range of the genus and, potentially, its scope as a zoonosis – seals, sea otters, dolphins and porpoises • An incident of laboratory-acquired infection suggests that this type is pathogenic for humans • Infection could result from occupational contact with infected seals or cetaceans. CLINICAL DIAGNOSIS Sanitary • Pasteurization of dairy products and use of protective clothing prevent human infection. More importantly, systematic identification and elimination of infected animals and vaccination of animals reduces the reservoir. Prevention • Eradication of brucellosis in cattle can be attempted by test and slaughter,active immunization of heifers with avirulent live strain 19,combined testing,segregation, and immunization.Cattle are examined by means of agglutination tests • Active immunization of humans against brucella infection is experimental.Control rests on limitation of spread and possible eradication of animal infection,pasteurization of milk and milk products, and reduction of occupational hazards wherever possible. Treatment • Brucella may be susceptible to tetracyclines or ampicillin. • Symptomatic relief may occur within a few days after treatment with these drugs is begun. • However ,because of their intracellular location,the organisms are not readily eradicated completely from the host. • For best results,treatment must be prolonged.Combined treatment with streptomycin and a tetracycline may be considered YERSINIA Enterobacteriaceae Genus Escherichia Genus Yersinia • Y. pestis • Y. enterocolitica • Y. pseudotuberculosis Biological Features – Small, 0.5-0.8 μm in width 1.0-2.0 μm in length. – Gram-negative rods. – Sometimes appearing as cocco bacilli. – Bipolar Staining:Retaining stain at the ends of cells. Biological Features • Cultural Features – Facultative anaerobes. – Optimal growth temperature range form 28˚C to 30˚C. – Optimal growth pH: 6.9~7.2. – Growth is more rapid in media containing blood or tissue fluids. – Nonmotile when grow at temperatures above 30 ˚C. Pathogenicity Transmission: Flea Bite Respiratory Tract Antigenic Structure • • • • F1 Antigen: V,W Antigen: Yersinia Outer membrane Protein (Yop) Murine Toxin (MT) 0.3%-0.4% formaldehyde Toxoid • Endotoxin (LPS) Ca2+ Dependent V-W Gene Gene Plasmid W Antigen V Antigen F1 Gene F1 Antigen Plasmid LPS Y. Pestis Virulence factors schematic diagram Pathogenesis Invade Lymph Nodes In Groin and Axilla Phagocyte Y. pestis Enter Pneumonic Plague Bubonic Plague Respiratory System Invade Blood Stream Septicemic Plague meningitis Pathogenicity • Clinical Forms : – Bubonic Plague: High fever, Swelling, Bleeding, Necrosis of lymph nodes – Pneumonic Plague: chills, cough, respiratory failure, circulatory collapse ——Black Death – Septicemic Plague: Fever (39-40 ˚C) , Shock , DIC Y.Enterocolitica & Y.Pseudotuberculosis • Gram negative, No capsule, No spore, Facultative anaerobes • V-W antigen • More than 50 serotypes of Y.Enterocolitica 6 serotypes of Y.Pseudotuberculosis • Diseases: – – – – Gastroenteritis terminal ileitis, appendicitis, mesenteric lymphadenitis, dermatitis contusiformia, arthritis Septicemia • Sanitary precautions, Antibiotic Epidemiology • Plague – Probably originated in Asia or central Africa. – One of the earliest record pandemics occurred in 542 B.C. – Three pandemics in the history. – 1989-1998:5440 cases, 681 dead. Immunity Humoral Immunity Cellular Immunity Antibody To: 1) F1 Ag 2) V,W Ag Phagocytose Promote phagocytose , agglutinate and kill bacteria Diagnosis • A. Specimens: – – – – B. Aspirates of lymph nodes Cerebrospinal fluid Blood Sputum Smears: Giemsa’s stain immunofluorescent stain Diagnosis • C. Culture: – All materials Cultured on blood agar and MacConkey’s agar and in infusion broth – Positive in 24 hours – Tentatively identified by biochemical reations Definite identified by immunofluorescence CAUTION: All cultures are highly infectious and must be handled with extreme caution Diagnosis • D. Serology: In patients who have not been previously vaccinated, a convalescent serum antibody titer of 1:16 or greater is presumptive evidence of Y.pestis infection.A titer rise in two sequential specimens confirms the serologic diagnosis. Treatment • Streptomycin • Tetracycline: alternative drug combination with streptomycin essential for control early in disease • Sulfonamides Summary of Yersinia infections Bacillus Spore-Forming Gram-Positive Bacilli: Bacillus Species • At least 48 species are known but only • B. anthracis and B. cereus cause defined diseases in humans. B. anthracis is responsible for the disease anthrax. This is a disease primarily of animals (zoonosis) but humans can acquire via handling, inhaling or ingesting contaminated animal products. B. cereus is predominantly responsible for food poisoning in humans. Bacitracin and polymyxin are two well-known antibiotics obtained from Bacillus species. Spores of many Bacillus species are resistant to heat, radiation, disinfectants and desiccation • It was from studies on anthrax that Koch established his famous postulates in 1876 • Pasteur (1881) developed a vaccine against anthrax B. anthracis Gram stain demonstrating spores B. anthracis, Colony on SBA “STICKY” Consistency of B. anthracis’ Colony on SBA Anthrax infections are classified by route of entry • Cutaneous • Gastrointestinal • Respiratory Cutaneous Anthrax • • • • • > 95% of naturally occurring cases Spores enter breaks in skin after contact with contaminated animal products Papule丘疹- Vesicle水泡- Ulcer - Eschar焦痂 Up to 20% case fatality rate if untreated Mortality with treatment < 1% • After a 2- to 3-day incubation period, a small pimple or papule appears at the inoculation site. • A surrounding ring of vesicles develops • Over the next few days, the central papule ulcerates, dries, and blackens to form the eschar Vesicles & Black Eschar Painless & Edema • The lesion is painless and is surrounded by marked edema that may extend for some distance • Pus and pain appear only if the lesion becomes infected by a pyogenic organism • Similarly, marked lymphangitis淋巴管炎and fever usually point to a secondary infection. Evolution of an anthrax eschar in a 4-year-old boy DAY 6 DAY 10 - 15 • Evolution of an anthrax eschar in a 4-year-old boy. (A&B) the lesion when first seen (day 0).Note the arm swollen from the characteristic edema. (C) Day 6. (D) Day 10. (E) Day 15. Although penicillin treatment was begun immediately and the lesion was sterile by about 24 hours, it continued to evolve and resolve as seen. Cutaneous anthrax Differential diagnosis Ecthyma gangrenosum Rat-bite fever Pseudomonas aeruginosa Streptobacillus moniliformis, Spirillum minor Ulceroglandular tularemia Francisella tularensis Plague Yersinia pestis Glanders Pseudomonas pseudomallei Rickettsialpox Rickettsia akari Orf Parapoxvirus Staphylococcal lymphadenitis Staphylococcus aureus Cutaneous tuberculosis Myocbacterium tuberculosis Leprosy Mycobacterium leprae Buruli ulcer Mycobacterium ulcerans Cutaneous anthrax For cutaneous and gastrointestinal anthrax, low-level germination occurs at the primary site, leading to local edema and necrosis Inhalation • Bacillus spores are inhaled and ingested by alveolar macrophages肺泡巨噬细胞 • These cells carry the bacteria to the regional lymph nodes, causing necrotic hemorrhaging which leads to death Gastrointestinal • Ingestion of contaminated meat produces systemic symptoms which can lead to death • Mortality by gastrointestinal anthrax may be 50% Gastrointestinal and pulmonary anthrax are both more dangerous than the cutaneous form because they are usually identified too late for treatment to be effective PATHOGENESIS • Anthrax infections result only if the bacteria produce a – i) capsule (poly-y-D-glutamic acid polypeptide) – ii) exotoxins – both encoded on plasmids • • • • three proteins protective antigen (PA) (82. 7 kDa) lethal factor (LF) (90.2 kDa) edema factor (EF) (88.9 kDa) ANTHRAX TOXINS 20 kDa PA PA LF EF Host Protease PA The complex (PA+LF or PA+EF) is internalized by endocytosis acidification of the endosome HOST CELL LF the LF or EF cross the membrane into the cytosol via PA-mediated ion-conductive channels Effects of anthrax exotoxins on macrophages • Edema toxin is a calmodulin钙调节蛋白dependent adenylate cyclase that increases intracellular levels of cyclic AMP (cAMP) on entry into most types of cell • This is believed to alter water homeostasis – resulting in massive edema Effects of anthrax exotoxins on macrophages • Lethal toxin is a zinc metallo-protease that causes a hyperinflammatory condition in macrophages – activating the oxidative burst pathway • release of reactive oxygen intermediates – production of proinflammatory cytokines • responsible for shock and death. • MAPKK denotes mitogenactivated protein kinase kinase •Endospores are phagocytosed by macrophages and germinate •Macrophages containing bacilli detach and migrate to the regional •Vegetative anthrax bacilli grow in the lymph node, creating regional hemorrhagic lymphadenitis •Bacteria spread through the blood and lymph and increase to high numbers, causing severe septicemia •High levels of exotoxins are produced that are responsible for overt symptoms and death. •In a small number of cases, systemic anthrax can lead to meningeal involvement by means of lymphatic or hematogenous spread •In pulmonary anthrax, peribronchial hemorrhagic lymphadenitis blocks pulmonary lymphatic drainage • leading to pulmonary edema Once they have been released from the macrophages, there is no evidence that an immune response is initiated against vegetative bacilli Protective immunity • Antibodies against protective antigen • Both the noncellular human vaccines and live-spore animal vaccines confer protection by eliciting antibodies to protective antigen • The poly-g-D-glutamic acid capsule of B anthracis is poorly immunogenic, and antibodies to the polysaccharide and other components of the cell wall are not protective. Species differences • Anthrax has been documented in a wide variety of warm-blooded animals • Some species, such as rats, chickens, and dogs, are quite resistant to the disease • Others (notably herbivores such as cattle, sheep, and horses) are very susceptible • Humans have intermediate susceptibility. reservoir of B anthracis is contaminated soil • Spores remain viable for long periods • Herbivores, the primary hosts, become infected when foraging in a contaminated region • Because the organism does not depend on an animal reservoir, it cannot readily be eradicated from a region – anthrax remains endemic in many countries • Humans become infected almost exclusively through contact with infected animals or animal products Cycle of infection in nature • As a susceptible animal with anthrax approaches death, its blood contains as many as 109 bacilli/ml • Necrosis of the walls of small blood vessels during the acute phase of the illness leads to hemorrhages and to characteristic bloody exudations from the mouth, nose, and anus, a highly diagnostic sign • These exudates carry vast numbers of the bacilli – sporulate on exposure to air – produce a heavily contaminated environmental site – potentially capable of infecting other animals for many years Handling of carcasses动物尸体 • Sporulation of B anthracis requires oxygen – therefore does not occur inside a closed carcass – regulations in most countries forbid postmortem examination of animals when anthrax is suspected – The vegetative cells in the carcass are killed in a few days by the process of putrefaction. • In endemic areas, animals that die suddenly should be handled cautiously • Livestock should be vaccinated annually. Do I look that I am going to die? Non-Industrial vs Industrial Anthrax • Nonindustrial anthrax • usually affects people who work with animals or animal carcasses – farmers, veterinarians, butchers – almost always cutaneous • Industrial anthrax • acquired from handling contaminated hair, hides, wool, bone meal, or other animal products – higher chance of being pulmonary as a result of the inhalation of spore-laden dust Transmission Clinical syndrome Who is at risk Injured skin or mucous membranes inoculated by spores from the soil or a contaminated animal or carcass Skin infection begins as a raised itchy bump and within 1-2 days develops into a vesicle and then a painless ulcer, usually 1-3 cm in diameter, with a characteristic black necrotic area in the center. Lymph glands in the adjacent area may swell. People in endemic areas in contact with infected animals of contaminated soils; people who work with animals materials (hides, fur, wool, hair) imported from endemic area, such as farmers, veterinarians, knackers, butchers and laboratorians. Intestinal anthrax The ingestion of poorly cooked meat or milk from infected animals Initial signs of nausea, loss of appetite, vomiting, and fever are followed by abdominal pain, vomiting of blood, and severe diarrhea. Pulmonary anthrax Inhalation of sporecontaining dust where animal hair or hides are being handled begins abruptly with high fever and chest pain, progresses rapidly to a systemic hemorrhagic pathology Cutaneous anthrax Bacillus Cereus蜡样芽胞杆菌 • B. cereus food poisoning results from the ingestion of preformed enterotoxins, producing predominantly vomiting and diarrhea. • The vomiting form is most often associated with ingestion of a heat stable toxin from contaminated rice, while the diarrheal form is most often associated with ingestion of a heat labile toxin from contaminated meat or vegetables B cereus virulence factors • A 38 to 46-kDa protein complex has been shown in animal models: – to cause necrosis of the skin or intestinal mucosa – to induce fluid accumulation in the intestine – a lethal toxin • Responsible for the necrotic and toxemic nature of severe B cereus infections and for the diarrheal form of food poisoning Bacillus cereus also produces two hemolysins Phospholipases produced by B cereus may act as exacerbating factors by degrading host cell membranes following exposure of their phospholipid substrates in wounds or other infections Bacillus Food Poisoning Two Distinct Types • Diarrheal type • diarrhea and abdominal pain • 8 to 16 hours after consumption of the contaminated food • Associated with a variety of foods, including meat and vegetable dishes, sauces, pastas, desserts, and dairy products • Emetic呕吐 disease • nausea and vomiting begin 1 to 5 hours after the contaminated food is eaten • Boiled rice that is held for prolonged periods at ambient temperature and then quick-fried before serving is the usual offender, although dairy products or other foods are occasionally responsible.