* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Short-Acting Я-Adrenergic Antagonist Esmolol Given at
Survey
Document related concepts
Remote ischemic conditioning wikipedia , lookup
Heart failure wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Electrocardiography wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Antihypertensive drug wikipedia , lookup
Cardiac surgery wikipedia , lookup
Jatene procedure wikipedia , lookup
Coronary artery disease wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Cardiac arrest wikipedia , lookup
Transcript
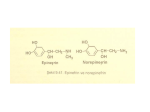
Short-Acting -Adrenergic Antagonist Esmolol Given at Reperfusion Improves Survival After Prolonged Ventricular Fibrillation Cheryl R. Killingsworth, DVM, PhD; Chih-Chang Wei, PhD; Louis J. Dell’Italia, MD; Jeffrey L. Ardell, PhD; Melody A. Kingsley; William M. Smith, PhD; Raymond E. Ideker, MD, PhD; Gregory P. Walcott, MD Downloaded from http://circ.ahajournals.org/ by guest on August 11, 2017 Background—High catecholamine concentrations are cytotoxic to cardiac myocytes. We hypothesized that myocardial interstitial catecholamine levels are greatly elevated immediately after long-duration ventricular fibrillation (VF), defibrillation, and reperfusion and that the short-acting -antagonist esmolol administered at reperfusion would protect against this catecholamine surge and improve survival. Methods and Results—In part 1 of this study, catecholamines from myocardial interstitial fluid (ISF) and aortic and coronary sinus plasma were quantified by use of 3H-labeled radioenzymatic assay in 8 open-chest, anesthetized pigs. Eight minutes of electrically induced VF was followed by internal defibrillation and reperfusion. By 4 minutes of VF, ISF norepinephrine increased significantly, from 1.3⫾0.3 to 7.4⫾2.4 ng/mL. Epinephrine increased significantly, from 0.4⫾0.2 to 1.5⫾0.7 ng/mL. ISF norepinephrine and epinephrine peaked at 219.2⫾92.1 and 63.7⫾25.1 ng/mL after defibrillation and reperfusion and decreased significantly to 12.2⫾3.5 and 6.7⫾3.1 ng/mL 23 minutes after defibrillation. Transcardiac catecholamine changes were similar. In part 2, 8 minutes of VF was followed by external defibrillation in anesthetized, closed-chest pigs. Animals received 1.0 mg/kg esmolol (n⫽8) or saline (n⫽8) intravenously at the start of cardiopulmonary resuscitation (CPR). Advanced cardiac life support, including CPR and epinephrine, was delivered to both groups. Esmolol before reperfusion improved return of spontaneous circulation and 4-hour survival (7/8 versus 3/8 survivors, 2 P⬍0.05). Conclusions—Transcardiac and ISF norepinephrine and epinephrine levels are briefly massively elevated after 8 minutes of VF, defibrillation, and reperfusion. A short-acting -antagonist administered immediately after defibrillation improves return of spontaneous circulation and 4-hour survival after this prolonged VF. (Circulation. 2004;109:24692474.) Key Words: catecholamines 䡲 cardiopulmonary resuscitation 䡲 norepinephrine 䡲 defibrillation E lectric countershock is highly effective in stopping ventricular fibrillation (VF) in patients with sudden cardiac death. However, return of spontaneous circulation (ROSC) is less common, occurring in ⬇76% of these patients, and survival to hospital discharge is much lower, at 28%.1 The mechanism(s) of myocardial dysfunction after resuscitation is poorly understood. Plasma catecholamines, an indirect measure of myocardial catecholamines, rise markedly with cardiac arrest to a level that can increase myocardial oxygen demand, aggravate myocardial ischemia, induce malignant arrhythmias, and be cytotoxic.2–6 Animal studies have suggested that -adrenergic receptor blockade may decrease postresuscitation myocardial dysfunction and improve survival.2,5 Despite studies reporting large increases in plasma catecholamines during cardiopulmonary resuscitation, cat- echolamine levels in the myocardium during global ischemia that occur with prolonged VF have been difficult to measure. In this study, we used microdialysis methods to quantify the magnitude and time course of release of the endogenous catecholamines norepinephrine and epinephrine into the interstitial fluid (ISF) of the left ventricle (LV) during prolonged VF when there is no blood flow and during reperfusion. In part 1, we hypothesized that intramyocardial tissue catecholamine levels are greatly elevated for a short time after prolonged VF, defibrillation, and reperfusion. In part 2, we hypothesized that the short-acting -antagonist esmolol at reperfusion protects against this catecholamine surge and improves survival after prolonged VF. Received November 18, 2003; revision received February 3, 2004; accepted February 6, 2004. From the Cardiac Rhythm Management Laboratory, Division of Cardiovascular Diseases, Departments of Medicine (C.R.K., C.-C.W., L.J.D., M.A.K., R.E.I., G.P.W.), Biomedical Engineering (W.M.S., R.E.I.), Veterans Affairs (L.J.D.), and Physiology (R.E.I., L.J.D.), University of Alabama at Birmingham, and the Department of Pharmacology (J.L.A.), East Tennessee State University, Johnson City. Dr Ideker has a research contract with Medtronic Physio-Control, Inc, Redmond, Wash. Correspondence to Cheryl R. Killingsworth, DVM, PhD, Cardiac Rhythm Management Laboratory, 1670 University Blvd, B140 Volker Hall, Birmingham, AL 35294-0019. E-mail [email protected] © 2004 American Heart Association, Inc. Circulation is available at http://www.circulationaha.org DOI: 10.1161/01.CIR.0000128040.43933.D3 2469 2470 Circulation May 25, 2004 Methods Animal Preparation Pigs were obtained from the University of Alabama at Birmingham Animal Resource Center and were managed in accordance with the guidelines established in the Position of the American Heart Association on Research Animal Use.7 The University of Alabama at Birmingham Institutional Animal Care and Use Committee approved the experimental protocols. Anesthesia was induced with intramuscular atropine (0.04 mg/kg), zolazepam-tiletamine (4.4 mg/kg), and xylazine (4.4 mg/kg). Animals were intubated, and anesthesia was maintained by inhalation of isoflurane (1.75% to 2.0%) delivered in 100% oxygen. Intravenous succinylcholine (3 to 6 mg/min) was administered before defibrillation. Animals were given intravenous 0.9% saline solution and mechanically ventilated. A lead II ECG was continuously monitored. Part 1: Effect of VF, Defibrillation, and Reperfusion on Catecholamines and Ventricular Function Downloaded from http://circ.ahajournals.org/ by guest on August 11, 2017 Eight mixed-breed pigs (41⫾4.2 kg) were studied. The chest was opened via median sternotomy and the heart suspended in a pericardial cradle. The left stellate ganglion was isolated for placement of stimulating electrodes. Catheters were placed in the descending aorta and into the coronary sinus (CS) for blood sampling. High-fidelity pressure transducers were inserted into the abdominal aorta and the LV for measurement of systemic arterial pressure and LV pressure. Two pairs of 2-mm piezoelectric transducers were placed on the endocardial surface of the LV on the major and minor axes. A tourniquet was placed around the inferior vena cava. The aortic root and right atrial appendage were cannulated for resuscitation by use of a cardiopulmonary bypass circuit. Intravenous heparin (10 000 U) was administered just before placement of the cannulae and repeated every hour (1000 U). Two microdialysis probes were inserted into the lateral wall of the LV myocardium midway between the apex and base of the heart.8 Two hours passed between probe placement and experimental measurements to allow cell damage products to wash out of the dialysis probes.9 Saline was infused through the probes continuously at a rate of 2.5 L/min, and 18.8% recovery was used in the calculation of ISF norepinephrine and epinephrine.8 Catecholamine concentrations were determined with the Biotrak catecholamine 3 H-labeled radioenzymatic assay (Amersham Pharmacia Biotech).8 ISF from the LV wall was collected through the microdialysis tubes for 10 minutes (baseline). ISF was then collected for 3 minutes during left stellate ganglion stimulation at 2 times the threshold that evoked an increase in systemic arterial pressure (5 ms, 7 to 15 mA). A 30-minute recovery period was followed by a second 10-minute baseline ISF dialysate collection. VF was then electrically induced and continued for 8 minutes. Eight minutes of VF was chosen because it is clinically relevant relative to emergency response system arrival1 and because by logistic regression models, this time period offers a realistic chance of influencing survival.10 ISF was collected continuously at 0 to 2, 2 to 4, 4 to 6, and 6 to 8 minutes of VF. The heart was defibrillated with a biphasic waveform at 20 to 30 J and internal paddles. Thirty seconds after successful defibrillation, cardiopulmonary bypass was started at 1 L/min to provide reperfusion without disrupting the dialysis catheters. ISF continued to be collected at 8 to 10, 10 to 12, 12 to 15, 15 to 18, 18 to 22, 22 to 26, and 26 to 31 minutes after the initiation of VF. After another 30-minute recovery period, a second left stellate stimulation was performed with ISF collection. Aortic and CS blood samples were collected at baseline and at 10, 15, 22, and 31 minutes after the initiation of VF and before and at the end of stellate stimulation. LV pressure and sonomicrometry dimension measurements were collected at baseline before VF induction and at the end of reperfusion with the animal off cardiopulmonary bypass to quantify cardiac function as determined by preload recruitable stroke work (PRSW).11 Data were collected during transient (15 seconds) preload reduction by vena cava occlusion. Cardiac function was measured over the same time course in 8 similarly instrumented, anesthetized control animals without induction of VF. At the end of the study, the animals were euthanized with intravenous potassium chloride solution. Part 2: Effect of the Short-Acting -Adrenergic Antagonist Esmolol on Survival After VF Sixteen mixed-breed pigs (30 to 40 kg) were anesthetized and instrumented as described above to measure systemic arterial and LV pressures. A third high-fidelity catheter was placed at the junction of the superior vena cava and the right atrium for measurement of central venous pressure and determination of coronary perfusion pressure. Animals were studied closed-chest with self-adhesive defibrillation electrodes located on the chest wall. Animals were randomized to receive either esmolol (1 mg/kg IV) or saline placebo after defibrillation. The investigator was blinded to the treatment. Eight minutes of electrically induced VF was followed by external biphasic waveform defibrillation beginning at 3 J/kg. If the initial shock failed, energy was increased in 1-J/kg increments. After defibrillation, esmolol or saline was injected as cardiopulmonary resuscitation (CPR) began. External compressions were delivered at 100 compressions per minute by the investigator and monitored by a CPR-plus device (Kelly Medical Products). Ventilation was delivered with a mechanical respirator. Intravenous epinephrine (0.01 mg/kg) was given every 3 minutes if systolic blood pressure was ⬍50 mm Hg. If after 1 hour, the animal did not maintain a systolic blood pressure ⬎50 mm Hg, dobutamine was infused (5 g · kg⫺1 · min⫺1) for 2 hours and then stopped. If the animal was successfully resuscitated, it was monitored for 4 hours after VF induction. The study was ended if there was no ROSC after 30 minutes of CPR. Statistical Analyses Results are expressed as the mean⫾SD. Continuous variables were compared by use of repeated-measures ANOVA. Differences between 2 variables were determined by use of Student’s t test. PRSW was constructed by linear regression of the beat-by-beat stroke work versus end-diastolic volume during vena cava occlusion.11 The slopes of the lines at baseline and at the end of the study were compared. Differences in the primary end point of survival at 4 hours were compared by use of a 2 test. Results were considered significant at a value of P⬍0.05. Results Part 1: ISF Norepinephrine and Epinephrine Measurements During Left Stellate Stimulation, VF, Defibrillation, and Reperfusion Baseline left stellate stimulation doubled ISF norepinephrine from 1.6⫾0.5 to 3.2⫾1.1 ng/mL after 3 minutes of left stellate stimulation (P⬍0.05). ISF epinephrine increased 4-fold from 0.3⫾0.1 to 1.1⫾0.5 ng/mL during the same stimulation period (P⬍0.05). By 2 minutes of VF, ISF norepinephrine had increased 5-fold from 1.3⫾0.3 to 6.8⫾2.9 ng/mL and remained elevated throughout VF (Figure 1). ISF norepinephrine after defibrillation and reperfusion increased to a peak 164 times the baseline ISF level (219.2⫾92.1 ng/mL, Figure 2). ISF norepinephrine then decreased with time but did not return to baseline during the 30 minutes of measurement. ISF epinephrine followed a trend similar to ISF norepinephrine. Baseline ISF epinephrine levels were 0.4⫾0.2 ng/mL and increased to 1.0⫾0.5 ng/mL by 2 minutes of VF (Figure 1), remaining elevated throughout VF (Figure 2). Defibrillation and reperfusion resulted in a similar increase in ISF epinephrine that peaked at 12 minutes (4 minutes after Killingsworth et al Esmolol and VF Survival After Catecholamine Surge Downloaded from http://circ.ahajournals.org/ by guest on August 11, 2017 Figure 1. ISF norepinephrine and epinephrine levels in 8 anesthetized, open-chest pigs during 8 minutes of VF. Values are mean⫾SD. defibrillation and reperfusion) at a level 170 times baseline and did not return to baseline levels during the study. After the massive catecholamine release that occurred after defibrillation and reperfusion, the ability of the sympathetic nervous system to release more catecholamines was tested 2471 Figure 3. Example of PRSW in 1 animal, an indicator of myocardial performance that is independent of load, geometry, and heart rate. Slope was decreased by 45.6% after VF and cardiopulmonary resuscitation CPR compared with baseline, indicating a severe functional deficit. with the second left stellate stimulation at the end of the study. After 3 minutes of stellate stimulation, ISF norepinephrine increased significantly, 1.3 times, from 2.3⫾0.7 to 3.1⫾1.1 ng/mL. ISF epinephrine increased significantly, 1.8 times, from 1.4⫾0.7 to 2.5⫾1.5 ng/mL. Arterial and CS Norepinephrine and Epinephrine Measurements Figure 2. ISF and plasma norepinephrine and epinephrine levels obtained from aorta and CS before and after 8 minutes of VF. Internal defibrillation was first attempted at 8 minutes, and reperfusion began 30 seconds after successful defibrillation. ISF catecholamine levels were lower than plasma levels at 10 and 12 minutes (2 to 4 minutes after defibrillation) but remained higher than plasma levels at all subsequent measurement points. *P⬍0.05 from baseline. Aortic norepinephrine and epinephrine both increased 3-fold after left stellate stimulation from 0.070⫾0.03 to 0.2⫾0.10 ng/mL and from 0.08⫾0.03 to 0.2⫾0.1 ng/mL, respectively (P⬍0.05). CS norepinephrine and epinephrine also increased significantly, from 0.1⫾0.08 ng/mL at baseline to 0.3⫾0.1 ng/mL and from 0.1⫾0.07 to 0.3⫾0.2 ng/mL after 3 minutes of left stellate stimulation. Aortic norepinephrine and epinephrine before VF were 0.11⫾0.05 and 0.14⫾0.04 ng/mL, respectively. CS norepinephrine and epinephrine were 0.13⫾0.07 and 0.17⫾0.09 ng/mL. Plasma catecholamines from both the aorta and CS increased greatly after defibrillation and reperfusion (Figure 2). Peak norepinephrine and epinephrine levels of 480.5⫾131.6 and 152.7⫾35.7 ng/mL, respectively, were measured in aortic plasma 10 minutes after the beginning of VF (⬇2 minutes after defibrillation and reperfusion, Figure 2). A similar peak was detected in CS plasma at the same time point (368.9⫾159.0 and 106.2⫾54.5 ng/mL, respectively). Norepinephrine and epinephrine concentrations in plasma then decreased but never reached baseline levels during the study (Figure 2). At the end of the study, a second left stellate stimulation resulted in significant increases in aortic norepinephrine from 0.7⫾0.3 to 1.0⫾0.4 ng/mL and epinephrine from 0.5⫾0.1 to 0.7⫾0.3 ng/mL. CS norepinephrine increased from 0.7⫾0.4 to 1.0⫾0.5 ng/mL (P⬍0.05), and epinephrine increased from 0.5⫾0.2 to 0.7⫾0.4 ng/mL. LV Function After prolonged VF, defibrillation, and resuscitation, global contractility was markedly decreased (Figure 3). The mean slopes of the stroke work–LV end-diastolic volume relation- 2472 Circulation May 25, 2004 Resuscitation Data Placebo Esmolol Mean No. of shocks 10⫾8 5⫾4 Total CPR time, min 27⫾15 13⫾13 Total CPR time (survivors only), min 9⫾8 9⫾7 Coronary perfusion pressure, mm Hg 15⫾7 17⫾7 1/3 2/7 Animals requiring dobutamine Downloaded from http://circ.ahajournals.org/ by guest on August 11, 2017 ship decreased from 35.2⫾13.62 mm Hg⫻mL at baseline to 11.1⫾9.26 mm Hg⫻mL 31 minutes after VF (P⬍0.01). The x intercept was unchanged. The peak ⫹dP/dt was unchanged from baseline (100.3%), but the peak ⫺dP/dt was decreased to 59.5% of baseline. Significant decreases from baseline were also measured relative to LV systolic pressure (69.4%), pulse pressure (69.4%), and stroke volume (45.8% of baseline). In a separate group of 8 control pigs, the PRSW slope after a similar time period was 92.7% of baseline, suggesting that time and anesthesia were insignificant factors in decreasing LV function. Part 2: Effect of Short-Acting -Adrenergic Blockade on Survival After Prolonged VF The survival rate was significantly higher for the esmolol group than for the placebo (Table). Seven of 8 animals survived 4 hours in the group receiving esmolol, whereas 3 of 8 animals survived to 4 hours in the control group. The maximum systolic arterial pressure after ROSC was significantly lower in the esmolol group (143⫾27 mm Hg, n⫽7) than the placebo group (174⫾13 mm Hg, n⫽3), suggesting that esmolol blunted the hemodynamic response to endogenous catecholamines on ROSC. All animals that had ROSC survived to 4 hours. There was a trend toward a greater number of shocks being delivered after initial successful defibrillation in the placebo group (9.6⫾8) compared with the esmolol group (5.2⫾4). Furthermore, the esmolol group received a total of 41 shocks during the study, whereas the control group received 75 shocks (Figure 4). There was no significant difference in the CPR time or coronary perfusion pressure between the 2 groups. The same proportion of animals in each group that had ROSC required dobutamine Figure 4. Histogram showing number of shocks delivered as a function of time since VF onset for control (black bars) and esmolol-treated animals (open bars). Same number of shocks was initially required to defibrillate both control and esmololtreated animals, but controls required significantly more shocks during resuscitation than esmolol-treated animals. support (Table). All animals were easily weaned from dobutamine after 2 hours of inotropic support. Systolic and diastolic function, as measured by peak ⫹dP/dt and peak ⫺dP/dt of the LV pressure trace, were not different between baseline and at 4 hours in the esmololtreated survivors (1142⫾221 versus 1176⫾235 and ⫺1403⫾157 versus ⫺1478⫾208), suggesting that esmolol treatment did not have a long-term effect on postresuscitation cardiac function in these animals. Discussion The major findings of this study are as follows. (1) ISF norepinephrine and epinephrine levels rise to ⬇150 times baseline in the first few minutes after 8 minutes of VF, defibrillation, and reperfusion, and (2) a single dose of the short-acting -adrenergic antagonist esmolol at reperfusion improves ROSC and survival in this swine model of prolonged VF and resuscitation. Other important findings include the following. (1) ISF norepinephrine and epinephrine increase 5-fold during VF; (2) plasma epinephrine and norepinephrine levels peak earlier and higher than ISF levels after reperfusion and then decrease rapidly to levels below ISF values, suggesting that in this model, plasma levels are not good estimates of ISF levels; and (3) both norepinephrine and epinephrine are released by cardiac nerves in the heart after prolonged VF and defibrillation. In vivo microdialysis techniques have made it possible to directly determine myocardial interstitial catecholamine levels. This is important because the sympathetic nerves innervating the heart produce both norepinephrine and epinephrine and can reuptake catecholamines extracted from the vascular compartment.8,12,13 Baseline ISF norepinephrine and epinephrine concentrations in this study were severalfold higher than arterial and CS plasma catecholamine levels. These data demonstrate the concentration gradient for catecholamine spillover into the circulation and are consistent with other studies in which catecholamine concentrations at sites of release are much higher than in plasma.8,14,15 Increased myocardial catecholamine release into the ISF has been reported during stellate ganglion stimulation and after infusions of angiotensin II, exogenous catecholamines, tyramine, and desipramine.8,13,15 Measurement of catecholamines by use of microdialysis methods has proved to be especially useful in settings of diminished blood flow to the tissue, such as during regional ischemia. Lameris et al12 reported that temporary coronary artery occlusion in anesthetized pigs results in a progressive increase in ISF catecholamines in the ischemic region. To the best of our knowledge, the present study is the first quantification of myocardial interstitial catecholamine levels during global ischemia induced by prolonged VF, defibrillation, and resuscitation. Significant increases in both ISF norepinephrine and epinephrine occurred during VF that were 1.5 to 2 times greater than catecholamine levels measured during electrical stimulation of sympathetic neurons and up to 5 times greater than baseline ISF catecholamine levels. Because there is essentially no blood flow during VF, these increases must have come directly from the heart. In addition, huge increases in arterial norepinephrine of 4000 times and Killingsworth et al Esmolol and VF Survival After Catecholamine Surge Downloaded from http://circ.ahajournals.org/ by guest on August 11, 2017 epinephrine of 1000 times baseline indicated a massive increase in sympathetic nervous activity and huge adrenomedullary neuroendocrine release after 8 minutes of hypoxia, hypotension, and global ischemia caused by VF. The marked increases in norepinephrine and epinephrine plasma CS levels were probably the end result of neuronal co-release and spillover because of failure of organ extraction and reuptake mechanisms. These data further indicate that measurement of plasma catecholamine levels alone does not provide an accurate dynamic estimate of catecholamine levels in the heart after defibrillation and subsequent reperfusion. Stimulation of the stellate ganglia at the end of the experiment after massive local and systemic release of catecholamines indicated that sympathetic nerve terminals remained functional after 8 minutes of global ischemia, but the absolute catecholamine increase was less than at the beginning of the experiment. In addition, ISF and plasma catecholamines remained elevated and had not returned to the initial baseline levels when measurements were obtained before the second stellate stimulation, indicating that both myocardial and systemic catecholamines were high 1 hour after the beginning of VF. Despite massive endogenous release of catecholamines during VF, exogenous epinephrine has been considered an essential drug in cardiopulmonary resuscitation.16 Several studies have suggested that the primary benefit of epinephrine is mediated through peripheral ␣-adrenergic vasoconstriction and increased coronary perfusion pressure. Epinephrinemediated -adrenergic effects may be harmful by increasing the myocardial oxygen consumption of the ischemic fibrillating heart at a time when metabolic demand of the heart is already increased and energy stores are low, predisposing to postdefibrillation dysfunction and cardiac arrhythmias.17–21 Exogenous epinephrine can also alter activity within the cardiac nervous system and thereby affect cardiac regulation.22 ISF catecholamine measurements in this study during reperfusion without exogenous epinephrine injection were of the magnitude reported to result in decreased ATP levels, arrhythmias, and irreversible myocyte damage and cell death.3,6,23 Furthermore, PRSW, which is a load-independent quantification of myocardial function, indicated significant myocardial dysfunction in the presence of massive catecholamine release. Other measurements of LV function, such as the peak ⫺dP/dt and stroke volume, were diminished and suggestive of diastolic stiffness. Although injection of epinephrine has been a mainstay of advanced cardiac life support, endogenous catecholamine levels may already be present at high levels in the earliest phase of resuscitation, when the heart is not functioning well mechanically to pump blood. Furthermore, some data show no improvement in survival after cardiac arrest in patients who receive epinephrine compared with no exogenous epinephrine.24 The phases of resuscitation after cardiac arrest need to be considered as the best drugs for long-term survival are established. The use of -blockers during and after experimental VF and resuscitation has been examined and has shown either benefit2,5 or no significant improvement in outcome.17 On the basis of the massive peak measured in the present studies 4 minutes after defibrillation and reperfusion, 2473 we theorized that the time of administration and duration of effect of -adrenergic blocking agents might be critical to favor the beneficial ␣-adrenergic receptor actions and inhibit the potentially detrimental -adrenergic receptor effects mediated by endogenous catecholamine release. Esmolol is a 1-adrenergic receptor antagonist with a half-life of 9 minutes. It has been used in a number of clinical situations in which rapid reversal of effect may be necessary, including during acute myocardial ischemia and during cardiopulmonary bypass surgery.25,26 Esmolol was chosen in this study because its period of action was similar to the duration of the catecholamine surge measured by tissue microdialysis after prolonged VF and resuscitation. We did see a blunting of the maximum systolic arterial blood pressure after ROSC, showing that esmolol did moderate the functional effects of the catecholamine surge. The percentage of survivors that required dobutamine support after resuscitation was the same in the 2 groups, suggesting that there was not a long-term decrease in cardiac function caused by -adrenergic receptor antagonism. It is inherently difficult to define the mechanisms of catecholamine cardiotoxicity in vivo because of complex alterations in blood flow and cardiac loading. However, studies in isolated hearts and cultured myocytes indicate that -adrenergic receptor activation results in cAMP-mediated calcium overload, which is fundamental to early myocyte death.3,27,28 Furthermore, the finding by Mann et al3 that norepinephrine could decrease myocyte protein synthesis suggests a mechanism for pump dysfunction in the presence of high levels of catecholamines. Catecholamines are important regulators of the response of the cardiovascular system to stress. Nonetheless, cardiac arrest and cardiopulmonary resuscitation are extreme forms of stress that lead to profoundly elevated cardiac ISF levels of catecholamines in the earliest stages of resuscitation, which can be cardiotoxic. Our data suggest that relatively short-duration -adrenergic receptor blockade in the first phase of resuscitation, when endogenous catecholamines are already extremely high may improve survival. Further study is needed to determine the adrenergic requirements of the damaged heart during the second phase of resuscitation, when adequate myocardial function is needed to support organ recovery and to improve long-term outcome from resuscitation. Limitations In this study, myocardial interstitial catecholamine levels were studied during and after a single duration of unsupported VF. Catecholamine levels may vary with unsupported fibrillation time. Additional studies are necessary to investigate this question. Esmolol was delivered at the time of reperfusion. It is unknown whether the beneficial effect will carry over if the drug is given later in the reperfusion period. It is also unclear whether the beneficial effects of esmolol reflected altered catecholamine release or were a manifestation of the transient blockade of the myocyte -adrenergic receptor. This information would be useful in applying the use of esmolol to CPR. Clinical Implications Although the pathophysiological significance of the massive accumulation of catecholamines in the myocardium after 2474 Circulation May 25, 2004 defibrillation and reperfusion is unclear, the catecholamine levels measured in this study can result in malignant arrhythmias and myocardial cell death.23 With newer biphasic automatic external defibrillators, our ability to successfully halt VF in sudden cardiac death victims has risen to an impressive 98%.23 Unfortunately, survival of these patients to hospital discharge is only ⬇30%, suggesting that much more work is necessary to understand how to maintain ROSC and myocardial function. The potential therapeutic usefulness of interventions such as esmolol at the time of resuscitation warrants further investigation. Furthermore, the universal use of exogenous epinephrine as the drug of first choice for cardiac resuscitation needs to continue to be evaluated. Acknowledgments Downloaded from http://circ.ahajournals.org/ by guest on August 11, 2017 This study was supported by National Institutes of Health research grants HL-67961 (Dr Ideker), HL-63775 (Dr Walcott), and HL-54816 (Dr Dell’Italia); an American Heart Association Grant-in-Aid (Drs Walcott and Ardell) and Beginning Grant-in-Aid (Dr Killingsworth); and the Office of Research and Development, Department of Veteran Affairs (Dr Dell’Italia). References 1. Schneider T, Martens PR, Paschen H, et al. Multicenter, randomized, controlled trial of 150-J biphasic shocks compared with 200- to 360-J monophasic shocks in the resuscitation of out-of-hospital cardiac arrest victims. Circulation. 2000;102:1780 –1794. 2. Ditchey RV, Rubio-Perez A, Slinker BK. Beta-adrenergic blockade reduces myocardial injury during experimental cardiopulmonary resuscitation. J Am Coll Cardiol. 1994;24:804 – 812. 3. Mann DL, Kent RL, Parsons B, et al. Adrenergic effects on the biology of the adult mammalian cardiocyte. Circulation. 1992;85:790 – 804. 4. Tang W, Weil MH, Sun S, et al. Progressive myocardial dysfunction after cardiac resuscitation. Crit Care Med. 1993;21:1046 –1050. 5. Tang W, Weil MH, Sun S, et al. Epinephrine increases the severity of postresuscitation myocardial dysfunction. Circulation. 1995;92: 3089 –3093. 6. Waldenstrom AP, Hjalmarson AC, Thornell L. A possible role of noradrenaline in the development of myocardial infarction. Am Heart J. 1978;95:43–51. 7. Position of the American Heart Association on research animal use. Circulation. 1985;71:849A– 850A. 8. Farrell DM, Wei C-C, Tallaj J, et al. Angiotensin II modulates catecholamine release into interstitial fluid of canine myocardium in vivo. Am J Physiol. 2001;281:H813–H822. 9. Akiyama T, Yamazaki T, Ninomiya I. In vivo monitoring of myocardial interstitial norepinephrine by dialysis technique. Am J Physiol. 1991;260: H1643–H1647. 10. Valenzuela TD, Roe DJ, Cretin S, et al. Estimating effectiveness of cardiac arrest interventions. Circulation. 1997;96:3308 –3313. 11. Glower DD, Spratt JA, Snow ND, et al. Linearity of the Frank-Starling relationship in the intact heart: the concept of preload recruitable stroke work. Circulation. 1985;71:994 –1009. 12. Lameris TW, de Zeeuw S, Alberts G, et al. Time course and mechanism of myocardial catecholamine release during transient ischemia in vivo. Circulation. 2000;101:2645–2659. 13. Lameris TW, de Zeeuw S, Duncker DJ, et al. Epinephrine in the heart: uptake and release, but no facilitation of norepinephrine release. Circulation. 2002;106:860 – 865. 14. Eisenhofer G. Concentrations of noradrenaline at neuronal uptake sites during sympathetic nervous inhibition and activation in rabbits. Neurochem Int. 1993;22:493– 499. 15. Lameris TW, van den Meiracker AH, Boomsma F, et al. Catecholamine handling in the porcine heart: a microdialysis approach. Am J Physiol. 1999;277:H1562–H1569. 16. Kloeck W, Cummins RO, Chamberlain D, et al. The universal advanced life support algorithm: an advisory statement from the Advanced Life Support Working Group of the International Liaison Committee on Resuscitation. Circulation. 1997;95:2180 –2182. 17. Berg RA, Otto CW, Kern KB, et al. High-dose epinephrine results in greater early mortality after resuscitation from prolonged cardiac arrest in pigs: a prospective, randomized study. Crit Care Med. 1994;22:282–290. 18. Ditchey RV, Horwitz LD. Metabolic evidence of inadequate coronary blood flow during closed-chest resuscitation. Cardiovasc Res. 1985;19: 419 – 425. 19. Ditchey RV, Lindenfeld J. Failure of epinephrine to improve the balance between myocardial oxygen supply and demand during closed-chest resuscitation in dogs. Circulation. 1988;78:382–389. 20. Foley PJ, Tacker WA, Wortsman J, et al. Plasma catecholamine and serum cortisol responses to experimental cardiac arrest in dogs. Am J Physiol. 1987;253:E283–E289. 21. Kern KB, Elchisak MA, Sanders AB, et al. Plasma catecholamines and resuscitation from prolonged cardiac arrest. Crit Care Med. 1989;17: 786 –791. 22. Armour JA. Intrinsic cardiac neurons involved in cardiac regulation possess alpha 1-, alpha 2-, beta 1- and beta 2-adrenoceptors. Can J Cardiol. 1997;13:277–284. 23. Schomig A, Richardt G. The role of catecholamines in ischemia. J Cardiovasc Pharmacol. 1990;16:S105–S112. 24. Herlitz J, Ekstrom L, Wennerblom B, et al. Adrenaline in out-of-hospital ventricular fibrillation: does it make any difference? Resuscitation. 1995; 29:195–210. 25. Scorsin M, Mebazaa A, Al Attar N, et al. Efficacy of esmolol as a myocardial protective agent during continuous retrograde blood cardioplegia. J Thorac Cardiovasc Surg. 2003;124:1022–1029. 26. Deng Y-K, Wei F, Li Z-L, et al. Esmolol protects the myocardium and facilitates direct version intracardiac operation with a beating heart. Circ J. 2002;66:715–717. 27. Opie LH, Walpoth B, Barsacchi R. Calcium and catecholamines: relevance to cardiomyopathies and significance in therapeutic strategies. J Mol Cell Cardiol. 1985;17:21–34. 28. Bloom S, Davis DL. Calcium as mediator of isoproterenol-induced myocardial necrosis. Am J Pathol. 1972;69:459 – 470. Short-Acting β-Adrenergic Antagonist Esmolol Given at Reperfusion Improves Survival After Prolonged Ventricular Fibrillation Cheryl R. Killingsworth, Chih-Chang Wei, Louis J. Dell'Italia, Jeffrey L. Ardell, Melody A. Kingsley, William M. Smith, Raymond E. Ideker and Gregory P. Walcott Downloaded from http://circ.ahajournals.org/ by guest on August 11, 2017 Circulation. published online May 3, 2004; Circulation is published by the American Heart Association, 7272 Greenville Avenue, Dallas, TX 75231 Copyright © 2004 American Heart Association, Inc. All rights reserved. Print ISSN: 0009-7322. Online ISSN: 1524-4539 The online version of this article, along with updated information and services, is located on the World Wide Web at: http://circ.ahajournals.org/content/early/2004/05/03/01.CIR.0000128040.43933.D3.citation Permissions: Requests for permissions to reproduce figures, tables, or portions of articles originally published in Circulation can be obtained via RightsLink, a service of the Copyright Clearance Center, not the Editorial Office. Once the online version of the published article for which permission is being requested is located, click Request Permissions in the middle column of the Web page under Services. Further information about this process is available in the Permissions and Rights Question and Answer document. Reprints: Information about reprints can be found online at: http://www.lww.com/reprints Subscriptions: Information about subscribing to Circulation is online at: http://circ.ahajournals.org//subscriptions/