* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
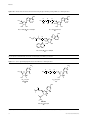
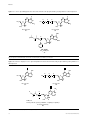
Download Review Nucleotide prodrugs for HCV therapy
Discovery and development of non-nucleoside reverse-transcriptase inhibitors wikipedia , lookup
Discovery and development of direct Xa inhibitors wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Discovery and development of direct thrombin inhibitors wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Discovery and development of cyclooxygenase 2 inhibitors wikipedia , lookup
Discovery and development of HIV-protease inhibitors wikipedia , lookup
Metalloprotease inhibitor wikipedia , lookup
Discovery and development of ACE inhibitors wikipedia , lookup
Discovery and development of neuraminidase inhibitors wikipedia , lookup
Discovery and development of integrase inhibitors wikipedia , lookup
Antiviral Chemistry & Chemotherapy 2011; 22:23–49 (doi: 10.3851/IMP1797) Review Nucleotide prodrugs for HCV therapy Michael J Sofia1* 1 Pharmasset, Inc., Princeton, NJ, USA *Corresponding author e-mail: [email protected] HCV infection is a significant worldwide health problem and is a major cause of hepatocellular carcinoma. The current standard of care, interferon and ribavirin, is only effective against a proportion of the patient population infected with HCV. To address the shortcomings of existing therapy, the development of direct acting antiviral agents is under investigation. The HCV RNA dependent RNA polymerase is an essential enzyme for viral replication and is therefore a logical target against which to develop novel anti-HCV agents. Nucleosides have been shown to be effective as antiviral agents for other viral diseases and therefore, have been investigated as inhibitors of HCV replication. The development of prodrugs of nucleoside 5′-monophosphates has been pursued to address limitations associated with poor nucleoside phosphorylation. This is required to produce the nucleoside 5′-triphosphate which is the anabolite that is the actual inhibitor of the polymerase enzyme. Prodrugs of nucleoside 5′-monophosphates have been developed that enable their delivery into cells and in vivo into the liver. The implementation of these prodrug strategies has ultimately led to the identification of several prodrugs of nucleoside 5′-monophosphates that are potent inhibitors of HCV replication in vitro. They have progressed into the clinic and the early data demonstrate greatly reduced viral load levels in HCV-infected patients. This review will survey the state of nucleotide prodrugs for the treatment of HCV. Introduction HCV is known to have infected approximately 180 million individuals worldwide [1]. It is estimated that of those infected with HCV approximately 80% will develop chronic liver disease and a significant proportion of those infected will eventually develop liver cirrhosis and subsequently hepatocellular carcinoma [2]. HCV is a single-stranded, positive sense RNA virus of the Flaviviridae. Six major viral genotypes with over 100 viral subtypes have been identified for HCV. Genotypes 1a and 1b are the most prevalent genotypes in the western world with genotypes 2 and 3 comprising 20–30% of this population. HCV genotypes 2–6 predominate in the developing world [3,4]. Because HCV replicates in the cytoplasm of infected cells by a membrane associated replication complex and the virus has an RNA genome with no DNA intermediate during replication, no genomic templates are stably integrated into the host genome. Therefore, virological cures are possible for HCV patients. This is in contrast to other viruses such as HIV and HBV where the viral genome is integrated into the host DNA and a virological cure is considered remote. However, for HCV-infected patients virological cures are made difficult due to the high rate of HCV viral replication and by the high spontaneous ©2011 International Medical Press 1359-6535 (print) 2040-2066 (online) mutation rate of the virus. This high mutation rate is a result of poor replication fidelity exhibited by the HCV polymerase and an apparent lack of proof reading [4]. The current therapy for treating chronic HCV infection consists of regular injections of α-interferon (IFN) with daily oral administration of ribavirin (RBV). This standard of care (SOC) regimen does not act by directly attacking the virus but functions by boosting the host immune response. For genotype 1 patients regular IFN/ RBV treatments for 48 weeks result in only 40–50% of patients achieving a sustained virological response (SVR) indicative of a cure [5,6]. However, for genotype 2 and 3 patients the SVR rates can be as high as 75%. It is also known that subpopulations which include individuals of African ancestry tend to respond less well to IFN/RBV treatments [7]. Recent genome-wide association studies have shown that a single nucleotide polymorphism 3kb upstream of the IL28B gene correlates with a significant difference in response to IFN therapy [8]. IL28B which encodes the type III interferon IFN-λ−3, is known to be upregulated by IFNs and by RNA viral infections. It has been shown that HCV patients who harbour a TT or TC allele in their IL28B gene tend to respond less well to IFN/ RBV treatment than do those having the CC genotype. 23 MJ Sofia Figure 1. Model of HCV NS5B RNA complex RNA template IN Nucleotide IN Thumb Fingers Palm RNA duplex OUT Ribbon structure of the HCV NS5B RNA dependent RNA polymerase showing the Palm, Finger and Thumb domains typical of a RNA polymerase. Also depicted is a bound template-primer strand of RNA and the nucleotide binding site. Patients who choose to undergo IFN/RBV therapy face not only the possibility of not responding to treatment but also must contend with the potential for multiple and sometimes serious side-effects that include influenza-like symptoms, fatigue, hemolytic anaemia and depression. The intolerable side effects can result in a high rate of drug discontinuations. Consequently, the modest cure rates and subpopulation differences combined with the side-effect profile for SOC have prompted an urgency to develop alternative novel, safe and effective therapies. As in the case of other viral diseases, the development of direct acting antivirals (DAAs) has become a focus. Development of small molecule agents to attack essential viral proteins has the potential benefit of reducing toxicities and side effects associated with manipulating host functions and hopefully such DAA therapies will not have the intolerable side effects exhibited by current SOC. The push to identify small molecule DAAs has also prompted the discussion around the possibility of eliminating or at least reducing the use of IFN/RBV from treatment regimens. Although clinical development of first generation DAAs has focused on combinations with SOC in the hope of both shortening duration of treatment and increasing the cure rate, the long-term desire is to completely eliminate the use of IFN/RBV from treatment regimens. Clearly this is an aspirational goal and a goal that can only be achieved if an immune component of therapy is not absolutely required to eliminate those vestiges of undetectable virus [9]. In addition, such a lofty goal can be realised if small 24 molecule DAAs either alone or more probably in combination can drive viral loads to undetectable limits and maintain those undetectable levels over the necessary course of therapy and after cessation of treatment without having viral breakthrough resulting from the emergence of resistant virus. As has been shown with HIV highly active antiretroviral therapy (HAART), the HCV treatment paradigm will likely require combinations of anti-HCV agents [7]. The desire is to suppress virus as rapidly and completely as possible in order to give the body’s natural immune system the opportunity to clear residual virus and to hold back emergence of resistant virus. However, what will comprise those ideal combinations of DAAs is yet to be determined and studies to clarify this question are under active discussion and investigation. HCV has a 9.6 kb genome of positive-stranded RNA. This genome encodes a precursor polyprotein that is processed into 10 functional proteins: three structural proteins and seven non-structural proteins [10]. Several of the non-structural proteins have been the focus of intensive efforts to identify small molecule DAA agents as inhibitors of HCV replication. Of the seven non-structural proteins, molecules that inhibit the functions of the NS3/4 protease, NS4A, NS4B, NS5A and NS5B RNA dependent RNA polymerase (RdRp) have advanced to the clinic [11–15]. The most advanced agents are the NS3/4 protease inhibitors telaprevir and boceprevir. Each of these compounds has completed Phase III clinical investigation and both have been shown to be efficacious in treating HCV infection when given in combination with SOC. However, each of these first generation protease inhibitors suffers from the lack of genotype coverage, undesired side effects, that may limit their usage, and the early emergence of resistant virus. It is therefore not surprising that even with the potential benefits of these first generation DAAs, warehousing of patients by physicians occurs in order to wait for approval of more effective and tolerable agents. The HCV RNA dependent RNA polymerase is an ~68 kd protein that has the typical palm-finger-thumb structural motif found in many viral polymerases (Figure 1) [16,17]. HCV polymerase is an essential enzyme involved in RNA replication. Phylogenetic analysis shows a 65% homology of HCV RdRp across genotypes and an 80% homology within a particular genotype [3]. The HCV polymerase active site is located in the palm domain where the conserved aspartic acid residue-containing GDD motif is located [18]. This conserved GDD motif is common to viral polymerases in general [18]. Through a divalent metal ion (Mg++ or Mn++) the GDD motif functions to coordinate the binding of the ribonucleoside triphosphate. The HCV polymerase catalyzes the addition of a single ribonucleoside triphosphate monomer to the 3′-end of the growing RNA chain by the ©2011 International Medical Press Nucleotide prodrugs for HCV therapy formation of a 3′,5′-phosphodiester linkage. To accomplish this process, the polymerase must simultaneously bind a template RNA strand, a primer RNA strand and a ribonucleoside triphosphate monomer [19,20]. Therefore, investigation of nucleoside analogues is a rational choice for the development of inhibitors of the HCV NS5B polymerase. Of all the DAAs under clinical investigation, nucleoside/nucleotide NS5B polymerase inhibitors hold the promise of pan-genotype coverage and a high barrier to development of resistant virus. As in the case of HIV infection where nucleosides have become the backbone of therapy (for example, TRUVADA® and Combivir®), HCV nucleosides/nucleotides are positioned to assume a similar role. To date, only nucleosides/nucleotides have demonstrated broad genotype coverage both in the laboratory and in human clinical studies [21]. In addition, to date, no pre-emergent resistant virus has been detected in clinical studies [22]. It is for these reasons that nucleosides/nucleotides are positioned to play a prominent role in developing HCV treatment paradigms. The HCV polymerase has been shown to be a uniquely selective polymerase as it relates to the development of nucleoside/nucleotide inhibitors. Over the last 10 years only two broad classes of nucleosides have emerged as inhibitors of this polymerase [23–25]. These include the 2′-methyl and the 4′-azido classes (Figure 2). However, these classes and subgroups within these classes have clearly differentiated themselves in preclinical and clinical studies. This differentiation is exemplified in their viral selectivity, viral resistance, overall safety and clinical efficacy profiles. Resistance associated with the 2′-methyl class of nucleosides is associated with the S282T amino acid alteration located in the finger domain of the HCV polymerase [26–29]. This mutation has been shown to be difficult to raise in vitro and has not been detected as a pre-existing mutation in clinical isolates [22]. Similarly, for the 4′-azido class, the S96T amino acid alteration has been identified in vitro but has not been observed in the clinic [27]. Implementation of a prodrug strategy has played a prominent role in the development of a number of nucleosides and nucleotides for the treatment of HCV infection [23,30]. These prodrugs have been developed to overcome, not only, bioavailability and stability issues but also to address key anabolism limitations important to nucleoside activation. Simple ester prodrugs of both the 2′-methylcytidine, 2′-α-fluoro-2′-β-Cmethylcytidine and 4′-azidocytidine nucleosides were developed to overcome both bioavailability issues and to curb undesirable metabolism [21,31,32]. Prodrugs of the phosphate group of nucleoside 5′-monophosphates were developed to address not only bioavailability Antiviral Chemistry & Chemotherapy 22.1 Figure 2. Two major classes of nucleosides that are known to be inhibitors of HCV polymerase. 2′-Methyl class BASE O HO HO X X = OH, F 4′-Azido class HO BASE O N3 HO X X = OH, F These classes include the 2′-methyl and the 4′-azido ribosides. issues but also poor in vitro and in vivo conversion of the parent nucleoside to the active nucleoside 5′-triphosphate [33]. Because nucleosides must be converted to their 5′-triphospates to be active as inhibitors of the HCV polymerase, they need to undergo a series of phosphorylation steps catalyzed by three separate kinases (Figure 3). These kinases convert the nucleoside first to the monophosphate, then to the diphosphate and finally to the active triphosphate. However, it is not uncommon that in the phosphorylation cascade, a nucleoside or its corresponding mono- or diphosphate is a poor substrate for one of the kinases. In particular, it is the first kinase in the phosphorylation cascade that is generally the most substrate selective. Therefore, it is not unusual that bypassing the first kinase results in achieving high levels of the active triphosphate. Because nucleoside monophosphates are enzymatically dephosphorylated and negatively charged, they do not readily enter cells and therefore are not desirable as drug candidates. To overcome the limitations of administering a nucleoside monophosphate-containing agent, prodrugs of the 5′-monophosphate nucleoside have been employed. Prodrugs of nucleoside monophosphates have been known for many years and a number of phosphate prodrug strategies have been developed to address the need to deliver a 5′-monophosphate nucleoside into the cell [33–35]. However, there have been few examples where a nucleotide prodrug has been shown to deliver the corresponding 5′-monophosphate in vivo to the desired site of action [33]. Often the prodrug moiety decomposes prior to achieving its objective because of either chemical or enzymatic instability in the gastrointestinal tract and/or plasma. The development of a nucleoside phosphate prodrug useful for the treatment of HCV faces several challenges. The nucleoside phosphate prodrug must have sufficient chemical stability to be formulated for oral 25 MJ Sofia Figure 3. Nucleoside kinase activation pathway HO O BASE BASE O Kinase 1 X HO O Kinase 2 BASE X Y HO Y O Kinase 3 X HO BASE X HO Y = Phosphate Y Nucleoside kinase activation pathway resulting in the nucleoside triphosphate which is the active substrate for a polymerase allowing incorporation of the nucleoside or nucleoside analogue into the growing RNA chain and thus resulting in inhibition of virus replication. Examples of kinases 1, 2, and 3 include deoxycytidine kinase (dCk), nucleoside monophosphate kinase (YMPK) and nucleoside disphosphate kinase (NDPK), respectively. administration. It must be stable to conditions of the gastrointestinal tract such that the prodrug reaches the site of absorption intact. The prodrug must have good absorption properties and must not undergo appreciable enzymatic degradation during the absorption phase. Once absorbed, the prodrug needs to have sufficient stability in the blood in order to reach the target organ: for example the liver in the case of HCV. The prodrug must then be transported into hepatocytes and release the free 5′-monophosphate nucleoside which can subsequently be converted to the active triphosphate derivative. Since HCV is a disease of the liver, and the liver is the first organ the prodrug encounters after absorption, HCV is an ideal disease for which to develop a targeted nucleotide prodrug strategy. Consequently, several of these nucleoside monophosphate prodrugs have advanced to the clinic and have demonstrated proof of concept for treating HCV. Here, the application of phosphate prodrugs in the development of nucleotide inhibitors of HCV and the status of nucleotide prodrugs under investigation for the treatment of HCV infection will be reviewed. Nucleotide phosphoramidates Nucleotide phosphoramidates were first disclosed by McGuigan et al. [36] as a prodrug strategy to deliver a nucleoside 5′-monophosphate for the treatment of HIV and cancer. The structure of a nucleotide phosphoramidate typically consists of a nucleoside 5′-monophosphate where the phosphate group is masked by appending an aryloxy group (usually a phenol) and an α-amino acid ester (Figure 4); however, other related constructs have also appeared. The 26 phosphate group is ultimately revealed by a sequence of enzymatic and chemical steps that requires either carboxyesterase or cathepsin A to cleave the terminal amino acid ester, intramolecular displacement of the phosphate phenol and then enyzmatic cleavage of the amino acid moiety by a phosphoramidase or histidine triad nucleotide-binding protein 1 (HINT 1) [37–39]. It is believed that the phosphoramidate prodrug construct increases lipophilicity of the nucleoside 5′-monophosphate and therefore increases cellular permeability and ultimately intracellular nucleotide concentrations. Since the phosphoramidate prodrug moiety contains a chiral phosphorus centre, issues arise with regard to development of a compound that consists of a mixture of isomers with implications arising from differential activity of each of the isomers, pharmacokinetics and manufacturing optimization, etc. In addition, the typical phosphoramidate contains a phenolic substituent that is released during metabolism to the free monophosphate. Successful development also considers the metabolic release of this phenolic substituent. Selective examples employing the phosphoramidate strategy to achieve kinase bypass for nucleoside inhibitors of HIV reverse transcriptase (RT) inhibitors, for example, ddA, d4T and d4A, showed that in vitro whole cell enhancement in potency could be achieved [40–42]. Although the phosphoramidate strategy was explored extensively to deliver nucleotides for the treatment of HIV and colon cancer [33,36], proof of concept in the clinic has yet to be reported. However, the phosphoramidate prodrug approach has proven to be a valuable strategy in the development of HCV nucleotide therapy. ©2011 International Medical Press Nucleotide prodrugs for HCV therapy Figure 4. The phosphoramidate prodrug decomposition pathway that results in the release of the nucleoside 5’-monophosphate O O R2 HN R1 R2 O O P O Carboxyesterase Nucleoside O or Cathepsin A O HN H O P O Nucleoside O Ar Ar Nucleotide phosphoramidate Spontaneous H N R2 O P O H20 Nucleoside O O O O R2 HN H O P O Nucleoside O HINT-1 (Phosphoramidase) O HO P O Nucleoside OH H 2′-C-Methyl ribonucleotide phosphoramidates The 2′-C-methylcytidine nucleoside, NM107 (1; Figure 5), was shown to be an inhibitor of HCV in cell culture (50% effective concentration [EC50] =1.23 µM) and its triphosphate (3) was demonstrated to be a potent inhibitor of the HCV polymerase enzyme (50% inhibitory concentration [IC50] =0.09–0.18 µM) acting as a nonobligate chain terminator [43]. NM107 also showed broad antiviral activity against not only HCV but also bovine virus diarrhoea virus (BVDV), yellow fever virus, dengue virus and West Nile virus [31]. To overcome bioavailability issues the 3′-valinate ester prodrug, NM283 (valopicitabine; 2) [31,44], was taken into clinical development. In a Phase I monotherapy study, NM283 demonstrated proof of concept delivering an ~1.2 log10 IU/ml reduction in viral load at a dose of 800 mg twice daily given over 14 days. Unfortunately, NM283 was discontinued because of significant gastrointestinal toxicity in Phase II studies [24,30]. Subsequent work on the development of the 2′-Cmethyl class of nucleosides focused on 5′-phosphate nucleotide prodrugs. It was observed that the 2′-Cmethylcytidine triphosphate (3) was highly active as an inhibitor of the NS5B polymerase, yet the parent Antiviral Chemistry & Chemotherapy 22.1 nucleoside NM107 (1) was only modestly active in the whole cell based replicon assay. Studies had shown that NM107-triphosphate (3) formation was inefficient, particularly because of poor conversion of the nucleoside to its monophosphate by 2′-deoxycytidine kinase [45]. Consequently, to circumvent this phosphorylation problem and potentially improve the therapeutic index by increasing nucleoside triphosphate levels in the liver, a phosphoramidate prodrug approach was investigated [45]. This effort lead to the identification of phosphoramidate derivative 4 (Figure 5; EC50≤0.5 µM) showing substantial increases in potency relative to NM283 [45]. The activity of compound 4 correlated with the levels of triphosphate produced in human hepatocytes and these levels were shown to be much higher than that seen with NM283 (2). In vivo studies assessing liver nucleoside triphosphate levels after oral administration in hamsters showed low triphosphate concentrations only twofold higher than obtained with NM283. Since substantial liver triphosphate levels were seen after subcutaneous administration in vivo and the compounds were shown to be stable in simulated gastric fluid, it was concluded that low oral bioavailability or metabolic degradation 27 MJ Sofia Figure 5. 2′-C-Methylcytidine nucleosides and nucleotide phosphoramidate prodrug inhibitors of HCV replication NH2 NH2 HO N O HO N HO O NH2 O O OH N N O OH O NM283 2 NM107 EC50=1.23 µM 1 NH2 O HO O P O OH O P O OH P O N N O O OH HO OH IC50=0.09–0.18 µM 3 NH2 NH2 O O O N H P O O N N O O O HO O O N H P O O O O HO OH EC50=0.22 µM, CC50=7 µM 4 N N OH EC50=0.24 µM 5 NH2 NH2 O O O N H P O O N N O OH HO O O O N H P O N N O OH OH EC50=8.2 µM, CC50=>100 µM NTP (human hepatocytes) AUC(0–4h)=1,720 µM•h 6 O HO OH NTP (human hepatocytes) AUC(0–4h)=190 µM•h 7 AUC, area under the curve; CC50, 50% cytotoxic concentration; EC50, 50% effective concentration; IC50, 50% inhibitory concentration. 28 ©2011 International Medical Press Nucleotide prodrugs for HCV therapy in the intestine was the reason for the lack of oral efficacy [45]. Another series of 2′-C-methylcytidine phosphoramidate prodrugs having an acyloxyethylamino phosphoramidate promoiety was studied (Figure 5) [46]. The phosphoramidate prodrug 5 provided up to a 30-fold improvement in HCV replicon potency over NM283 (2), and it was also shown that this activity correlated to levels of nucleoside triphosphate in rat and human hepatocytes. However, when administered orally to rats these phosphoramidates demonstrated no improvement in production of triphosphate in rat liver relative to NM283 (2). These results put into question the oral bioavailability and conversion of these prodrugs to the nucleoside triphosphate in the liver. Phosphoramidate monoesters of 2′-C-methylcytidine were also explored (Figure 5) [45]. In this case the amidate moiety was either an α-amino acid (6) or an acyloxyethylamino substituent (7). Although phosphoramidate monoester 6 was shown to have inferior replicon potency relative to its phenolic ester counterpart (<200fold) it showed higher levels of triphosphate formation in human hepatocytes. Formation of nucleoside triphosphate levels were observed in hepatocytes of various species for both 6 and 7 but the phosphoramidate monoester 6 was shown to be superior to 7. In both cases, liver levels of nucleoside triphosphate 3 were achieved in vivo after subcutaneous administration but not after oral administration suggesting a lack of oral bioavailability. Other 2′-C-methyl nucleosides containing purine bases were also investigated as inhibitors of HCV, including the 7-deaza-2′-C-methyladenosine derivative MK0608 (8) which showed potent inhibition of HCV replication in vitro (EC50=0.25 µM) and demonstrated SVR in an HCV-infected chimpanzee animal model [47,48]. Although MK0608 (8) was never progressed into clinical development, it did demonstrate the potential of purine nucleosides as inhibitors of HCV. Subsequently, phosphoramidate prodrugs of 2′-C-methyl purine analogues were studied to determine if improvement in potency could be achieved by kinase bypass. Although application of the phosphoramidate prodrug strategy was not successful for the adenosine derivative 11 (Figure 6), its application to the guanosine analogue 12 resulted in an 84-fold increase in activity in the replicon assay relative to the parent nucleoside (Figure 7) [49]. This result was rationalized by the observation that the guanosine triphosphate (13; IC50=0.13 µM) was more potent as an inhibitor of the HCV polymerase than the adenosine triphosphate (10; IC50=1.9 Figure 6. 2′-C-Methyladenosine nucleoside and nucleotide phosphoramidate prodrug inhibitors of HCV replication NH2 HO O HO N N HO N OH O HO P OH O O P OH O O P O O N OH HO N N N OH EC50=0.3 µM, CC50=>50 µM 9 NH2 N N N O HO EC50=0.25 µM, CC50=>50 µM 8 NH2 N CH3 EtO O OH IC50=1.9 µM, EC50=0.3 µM 10 N H O P NH2 N O O N O HO N N OH EC50=0.25 µM, CC50=>50 µM 11 CC50, 50% cytotoxic concentration; EC50, 50% effective concentration; IC50, 50% inhibitory concentration. Antiviral Chemistry & Chemotherapy 22.1 29 MJ Sofia Figure 7. 2′-C-Methylguanosine nucleoside and nucleotide phosphoramidate prodrug inhibitors of HCV replication O N HO N O HO O NH N HO NH2 O P O OH O P O P OH R2 R1O OH O P O N O HO N NH N NH2 OH IC50=0.13 µM 13 O N O O HO O HN O OH EC50=3.5 µM 12 O N CH3 NH N O NH2 O HN O P O HO 14 R1=CH2CH2Ph, R2=Ala EC50=0.08 µM, CC50=>100 µM 15 R1=o-CIPh, R2=Val EC50=0.43 µM, CC50=>100 µM P N N N NH2 OH INX-08189 EC50=0.010 µM, CC50=7 µM 16 O N O HN O O N O O O O OH OCH3 N S HO NH N NH2 OH OH IDX184 EC50=0.4 µM, CC50>100 µM 17 CC50, 50% cytotoxic concentration; EC50, 50% effective concentration; IC50, 50% inhibitory concentration. µM), yet in the whole cell replicon assay the adenosine analogue (9; EC50=0.3 µM; Figure 6) was more potent than the guanosine analogue (12; EC50=3.5 µM; Figure 7). Therefore, this finding coupled with the observation that low levels of triphosphate were detected in cells for the guanosine nucleoside relative to that for the adenosine nucleoside hinted at poor phosphorylation in the case of the guanosine nucleoside. The development of the 2′-C-methylguanosine phosphoramidate derivative was further explored by systematically evaluating structural modifications to the phosphoramidate moiety in an effort to achieve increased HCV replicon potency, plasma stability across multiple species, and appropriate relative stability in intestinal and liver S9 preparations. Ultimately, these studies were able to identify 2′-C-methylguanosine phosphoramidate 30 prodrugs 14 and 15 (Figure 7) [50]. These prodrugs contained benzyl or alkyl l-alanine and l-valine amino acid ester moieties and a naphthyl phosphate ester and exhibited a 10–30-fold enhancement in HCV replicon potency with acceptable plasma and intestinal S9 stability suitable for progression into in vivo studies. However, when mice were dosed orally in order to assess liver levels of the nucleoside triphosphate 13, the 2′-C-methylguanosine phosphoramidates 14 and 15 (Figure 7) produced substantial liver levels of triphosphate but these levels were not significantly improved over that observed when mice were dosed with the parent guanosine nucleoside 12. Further investigation of the 2′-C-methylguanosine phosphoramidate series led to the evaluation of substitution at the C-6-position of the guanosine base with the ©2011 International Medical Press Nucleotide prodrugs for HCV therapy intention of increasing lipophilicity and thus improving cellular uptake relative to the natural guanosine derivative 12. This led to the identification of the C-6-O-methyl derivative INX-08189 (16; Figure 7) containing both a neopentyl ester on the l-alanyl amino acid moiety and a naphthyl ester on phosphorus of the phosphoramidate [51]. In INX-08189 (16), in addition to metabolic conversion of the phosphoramidate pro-moiety to the 5′-monophosphate, the 6-O-methyl group of the purine base is metabolized to the guanine base. INX-08189 (16) demonstrated exceptional potency in the HCV 1b replicon assay (EC50=0.01 µM; CC50=7 µM), was active against genotype 1a and 2 replicons and produced substantial levels of the guanosine triphosphate 13 in primary human hepatocytes over 48 h. The known 2′-Cmethyl nucleoside S282T mutant replicon was shown to be moderately resistant (3- to 10-fold) to INX-08189 (16) [52]. No difference in HCV replicon potency was observed for each of the individual diastereoisomers of INX-08189 (16), thus INX-08189 was advanced into clinical development as a mixture of isomers at the phosphorus centre of the prodrug. In a single-ascending-dose Phase Ia study in healthy volunteers administered doses ranging from 3 mg to 100 mg, INX-08189 (16) was shown to be generally well tolerated at all doses with no drug-related serious adverse events and pharmacokinetics (PK) supporting once daily oral dosing [53,54]. INX-08189 was progressed into a Phase Ib study in treatment-naive genotype 1 HCV patients dosed once daily at either 9 mg or 25 mg. Antiviral activity was observed with a mean HCV RNA reduction of -0.71 and -1.03 log10 IU/ml, respectively [54,55]. These early human data demonstrated clinical proof of concept for INX-08189 and consequently, the compound is continuing to undergo clinical evaluation. Another application of phosphoramidate prodrug technology for delivering a 2′-C-methylguanosine 5′-monophosphate is exemplified by IDX184 (17; Figure 7) [56]. In this case the phosphoramidate prodrug moiety utilized a benzyl amine in place of an amino acid for the amine substituent and an S-acetyl-2-thioethyl moiety (SATE) as the phosphate ester substituent in place of the more common aryl group. The SATE group is a known phosphate ester prodrug construct [33,57,58]. Mechanism for release of the desired 2′-C-methylguanosine 5′-monophosphate is believed to involve both CYP450dependent and independent processes. Based on the prodrug structure one would anticipate that prodrug release would involve cleavage of the terminal thioester followed by loss of the phosphate ester via intramolecular attack of the free thiol group releasing the phosphate and an equivalent of episulfide, then enzymatic cleavage of the benzyl phosphoramide. However, a detailed mechanism for the IDX184 (17) prodrug cleavage has not been Antiviral Chemistry & Chemotherapy 22.1 reported to date. In the whole cell HCV replicon assay IDX184 (17) was shown to be a potent inhibitor of HCV replication (EC50=0.4 µM; CC50>100 mM) and was also active in the genotype 2a JFH1 replicon (EC50=0.6–11 µM) [59]. At a concentration of 2.5 µM it cleared HCV replicon RNA after 14 days of treatment. Like other 2′-C-methyl nucleosides it was shown to select for the NS5B S282T mutation in the HCV replicon. In combination with the protease inhibitor IDX320, IFN-α, or RBV, IDX184 exhibited an additive or synergistic profile [60]. When administered orally to cynomolgus monkeys, IDX184 (17) produced high live triphosphate levels relative to oral administration of the parent nucleoside with what appears to be high hepatic extraction. Subsequent studies in a HCV-1-infected chimpanzee model showed that oral administration of IDX184 (17) at 10 mg/kg over 3 days produced a median viral load decline of approximately -2.3 log10 at day 3 and 4 [61]. Consequently, IDX184 (17) was progressed into the clinic and in a Phase Ia single ascending dose study was shown to be generally safe and well tolerated at oral doses from 5 mg to 100 mg [56]. PK assessment supported a liver targeting mechanism for the compound. In a Phase Ib monotherapy study in HCV genotype-1-infected patients, IDX184 (17) was administered at doses from 25 to 100 mg once a day for 3 days. Day 4 viral load assessment showed that at the highest dose of 100 mg, a -0.74 log10 IU/ml reduction in viral load was observed [62]. Following the positive Phase I clinical results, a Phase II study was initiated in which IDX184 (17), at doses from 50–200 mg, was combined with pegylated IFN and RBV for 14 days in treatment-naive HCV genotype 1 patients [63]. At day 14 viral load reductions of -2.7 to -4.1 log10 IU/ml were achieved with no adverse events attributed to IDX184 (17) and with no detection of resistant virus [64]. However, in an attempt to evaluate the efficacy of a DAA combination of IDX184 (17) with the NS3 protease inhibitor IDX320, three serious adverse events were reported in a drug–drug interaction study in healthy volunteers and consequently, the programme was placed on clinical hold in September 2010, awaiting resolution of the cause of the adverse events [65,66]. In February 2011, the US Food and Drug Administration (FDA) removed full clinical hold for IDX184 (17) owing to evidence that the toxicity was likely caused by IDX320, and the programme was placed on partial clinical hold. Initiation of a Phase IIb 12-week trial of IDX184 (17) in combination with pegylated IFN and RBV is anticipated in the second half of 2011 [67]. 4′-Azido ribonucleotide phosphoramidates The 4′-C-azidocytidine nucleoside R1479 (18; Figure 8) was reported to be an inhibitor of HCV replication (EC50=1.28 µM) and subsequently its 2′,3′,5′-tri-Oisobutryate ester prodrug R1626 (balapiravir; 20) was 31 MJ Sofia Figure 8. 4′-Azido cytidine nucleoside and nucleotide phosphoramidate prodrug inhibitors of HCV replication NH2 HO O O N N NH2 HO O N3 HO P O O OH P O O P OH O O O OH N3 HO OH EC50=1.28 µM, CC50=>100 µM R1479 18 N N OH IC50=0.32 µM 19 NH2 NH2 O O O N N O O N3 O Ph O O O N H P O O O O N3 HO O N N OH O CH3 R1626 EC50=0.51 µM, CC50=>100 µM 20 21 CC50, 50% cytotoxic concentration; EC50, 50% effective concentration; IC50, 50% inhibitory concentration. taken into clinical development as a treatment for HCV infection [32,68]. R1479 was shown to have reduced activity against the S96T HCV polymerase mutant [27]. In a Phase Ib monotherapy clinical study in HCVinfected patients dosed with 500, 1,500, 3,000 and 4,500 mg twice daily for 14 days, R1626 (20) demonstrated a mean decrease in viral load of -0.3, -1.2, -2.6 and -3.7 log10 IU/ml, respectively, thus providing proof of concept for this class of nucleosides. R1626 was subsequently taken into a Phase IIa study in genotype 1 treatment-naive patients where at a dose of 1,500 twice daily in combination with IFN/RBV a -5.2 log10 IU/ml reduction in viral load with an 81% rapid virological response (RVR) was observed after 28 days of therapy [30]. However, dosing was limited by neutropenia and in a Phase IIb study in combination with IFN/RBV, the development of R1626 (20) was discontinued as a result of significant haematological adverse events [69]. In an effort to increase the potency of R1479 (18), a 5′-phosphoramidate nucleotide prodrug strategy 32 was investigated [70]. It was hoped that the increased lipophilicity introduced by the phosphoramidate group might result in enhanced passive diffusion and therefore, increased intracellular levels of the monophosphate and ultimately of the active triphosphate thus leading to a significant in vitro and in vivo advantage. In addition, if liver targeting was possible, as was described for other nucleotide prodrugs, there existed a possibility that the liabilities associated with R1479 (18) could be ameliorated by limiting systemic exposure. After an extensive structure–activity relationship (SAR) study, it was noted that preparation of the 5′-phosphoramidate derivative 21 (Figure 8) did not provide any appreciable improvement in potency over the nucleoside R1479 (18), indicating that this nucleoside is already efficiently phosphorylated and that application of the phosphoramidate technology was not able to boost its potency [70]. Further evaluation of the 4’-azido pyrimidine class of nucleosides investigated the 4′-C-azidouridine ©2011 International Medical Press Nucleotide prodrugs for HCV therapy Figure 9. 4′-Azido uridine nucleoside and nucleotide phosphoramidate prodrug inhibitors of HCV replication O O HO O HO O N3 HO O NH N O P O OH P O O P OH O O HO EC50=>100 µM, CC50=>100 µM 22 O OH N3 OH NH N OH IC50=0.22 µM 23 O Ph O O O N H P O O O N3 HO NH N O OH EC50=0.22 µM, CC50=>100 µM 24 CC50, 50% cytotoxic concentration; EC50, 50% effective concentration; IC50, 50% inhibitory concentration. derivative 22 (Figure 9) [71]. It was shown that the 4′-Cazidouridine (22) was inactive as an HCV inhibitor as determined in the cell-based replicon assay. However its 5′-triphosphate analogue (23) demonstrated inhibition of the HCV polymerase enzyme (IC50=0.22 µM) similar to that of the triphosphate 19 (IC50=0.32 µM) of the cytidine derivative 18. Speculating that poor substrate activity for cellular kinases was contributing to the lack of whole cell activity of the 4′-C-azidouridine (22), a series of 5′-phosphoramidate prodrugs was prepared. SAR around the phosphoramidate moiety showed that the nature of the substituents on the phosphoramidate had a dramatic effect on activity in the HCV whole cell assay. Since the compounds were prepared as a mixture of diastereomers at the phosphorus centre of the phosphoramidate moiety, the diastereomers were separated chromatographically but no difference in potency was observed. Improvements in activity of >450-fold were reported, with the most active compound in the series having the 1-naphthyl l-alanine benzyl ester phosphoramidate (24) substitution pattern (EC50=0.22 µM). To date, no reports have appeared that indicate further progression of a 4′-C-azidouridine 5′-phosphoramidate prodrug beyond in vitro studies. Antiviral Chemistry & Chemotherapy 22.1 In the 4′-azido riboside class of nucleosides, the phosphoramidate prodrug strategy was also applied to the adenosine derivative 25 (Figure 10) [72]. As in the case of the 4′-C-azidouridine, the 4′-C-azidoadenosine (25) was shown to be inactive in the HCV replicon assay with the assumption that the lack of activity was due to its inability to be phosphorylated by cellular kinases. It was hypothesized that delivering a monophosphate prodrug derivative would both bypass the non-productive monophosphorylation step and also reduce the extent of intracellular metabolism by deamination. Preparation of a series of phosphoramidate derivatives led to the identification of several compounds, like compound 27, that showed submicromolar EC50 values (0.22–0.59 µM) in the HCV replicon assay and were devoid of cellular toxicity; however, no additional data was provided to support protection from deamination nor translation of the in vitro data into an in vivo setting. 2′-α-Fluoro-2′-β-C-methyl nucleotide phosphoramidate prodrugs In 2005, the first disclosure of a 2′-α-fluoro-2′-βC-methylcytidine nucleoside was made with the 33 MJ Sofia Figure 10. 4′-Azido adenosine nucleoside and nucleotide phosphoramidate prodrug inhibitors of HCV replication NH2 N HO O N N3 HO O N HO N O P O O P OH O P OH O O HO Ph N N N OH N3 OH EC50=>100 µM, CC50=>100 µM 25 NH2 N OH IC50= Not determined 26 O O N H O P O NH2 N O N N O N N3 HO OH EC50=0.22 µM, CC50=>100 µM 27 CC50, 50% cytotoxic concentration; EC50, 50% effective concentration; IC50, 50% inhibitory concentration. Figure 11. 2′-α-F-2′-β-C-Methylcytidine nucleoside inhibitors of HCV replication NH2 HO O HO N NH2 O N HO O O P O OH O P O OH P O O HO N O OH F EC90=4.5 µM PSI-6130 28 N F IC50=0.34 µM 29 NH2 O O O O O N N O F RG7128 EC50=0.32–0.98 µM 30 CC50, 50% cytotoxic concentration; EC50, 50% effective concentration; EC90, 90% effective concentration; IC50, 50% inhibitory concentration. 34 ©2011 International Medical Press Nucleotide prodrugs for HCV therapy publication of PSI-6130 (28; Figure 11) [73,74]. This class of nucleosides demonstrated unique characteristics in vitro that would eventually translate into the clinic. PSI-6130 (28) was shown to be an inhibitor of HCV replication in the cell-based replicon assay (90% effective concentration [EC90]=4.5 µM) and its triphosphate (29) was shown to be a potent inhibitor of the HCV NS5B polymerase (IC50=0.34 µM) with an intracellular half-life of approximately 6 h. This class of nucleosides demonstrated exquisite selectivity for HCV versus other Flaviviruses, other RNA viruses as well as for HIV and HBV. The class showed broad genotype coverage inhibiting the HCV polymerase from genotypes 1 to 6. Triphosphate 29 was also shown not to be an inhibitor of any of the human DNA or RNA polymerases. PSI-6130 (28) demonstrated a safety profile that did not exhibit any of the toxicology signals common to nucleosides including bone marrow and mitochondrial toxicity. Although the known 2′-C-methyl nucleoside S282T amino acid alteration in the HCV polymerase was shown to be resistant to PSI-6130 (28), the level of resistance was demonstrated to be relatively minimal (threefold) and difficult to raise in vitro [27,75]. However, in clinical studies PSI-6130 (28) delivered modest oral bioavailability and a significant amount of the drug was metabolized to an inactive uridine metabolite [21]. To rectify these shortcomings a prodrug was eventually developed leading to the simple 3′,5′-di-O-isobutyrate diester prodrug RG7128 (mericitabine; 30) [21]. RG7128 (30) demonstrated proof of concept in the clinic when administered alone or in combination with SOC [21,24,30]. In a Phase Ib multiple ascending dose study in 40 genotype 1 non-responder HCV-infected patients, RG7128 (30) was dosed at 750 mg and 1,500 mg either once daily or twice daily. The study demonstrated a dose-dependent reduction in HCV RNA levels and also showed that twice daily dosing was preferred. The 1,500 mg twice daily dose of RG7128 (30) resulted in the maximum antiviral effect producing a -2.7 log10 IU/ml drop in viral load after 14 days of monotherapy. Subsequently, two Phase IIa studies in combination with SOC (Pegasys® and Copegus®) were initiated, one in genotype 1 treatment-naives and another in genotype 2,3 non-responder HCV-infected patients. The genotype 2/3 study was the first non-genotype 1 patient study initiated with a DAA. In the Phase IIa study treating genotype 1 patients, doses of 500, 1,000 and 1,500 mg twice daily were investigated over 28 days. Study results showed that the 1,000 and 1,500 mg doses provided the best viral load reduction and were essentially equally effective producing RVRs of 85% and 88% and viral load reductions of -5.05 and -5.09 log10 IU/ml, respectively. In the genotype 2/3 patient study at a dose of 1,500 mg twice daily over 28 days, a -5 log10 IU/ml reduction in viral load was observed which translated Antiviral Chemistry & Chemotherapy 22.1 into a 90% RVR. The results of the genotype 2/3 patient study demonstrated for the first time that a DAA could be effective in the clinic in this patient population and corroborated the broad genotype coverage predicted by the in vitro data. In both genotype 1 and genotype 2/3 patient studies there were no significant adverse events reported and the safety was described as no different than placebo with SOC. In both the genotype 1 and genotype 2/3 Phase IIa clinical studies where RG7128 (30) was dosed in combination with SOC no viral breakthroughs were observed over the course of therapy. Subsequent clonal analyses showed that there was no identified NS5B S282T variants in the treatment groups nor was there any pre-existing S282T viral variants observed at baseline in the patient population studied [75]. These data support the high barrier to resistance observed in the preclinical studies for this nucleoside class. RG7128 (30) progressed into a Phase IIb clinical study evaluating 408 genotype 1 and 4 HCV patients when dosed with 500 or 1,000 mg twice daily RG7128 (30) in combination with SOC over 12 or 24 weeks assessing SVR as the primary end point. An interim 12-week analysis was reported which showed an average 83% complete early virological response (cEVR) for the 1,000 mg RG7128 12-week regimen and no evidence of drug resistance [22,76]. RG7128 (30) is expected to complete the Phase IIb study in the near term. With the hope of demonstrating proof of concept that the combination of two DAAs could lead to an IFN-sparing clinical regimen, RG7128 (30) was combined with a protease inhibitor RG7227 (danoprevir) in a 14-day clinical study in genotype 1 patients to assess safety and viral kinetics [77]. The study demonstrated that combination of the two DAAs was safe and well tolerated and that at the highest dose levels (1,000 mg RG7128 and 900 mg danoprevir) in both treatmentnaive and IFN-null responders, viral load declines of -5.1 log10 IU/ml and -4.9 log10 IU/ml were observed. This combination produced a viral decline that is quantitatively similar to that observed when each individual agent is combined with IFN and RBV. These results demonstrated that in the clinic the combination of a nucleoside HCV polymerase inhibitor and a protease inhibitor has the potential to provide a promising combination for future HCV therapy. Metabolism studies on the cytidine nucleoside PSI6130 (28; Figure 11) showed that not only was the nucleoside converted to its active triphosphate derivative 29, but that it was also converted to the uridine nucleoside PSI-6206 (31; Figure 12) in vivo [78,79]. However, this uridine nucleoside was shown not to be active as an inhibitor of HCV replication in the replicon assay. Subsequent studies indicated that the triphosphate of PSI-6206 (32; Figure 12) was a potent 35 MJ Sofia inhibitor of the HCV NS5B polymerase with an IC50=1.19 µM and that this triphosphate had a long desirable intracellular half-life of 36 h [80]. Study of the phosphorylation kinetics of the uridine nucleoside 31 showed that its lack of activity was related to the lack of conversion to its 5′-monophosphate derivative and that subsequent conversion of the 5′-monophosphate to the corresponding 5′-diphosphate and -triphosphate derivatives was facile. In an attempt to take advantage of the long intracellular half-life of the uridine triphosphate 32 with the hope of potentially developing a once-daily drug, a prodrug of the uridine 5′-monophosphate was pursued. Similar to the nucleosides discussed above, a phosphate prodrug approach was investigated to overcome the enzymatic blockade at the monophosphorylation step and effectively deliver the uridine nucleoside 5′-monophosphate. A phosphoramidate prodrug strategy was implemented with the expectation that first pass metabolism of the prodrug moiety in the liver would achieve efficient delivery to the liver of the uridine nucleoside 5′-monophosphate [21,81,82]. Extensive SAR around the phosphoramidate moiety showed that the preferred substitution to achieve submicromolar whole cell HCV replicon potency without cellular toxicity required a small branched alkyl ester at the terminal carboxylate ester moiety, a small alkyl group at the alpha position of the amino acid substitutent and a simple phenoxy or halogenated phenoxy phosphate ester group. To achieve the required delivery of the prodrug to the liver and subsequent release of the nucleoside 5′-monophosphate in hepatocytes, stability studies in simulated gastric fluids (SGF) and simulated intestinal fluids (SIF) and stability on exposure to liver S9 fractions were carried out. Several compounds provided the desired profile which showed good stability in SGF, SIF and blood but a short half-life in liver S9 fractions, thus supporting the expectation that the phosphoramidate strategy could enable liver targeting. The 2′-α-F-2′-β-C-methyluridine 5′-phosphoramidates were subsequently shown to generate high concentrations of the corresponding nucleoside triphosphate 32 in primary hepatocytes of rat, dog, monkey and human, and eventually showed in rat and dog PK studies that the phosphoramidates could get to the liver and generate high levels of the uridine nucleoside triphosphate 32 there. The lead compound Figure 12. 2′-α-F-2′-β-C-Methyluridine nucleoside and nucleotide phosphoramidate prodrug inhibitors of HCV replication O HO O HO O O NH N HO O P OH O O O P O OH P O O O OH F HO NH N F IC50=1.19 µM PSI-6206 EC50=>100 µM 31 32 O O O O O N H P O O N O O HO NH F PSI-7851 EC90=0.52 µM, CC50=>100 µM, 33 O O O N H P O O N O O HO NH F PSI-7977 EC90=0.42 µM, CC50=>100 µM, 34 CC50, 50% cytotoxic concentration; EC50, 50% effective concentration; EC90, 90% effective concentration; IC50, 50% inhibitory concentration. 36 ©2011 International Medical Press Nucleotide prodrugs for HCV therapy PSI-7851 (33) was subsequently shown to be devoid of mitochondrial, bone marrow and general cellular toxicities. Combination studies with either IFN and/ or RBV and other DAAs such as protease, non-nucleoside polymerase, or NS5A inhibitors showed that PSI7851 (33) was either additive or synergistic in its effect on HCV replication in the replicon assay [83]. When evaluated in the S282T mutant replicon known to be resistant to 2′-C-methyl nucleosides, PSI-7851 (33) demonstrated a 16.4-fold resistance relative to wild type [83,84]. PSI-7851 (33), a 1:1 mixture of diastereomers at the phosphorus centre of the phosphoramidate moiety, was chosen for further development and progressed into Phase I human clinical trials. In a single ascending dose clinical study of healthy volunteers receiving doses up to 800 mg once daily, PSI7851 (33) was demonstrated to be generally safe and well-tolerated, with no dose-limiting toxicities. In addition, PK results exhibited a systemic exposure profile that was described as consistent with rapid uptake of the drug by the liver and low plasma exposure to the prodrug PSI-7851 (33). PSI-7851 (33) was progressed into a 3-day multiple ascending dose study in treatmentnaive, HCV-infected patients with the object of evaluating safety, PK and effect on viral load. HCV RNA levels declined in a dose dependent manner with the maximal mean change from baseline of -1.95 log10 IU/ml at the 400 mg dose. In addition, no pre-existing or treatmentemergent S282T resistant virus was detected, nor was there evidence of viral resistance after therapy [21]. With proof of concept demonstrated in HCV patients, further development of the 2′-α-F-2′-β-Cmethyluridne phosphoramidate proceeded using PSI7977 (34; EC90=0.42 µM) the more active (>10-fold) single diastereomer of PSI-7851 (33) [81]. PSI-7977 (34) was obtained by direct crystallization from the diastereomeric mixture and it was shown to have the Sp stereochemistry at the phosphorus atom thus representing the first example of the crystallization and x-ray structure determination of a nucleotide phosphoramidate. In a 28-day Phase IIa clinical study in genotype 1 treatment-naive HCV patients, in which PSI-7977 (34) was dosed at either 100, 200 or 400 mg once daily in combination with pegylated IFN/RBV, RVR rates of 88%, 94% and 93%, respectively, were observed [85,86]. In addition, no viral resistance was detected nor were there any significant adverse events reported. Recently, a 14-day monotherapy study with PSI-7977 (34) showed that HCV genotype 1 treatment-naive patients achieved an average -5.0 log10 decline in viral load with 88% of patients reaching undetectability (<15 IU/ml) after 14 days. This result is a significant improvement from what was observed for the diastereomeric mixture PSI-7851 (33) [87]. PSI-7977 (34) has entered Phase IIb clinical trials and Antiviral Chemistry & Chemotherapy 22.1 it is being evaluated in combination with IFN/RBV for its effects in genotype 1, 2 and 3 patients. In genotype 1 patients treated with PSI-7977 (34), 47 of 48 patients (98%) at 200 mg once daily and 46 of 47 patients (98%) at 400 mg once daily achieved an RVR at week 12 with no viral breakthrough and a promising safety profile [88]. In genotype 2 and 3 HCV patients a 400 mg once daily dose of PSI-7977 (34) in combination with IFN/RBV achieved an SVR 12 weeks after cessation of therapy in 96% of patients treated [88]. In addition to the continued Phase IIb study in combination with abbreviated durations of IFN/RBV, PSI-7977 (34) is being studied in combination with other nucleosides (PSI-352938) and with the NS5A inhibitor BMS-790052 [15,89]. Further structure–activity relationship studies around the 2′-α-F-2′-β-C-methyl class of nucleosides had shown that the guanosine derivative 35 (Figure 13) was weakly active (EC90=69.2 µM) as an inhibitor of HCV in the replicon assay; however, the guanosine triphosphate 36 was shown to be a good inhibitor of the HCV RNA polymerase (IC50=5.94 µM; Figure 13) [90,91]. It was also demonstrated that the guanosine triphosphate 36 was equipotent as an inhibitor of both the wild type and S282T mutant HCV polymerase: the first example of such a profile for a 2′-C-methyl nucleoside. In order to try and improve potency of this guanosine nucleoside, speculating that the first monophosphorylation was limiting, preparation of phosphoramidate prodrugs of the 2′-α-F-2′-β-C-methylguanosine 5′-monophosphate along with modifications at the C-6 position of the guanosine base were pursued [91]. This double prodrug strategy, where in addition to the metabolism of the phosphoramidate, the C-6-O-methyl group of the base is metabolized to give guanine, ultimately led to a series of HCV inhibitors having low nanomolar potency with acceptable in vitro safety and stability profiles. Selected compounds were shown to produce high levels of the guanosine triphosphate 36 in primary human hepatocytes and a radiolabelled study showed that a guanosine phosphoramidate derivative provided a desirable 3.5–4.8:1 liver to plasma ratio. The single Sp phosphoramidate diastereomer, PSI-353661 (37; EC90=8 nM; Figure 13) was obtained by crystallization from the diastereomeric mixture and was shown to be the more potent of the two possible diastereomers. In vitro studies with IFN or RBV and other DAAs demonstrated that PSI-353661 (37) could be combined to produce an additive or synergistic antiviral effect [92]. PSI-353661 (37) was selected as a clinical development candidate for the treatment of HCV infections and is awaiting the start of clinical evaluation. Another 2′-α-F-2′-β-C-methyl nucleoside having a 7-ethynyl-7-deaza adenosine base unit (38; Figure 14) was reported to be an efficient inhibitor of the 37 MJ Sofia Figure 13. 2′-α-F-2′-β-C-Methylguanosine nucleoside and nucleotide phosphoramidate prodrug inhibitors of HCV replication O N HO O HO O NH N N HO NH2 O P O O P OH O P OH O NH N O N OH F HO EC90=69.2 µM 35 O N NH2 F IC50=5.94 µM 36 O O N H O OMe N P O N N O N O HO NH2 F PSI-353661 EC90=0.008 µM 37 CC50, 50% cytotoxic concentration; EC90, 90% effective concentration; IC50, 50% inhibitory concentration. Figure 14. 7-Deaza-7-ethynyl-2′-α-F-2′-β-C-methyladenosine nucleoside and nucleotide phosphoramidate prodrug inhibitors of HCV replication NH2 NH2 HO O HO O N N HO N O P O OH P OH O O P O O OH F HO EC50=24 µM 38 N N N F IC50=0.4 µM 39 NH2 R O O O N H P O O O N N N Ar HO F R=Me, Ph Ar=Ph, p-Me-Ph, p-F-Ph, p-MeO-Ph, 1-naphthyl, 2-naphthyl No observed activity 40 EC50, 50% effective concentration; IC50, 50% inhibitory concentration. 38 ©2011 International Medical Press Nucleotide prodrugs for HCV therapy Figure 15. C-6-Hydrazido sulfonamide 2′-C-methyladenosine and 2′-C-methyl-2-amino-adensosine nucleoside and nucleotide cyclic phosphate prodrug inhibitors of HCV replication HN N HO HO HN N HO R1 P O 41 R1=H EC50=300 µM, CC50>300 µM 42 R1=NH2 EC50=92 µM, CC50>300 µM OH O N O O SO2CH3 N N P R1 43 R1=H EC50=100 µM 44 R1=NH2 EC50=23.8 µM HN HO N OH HO SO2CH3 N N O OH O N N O N N O SO2CH3 N N N R1 OH 45 R1=H EC50=68.5 µM 46 R1=NH2 EC50=1.7 µM HN N N O O O n-Pr O S O P O O N SO2CH3 N N R1 OH 47 R1=H EC50=0.039 µM, CC50>50 µM 48 R1=NH2 EC50=0.008 µM, CC50>50 µM CC50, 50% cytotoxic concentration; EC50, 50% effective concentration. HCV NS5B polymerase (IC50=0.4 µM) yet its activity in a whole cell Huh7 replicon assay was reported to be low (EC50=24 µM) [93]. Consequently, a small series of phosphoramidate prodrugs (40) of 2′-α-F2′-β-C-methyl-7-ethynyl-7-deaza adenosine (38) was reported, however, no antiviral activity was observed for these prodrugs and the series was not progressed further [94]. Antiviral Chemistry & Chemotherapy 22.1 3′,5′-Cyclic phosphate nucleotide prodrugs The use of a 3′,5′-cyclic phosphate prodrug construct for delivering a nucleoside 5′-monophosphate into cells is a relatively uncommon prodrug motif [95–97]. Its application has only recently been selectively applied in the development of nucleotide inhibitors for HCV polymerase. Little is known about the mechanism by which these cyclic phosphates are metabolized to the 39 MJ Sofia Figure 16. 2′-C-Methylcytidine-3′,5′-cyclic phosphoramidate prodrug inhibitor of HCV replication NH2 O O N N O O P NH EtO O OH O Slow eluting diastereomer EC50=4 µM, CC50=>100 µM 49 CC50, 50% cytotoxic concentration; EC50, 50% effective concentration. Figure 17. O-6-Ethyl-2′-α-F-2′-β-C-Methylguanosine3′,5′-cyclic phosphate prodrug inhibitor (PSI-352938) of HCV replication OEt N O O O P O O N N N NH2 F PSI-352938 EC90=1.37 µM, CC50=>100 µM 50 CC50, 50% cytotoxic concentration; EC90, 90% effective concentration. desired 5′-monophosphates; however, based on the reported HCV data and recent clinical data [98], it is clear that this approach is an effective prodrug strategy for delivering in vivo a nucleoside 5′-monophosphate into cells. 2′-C-Methyl nucleotide 3′,5′-cyclic phosphate prodrugs The first reported example of a 3′,5′-cyclic phosphate prodrug construct for inhibiting HCV was reported in an attempt to develop a set of 2′-C-methyl ribonucleosides with modified purine bases where the purine base has a hydrazido sulfonamide at the C-6 40 position (Figure 15) [99]. The parent nucleosides 41, 42 were essentially inactive (EC50≥92 µM) as inhibitors of HCV replication in the replicon assay and subsequently were shown not to be substrates for adenosine kinase, suggesting an inability to be phosphorylated to the respective monophosphates 43 and 44. Preparation of the 3′,5′-cyclic phosphate derivatives 45, 46 resulted in significant improvement in replicon potency (EC50s=68.5 and 1.7 µM) relative to both the 5′-monophosphate derivatives 43 and 44 (EC50s=100 and 23.8 µM) and the parent nucleosides 41 and 42. This suggests that even though 44 and 45 retain a phosphate monoacid, the cyclic phosphate construct sufficiently reduces the polarity relative to the 5′-monophosphates 43 and 44 to improve cell penetration. To further enhance cell permeability characteristics, the acid of the cyclic phosphates was converted to the SATE 47 and 48 resulting in substantial improvement in HCV replicon potency [99]. The SATE cyclic phosphate prodrugs 47 and 48 demonstrated a 7,000- to 11,000-fold improvement in potency (EC50s=0.463 to 0.008 µM) relative the parent nucleosides with no observed cytotoxicity. Further development of these novel inhibitors has not been reported. Another application of the 3′,5′-cyclic phosphate prodrug approach focused on the study of 3′,5′-cyclic phosphoramidates in an attempted to improve the activity of 2′-C-methylcytidine (Figure 16) [100]. It was postulated that relative to the 5′-phosphoramidate prodrug, the 3′,5′-cyclic version would reduce the molecules rotational degrees of freedom and potentially improve entry into cells. In addition, use of a cyclic phosphate would remove the need for a phenolic substituent on the phosphoramidate moiety. Preparation of 2′-Cmethycytidine 3′,5′-cyclic phosphoramidates like 49 (Figure 16) lead to prodrugs which were a mixture of diastereomers that were ultimately separated chromatographically. In some cases there was a substantial difference in activity between the prodrug diastereomers, however, in no case did any of the prodrugs demonstrate significant improvement in anti-HCV activity over 2′-Cmethylcytidine itself. Contrary to the results from the HCV replicon assay, several cyclic phosphoramidate analogues did produce enhanced levels of nucleoside triphosphate when incubated with primary human hepatocytes (two- to sevenfold). Subsequent in vivo studies in the hamster showed a lack of oral bioavailability as measured by the levels of nucleoside triphosphate in the liver, possibly due to poor absorption or gastrointestinal instability. However, subcutaneous administration did show a 10- to 50-fold improvement in the levels of nucleoside triphosphate relative to that produced with 2′-C-methylcytidine. No further developments on this class of nucleotide prodrugs have been reported. ©2011 International Medical Press Nucleotide prodrugs for HCV therapy 2′-α-F-2′-β-C-Methyl nucleotide 3′,5′-cyclic phosphate prodrugs The 3′,5′-cyclic phosphate prodrug strategy was also applied to the 2′-α-F-2′-β-C-methyl class of nucleosides and ultimately resulted in the clinical development candidate PSI-352938 (50; Figure 17) [101]. PSI-352938 (50) demonstrated for the first time human clinical proof of concept for use of the cyclic phosphate prodrug approach to deliver a 5′-monophosphate [98]. In the case of the 2′-α-F-2′-β-C-methyl class of nucleosides the 3′,5′-cyclic phosphate prodrug approach was explored in an attempt to deliver 6-substituted guanosine derivatives [101]. This approach was taken because the parent 2′-α-F-2′-β-C-methylguanosine nucleoside 35 was a weak inhibitor in the HCV replicon assay even though its corresponding 2′-α-F-2′β-C-methylguanosine triphosphate (36) was a good inhibitor of the HCV polymerase (IC50=5.94 µM) and equipotent against both the wild type and S282T mutant polymerases. In the case of the 2′-α-F-2′-βC-methylguanosine series, unlike in the case of other 2′-C-methyl nucleosides, simple alkyl and aryl esters of the 3′,5′-cyclic phosphate were explored and SAR optimization focused around both the 6-position of the guanosine base and the phosphate ester moiety. These 2′-α-F-2-β-C-methyl 6-substituted guanosine 3′,5′- cyclic phosphate esters were shown to be metabolized to the 2′-α-F-2-β-C-methylguanosine triphosphate (36); however, the mechanistic details of the metabolism have yet to be reported. It was ultimately determined based on potency, cytotoxicity profile, gastrointestinal stability characteristics, and triphosphate production in vitro and in vivo that the Rp diastereomer PSI-352938 was the optimal candidate for clinical development. PSI-352938 was shown to have an EC90=1.37 µM with no cytotoxicity in multiple cell lines nor any observed mitochondrial toxicity or bone marrow toxicity when tested up to 100 µM in vitro. When tested against the HCV NS5B polymerase isolated from genotypes 1 to 4, the triphosphate 36 of PSI-352938 (50) was shown to be equipotent against all genotypes and PSI-352938 demonstrated in the replicon assay to be additive or synergistic in combination with IFN and RBV and with other DAAs [83,102]. In addition, the combination of PSI-352938 and the uridine nucleotide phosphoramidate PSI-7977 (34; Figure 12) was shown to effectively clear both the wild type and S282T mutant replicons [102,103]. In vitro and in vivo studies showed that high triphosphate levels were produced in primary human hepatocytes and in rat liver (area-under-the-curve [AUC0-24]=41,893 ng h/g) when PSI-352938 (50) was administered orally at a 50 mg/kg dose. PSI-352938 (50) Table 1. Nucleoside/nucleotide prodrugs: comparison of Phase I and Phase IIa human clinical trial resultsa Drug (compound number) Class Inhibition HCV replicon, µM CC50, µM Monotherapy Combination with GT 1 HCV patients SOC GT 1 HCV patients Maximum effect Log10 IU/ml, Maximum effect Log10 IU/ml, Status of dose, mg daysb dose, mg daysb program Nucleoside prodrugs RG7128 2′-F,2′-methyl EC90=2.5 >100 1,500 -2.7 (14) 1,000 -5.05 (28) (30) cytidine (twice daily) (twice daily) NM283 2′-OH,2′-methyl EC50=1.23 >100 800 -1.2 (14) 800c >-4 (28) (2) cytidine (twice daily) (twice daily) >100 4,500 -3.7 (14) 1,500 -5.2 (28) R1626 4′-azido EC50=1.28d (20) cytidine (twice daily) (twice daily) Nucleotide prodrugs PSI-7977 2′-F,2′-methyl EC90=0.42 >100 400e -1.95e (3) 400 -5.3 (28) (34) uridine PA (once daily) (once daily) PSI-352938 2′-F,2′-methyl EC90=1.37 >100 200 -4.64 (7) ND ND (50) guanosine 3′,5′- (once daily) cyclic phosphate IDX184 2′-OH,2′-methyl EC50=0.4 >100 100 -0.74 (3) 150 -4.1 (28) (17) guanosine PA (once daily) (once daily) INX-08189 2′-OH,2′-methyl ED50=0.01 7 25 -1.03 (7) ND ND (16) guanosine PA (once daily) Completed Phase II Discontinued GI toxicity Discontinued haematological toxicity Ongoing Phase IIb study Ongoing Phase Ib study Full clinical hold changed to partial clinical hold Ongoing Phase Ib study Comparison across clinical studies. bNumbers in brackets represent number of days on the drug. cCombination with interferon only no ribavirin present. dNo direct 50% effective concentration (EC50) available, represents EC50 of the parent nucleoside R1479. eResult from PSI-7851 the diastereomeric mixture. CC50, 50% cytotoxic concentration; EC90, 90% effective concentration; GI, gastrointestinal; GT, genotype; ND, no data available; PA, 5′-Phosphoramidate; SOC, standard of care. a Antiviral Chemistry & Chemotherapy 22.1 41 MJ Sofia Figure 18. HepDirect prodrug technology and the prodrug decomposition pathway. Ar O O P O Cytochrome P450 Nucleoside Ar OH O O P O Nucleoside O O HepDirect Prodrug HO Spontaneous Ar O P O Nucleoside Spontaneous O O O Ar O HO P O Nucleoside OH was taken into a Phase I clinical study and was shown to be generally safe and well-tolerated when dosed up to 1,600 mg in healthy volunteers [104]. In a multiple ascending dose study in HCV genotype-1-infected patients, PSI-352938 was administered at doses of 100, 200 and 300 mg once daily and 100 mg twice daily over 7 days to assess safety, tolerability and antiviral response [98]. In the 40 patients studied HCV RNA levels declined consistently through the 7-day treatment period with a mean HCV viral load change from baseline of -4.31 log10 IU/ml, -4.65 log10 IU/ml, -3.94 log10 IU/ml and -4.59 log10 IU/ml for patients receiving 100 mg once daily, 200 mg once daily, 300 mg once daily and 100 mg twice daily. For those patients receiving the 200 and 300 mg doses 11 of 16 patients achieved HCV viral load below the limit of quantification. In addition, there were no reported adverse events in this study [98]. The early potency achieved by PSI-352938 (50) is comparable to that observed with NS3 protease inhibitors and PK parameters supported once-daily dosing. These results represent the first demonstration of clinical efficacy for a cyclic phosphate prodrug and represent the most potent nucleoside evaluated to date in a clinical setting (Table 1). PSI-352938 (50) was further evaluated in a dual nucleotide DAA combination study with the pyrimidine nucleotide prodrug PSI-7977 (34) to evaluate the need for IFN in the treatment of HCV infection [87]. In this proof of concept study, no significant PK interactions between PSI-352938 (50) and PSI-7977 (34) were 42 observed and no viral breakthrough was detected during therapy. This dual nucleotide combination demonstrated at day 14 a -4.6–5.5 log10 decline in viral load with an average of 94% of patients having viral loads below the limit of detection (<15 IU/ml). This study is the first proof of concept that two nucleosides/nucleotides can be combined as a possible DAA treatment regimen. A Phase II study is being planned for this dual nucleotide DAA combination [87]. Cyclic 1-aryl-1,3-propanyl nucleotide phosphate esters: HepDirect prodrugs Preparation of a nucleoside 5′-monophosphate cyclic diester using a 1-aryl-1,3-propanyl prodrug moiety to accomplish liver targeting has been called HepDirect prodrug technology [33,105]. HepDirect technology was developed specifically to deliver drugs to the liver by taking advantage of liver-specific enzymes which would cleave the prodrug moiety thus revealing the active drug in hepatocytes. HepDirect prodrugs have been reported to be very stable in aqueous solutions, blood and non-hepatic tissues other than that of the gastrointestinal tract. For HepDirect technology, activation is achieved via a cytochrome P450 mediated oxidation (Figure 18). The described mechanism for nucleoside prodrug cleavage starts with hydroxylation of the C-4 tertiary carbon of the pro-moiety by CYP3A4 followed by rapid ring opening and then β-elimination to reveal the nucleoside monophosphate and an aryl vinyl ketone byproduct. Although ©2011 International Medical Press Nucleotide prodrugs for HCV therapy Figure 19. HepDirect prodrug analogues of 2′-C-methyladenosine nucleoside monophosphates O P O NH2 N O O O HO Cl N N N OH NTP levels in rat hepatocytes =389 nmol/g 51 O Cl P O NH2 N O O O H2N O N N OH O NTP levels in rat hepatocytes =117 nmol/g 52 release of aryl vinyl ketone as a byproduct of HepDirect cleavage raises toxicological concerns, it is hypothesized that this byproduct would be rapidly scavenged by high levels of glutathione in the liver, thus minimizing the associated risks. The HepDirect liver targeting prodrug approach was demonstrated in the clinic with pradefovir, a HepDirect prodrug version of adefovir [33]. 2′-C-Methyladenosine HepDirect prodrugs 2′-C-Methyladenosine (9; Figure 6; EC50=0.3 µM) was shown to be active as an inhibitor of HCV replication in the subgenomic replicon assay, however it was also determined to be an efficient substrate for adenosine deaminase, thus limiting its potential in vivo [106,107]. Since it was hypothesized that preparation of a 5′-monophosphate prodrug would block deamination and increase therapeutic levels of 2′-C-methyladenosine triphosphate (10) in the liver, a HepDirect prodrug approach was investigated. An SAR study evaluated activation in rat hepatocytes by modifications to the aryl substituent of the HepDirect prodrug moiety [108]. This assessment showed that the prodrugs were generally efficiently cleaved and converted to the nucleoside triphosphate 10 (Figure 6) particularly those with halogen substituted phenyl 51 or pyridyl groups (Figure 19). In vivo intravenous administration Antiviral Chemistry & Chemotherapy 22.1 O N Cl P O NH2 N O N O O O N N O O NTP levels in rat hepatocytes =296 nmol/g 53 was able to achieve substantial levels of nucleoside triphosphate in rat liver, but oral administration resulted in a > fivefold lower level of liver triphosphate and only a 5% oral bioavailability. To circumvent this bioavailability issue the HepDirect prodrug approach was combined with other prodrug groups that would protect the 2′- and/ or the 3′-hydroxyl groups or the N-6 amino group of the base (Figure 19). The combination of HepDirect with a 2′,3′-cyclic carbonate 53 appeared to be the most fruitful in that it provided a 2- to 10-fold improvement in the amount of nucleoside triphosphate observed in the liver compared to compounds incorporating only the HepDirect moiety. Unfortunately, no inhibition of HCV replicon activity was provided for any of the HepDirect derivatives to compare their potency to other prodrug approaches and no additional data have been forthcoming on the status of these agents. Bis(S-acyl-2-thioethyl) nucleotide phosphate prodrugs: SATE prodrugs The bis(S-acyl-2-thioethyl) phosphate strategy (SATE) has been applied widely in the field of nucleoside 5′-phosphate prodrugs [33,34]. As described above in the case of IDX184 which contains a SATE monoester, an acid and an equivalent of episulfide is liberated as a result of each thioester cleavage (Figure 20). Episulfide 43 MJ Sofia Figure 20. SATE prodrug decomposition pathway O O R S R S O O O P O R Esterase Nucleoside S HS O O O P O Nucleoside O SATE Prodrug O R O O HO P S O Esterase Nucleoside S S O HO HO P O Nucleoside Figure 21. SATE prodrug of the 5′-monophosphate of 7-deaza-7-ethynyl-2′-α-F-2′-β-C-methyladenosine O NH2 S O O O P S N O O HO N N F O EC50=8 µM 54 EC50, 50% effective concentration. is highly reactive and is a known mutagen, consequently it has been assumed that release of such an 44 agent from a pronucleotide compound would be of some concern [109]. The only reported application of the bis-SATE prodrug method to nucleotide HCV inhibitors was toward 2′-α-F-2′-β-C-methyl 7-ethynyl-7-deaza adenosine 38 [94]. In this case the activity of the SATE prodrug 54 (EC50=8 µM) was threefold better than the parent nucleoside (Figure 21). However, no additional data or progress has been reported for this compound or on the use of bis-SATE prodrugs for delivering nucleotides for inhibiting HCV. Discussion Never before has the use of phosphate prodrugs played such a significant role in the discovery and development of nucleoside-based drugs as has been the case in the area of inhibitors of HCV. The translation of multiple examples of phosphate prodrugs of nucleotides into the clinic and the demonstration of clinical proof of concept and beyond are equally unprecedented. HCV has offered a unique opportunity to apply phosphate prodrug technology. Since HCV is a disease of the liver, drug hunters are able to leverage the robust characteristics ©2011 International Medical Press Nucleotide prodrugs for HCV therapy of liver metabolism and first pass metabolism as the means for achieving target organ drug levels and obviating the need for maintaining high circulating levels of a potentially unstable prodrug. However, because of liver targeting, decomposition of the phosphate prodrug to the desired nucleoside 5′-monophosphate does release prodrug byproducts into the liver at potentially high concentrations. This release of sometimes potentially reactive or toxic metabolites does pose a concern in developing prodrugs of nucleoside monophosphates. This concern is particularly relevant to HCV patients with advanced liver disease where liver function is compromised and therefore, may have a lower capacity to clear or process metabolites. Some of the prodrug strategies implemented for delivering nucleoside monophosphates such as the SATE or HepDirect strategies are known to release reactive metabolites that should be of concern in assessing their long term safety potential. Other prodrug strategies such as the phosphoramidate release different metabolites such as phenol or naphthol. Although phenol is readily cleared by the liver, the toxicity of substituted phenols may be of concern in deciding to progress a nucleotide prodrug forward into development [110,111]. Each of these liability concerns must be put into context of dose and PK. It is possible to circumvent any toxicity concerns if the dose of drug administered is sufficiently small to not warrant concern or if the PK profile of the metabolites limits exposure. Clearly the increased potency of these nucleotide prodrugs relative to the first generation nucleoside HCV inhibitors increases the therapeutic index and reduces the chances of observing undesired side effects. However, long term animal toxicity studies and clinical safety assessment over extended dosing periods will ultimately address the concerns over any potential human toxicity. The nucleotide phosphate prodrugs which have entered clinical study have demonstrated superiority over the first generation nucleoside inhibitors developed for treating HCV (Table 1). The use of the nucleoside 5′-monophosphate prodrugs has demonstrated dramatic potency enhancements relative to first generation nucleoside inhibitors in the cellular subgenomic replicon assay, higher levels of triphosphate in whole cells and in livers of animals when dosed orally. To a large extent this improved in vitro and whole animal profile has translated into the clinical setting. In the clinic, dosing frequency has been reduced from twice daily to once daily, drug load has been greatly reduced and impressive viral load declines and RVR rates have been achieved. However, in vitro HCV replicon potency alone can not itself justify the robust initial clinical results observed for these phosphate prodrugs. The least potent inhibitor in the subgenomic replicon assay, PSI-352938 (50; Figure 17), produced the greatest viral load decline in Antiviral Chemistry & Chemotherapy 22.1 human monotherapy studies, therefore issues such as PK must be playing a critical role in the overall human clinical response. How the human body handles these prodrugs is yet to be determined. A question that remains unanswered is whether there are specific transporters that play a clinically meaningful role in prodrug uptake into the liver or transport through the gut. It has been suggested that nucleosides/nucleotides will eventually become the backbone of anti-HCV therapy, as they have for HIV HAART. Their broad genotype coverage and resistance profile put them in a unique position and make them the drug class of choice to combine with DAAs of different mechanisms of action. With the identification of both purine and pyrimidine nucleotide prodrugs with different resistance profiles and metabolic pathways, the possibility also exists for combining two nucleosides/nucleotides into one regimen as has been done in the HIV field. Studies to explore different combinations of DAAs including dual nucleotide combinations have begun. Results in a 14-day dual nucleotide proof of concept study with PSI-7977 (34) and PSI-352938 (50) appear promising. It is the hope that these studies will pave the way forward for potentially new therapeutic paradigms in the treatment of HCV which include the elimination of IFN. However, issues such as safety, tolerability and the extent of a SVR after 12 or 24 weeks of therapy will help determine which of the current and future nucleotide produgs will successfully reach the market. At this stage long term safety is still unknown and only PSI-7977 (34) has demonstrated an SVR in the clinic, but only on a small patient population. Longer term studies are underway to better define the risk:benefit profile of these agents. However, these agents provide great hope for patients suffering from HCV. Disclosure statement MJS is an employee of Pharmasset, Inc. and receives salaried compensation in his role as Vice President of Chemistry. References 1. WHO. Hepatitis C. Geneva: World Health Organization 2000. 2. Lavanchy D. The global burden of hepatitis C. Liver Int 2009; 29 Suppl 1:74–81. 3. Simmonds P. Genetic diversity and evolution of hepatitis C virus – 15 years on. J Gen Virol 2004; 85:3173–3188. 4. Le Guillou-Guillemette H, Vallet S, Gaudy-Graffin C, et al. Genetic diversity of the hepatitis C virus: impact and issues in the antiviral therapy. World J Gastroenterol 2007; 13:2416–2426. 5. Zeuzem S, Berg T, Moeller B, et al. Expert opinion on the treatment of patients with chronic hepatitis C. J Viral Hepat 2009; 16:75–90. 45 MJ Sofia 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. 22. 23. 24. 25. 26. 46 Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002; 347:975–982. Manns MP, Foster GR, Rockstroh JK, et al. The way forward in HCV treatment – finding the right path. Nat Rev Drug Discov 2007; 6:991–1000. Ge D, Fellay J, Thompson AJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature 2009; 461:399–401. Gremion C, Cerny A. Hepatitis C virus and the immune system: a concise review. Rev Med Virol 2005; 15:235–268. Reed KE, Rice CM. Overview of hepatitis C virus genome structure, polyprotein processing, and protein properties. Curr Top Microbiol Immunol 2000; 242:55–84. Sulkowski MS. Specific targeted antiviral therapy for hepatitis C. Curr Gastroenterol Rep 2007; 9:5–13. Kwong AD, McNair L, Jacobson I, et al. Recent progress in the development of selected hepatitis C virus NS3.4A protease and NS5B polymerase inhibitors. Curr Opin Pharmacol 2008; 8:522–531. Einav S, Gerber D, Bryson PD, et al. Discovery of a hepatitis C target and its pharmacological inhibitors by microfluidic affinity analysis. Nat Biotechnol 2008; 26:1019–1027. Einav S, Sobol HD, Gehrig E, et al. The hepatitis C virus (HCV) NS4B RNA binding inhibitor clemizole is highly synergistic with HCV protease inhibitors. J Infect Dis 2010; 202:65–74. Gao M, Nettles RE, Belema M, et al. Chemical genetics strategy identifies an HCV NS5A inhibitor with a potent clinical effect. Nature 2010; 465:96–100. Bressanelli S, Tomei L, Roussel A, et al. Crystal structure of the RNA-dependent RNA polymerase of hepatitis C virus. Proc Natl Acad Sci U S A 1999; 96:13034–13039. Bressanelli S, Tomei L, Rey FA, et al. Structural analysis of the hepatitis C virus RNA polymerase in complex with ribonucleotides. J Virol 2002; 76:3482–3492. Lesburg CA, Cable MB, Ferrari E, Hong Z, Mannarino AF, Weber PC. Crystal structure of the RNA-dependent RNA polymerase from hepatitis C virus reveals a fully encircled active site. Nat Struct Biol 1999; 6:937–943. Zhong W, Uss AS, Ferrari E, et al. De novo initiation of RNA synthesis by hepatitis C virus nonstructural protein 5B polymerase. J Virol 2000; 74:2017–2022. Butcher SJ, Grimes JM, Makeyev EV, et al. A mechanism for initiating RNA-dependent RNA polymerization. Nature 2001; 410:235–240. Sofia MJ, Furman PA, Symonds WT. 2′-F-2′-C-methyl nucleosides and nucleotides for the treatment of hepatitis C virus: from discovery to the clinic. In RSC Drug Discovery Ser. Edited by JC Barrish, PH Carter, PTW Cheng and R Zahlers. Royal Society of Chemistry; 2010. pp. 238-266. Le Pogam S, Yan JM, Kosaka A, et al. No evidence of drug resistance or baseline S282T resistance mutation among GT1 and GT4 HCV infected patients on nucleoside polymerase inhibitor RG7128 and PEG-INF/ RBV combination treatment for up to 12 weeks: interim analysis from the propel study. 61st Annual Meeting of the American Association for the Study of Liver Diseases. 29 October - 2 November 2010, Boston, MA. Furman PA, Lam AM, Murakami E. Nucleoside analog inhibitors of hepatitis C viral replication: recent advances, challenges and trends. Future Med Chem 2009; 1:1429–1452. Brown NA. Progress towards improving antiviral therapy for hepatitis C with hepatitis C virus polymerase inhibitors. Part I: nucleoside analogues. Expert Opin Investig Drugs 2009; 18:709–725. Bobeck DR, Schinazi RF, Coats SJ. Advances in nucleoside monophosphate prodrugs as anti-HCV agents. Antivir Ther 2010; 15:935–950. Dutartre H, Bussetta C, Boretto J, et al. General catalytic deficiency of hepatitis C virus RNA polymerase with an S282T mutation and mutually exclusive resistance towards 2′-modified nucleotide analogues. Antimicrob Agents Chemother 2006; 50:4161–4169. 27. Le Pogam S, Jiang WR, Leveque V, et al. In vitro selected Con1 subgenomic replicons resistant to 2′-C-methylcytidine or to R1479 show lack of cross resistance. Virology 2006; 351:349–359. 28. Migliaccio G, Tomassini JE, Carroll SS, et al. Characterization of resistance to non-obligate chain-terminating ribonucleoside analogs that inhibit hepatitis C virus replication in vitro. J Biol Chem 2003; 278:49164–49170. 29. Ali S, Leveque V, Le Pogam S, et al. Selected replicon variants with low-level in vitro resistance to the hepatitis C virus NS5B polymerase inhibitor PSI-6130 lack crossresistance with R1479. Antimicrob Agents Chemother 2008; 52:4356–4369. 30. Burton JR, Jr. HCV NS5B polymerase inhibitors. Clin Liver Dis 2009; 13:453–465. 31. Pierra C, Amador A, Benzaria S, et al. Synthesis and pharmacokinetics of valopicitabine (NM283), an efficient prodrug of the potent anti-HCV agent 2′-C-methylcytidine. J Med Chem 2006; 49:6614–6620. 32. Brandl M, Wu X, Holper M, et al. Physicochemical properties of the nucleoside prodrug R1626 leading to high oral bioavailability. Drug Dev Ind Pharm 2008; 34:683–691. 33. Hecker SJ, Erion MD. Prodrugs of phosphates and phosphonates. J Med Chem 2008; 51:2328–2345. 34. Ariza ME. Current prodrug strategies for the delivery of nucleotides into cells. Drug Des Rev Online 2005; 2:373–387. 35. Mackman RL, Cihlar T. Prodrug strategies in the design of nucleoside and nucleotide antiviral therapeutics. Annu Rep Med Chem 2004; 39:305–321. 36. Mehellou Y, Balzarini J, McGuigan C. Aryloxy phosphoramidate triesters: a technology for delivering monophosphorylated nucleosides and sugars into cells. ChemMedChem 2009; 4:1779–1791. 37. Chou TF, Cheng J, Tikh IB, et al. Evidence that human histidine triad nucleotide binding protein 3 (Hint3) is a distinct branch of the histidine triad (HIT) superfamily. J Mol Biol 2007; 373:978–989. 38. Chou TF, Baraniak J, Kaczmarek R, et al. Phosphoramidate pronucleotides: a comparison of the phosphoramidase substrate specificity of human and Escherichia coli histidine triad nucleotide binding proteins. Mol Pharm 2007; 4:208–217. 39. Chou TF, Bieganowski P, Shilinski K, et al. 31P NMR and genetic analysis establish hinT as the only Escherchia coli purine nucleoside phosphoramidase and as essential for growth under high salt conditions. J Biol Chem 2005; 280:15356–15361. 40. McGuigan C, Cahard D, Sheeka HM, De Clercq E, Balzarini J. Phosphoramidate derivatives of d4T with improved anti-HIV efficacy retain full activity in thymidine kinase-deficient cells. Bioorg Med Chem Lett 1996; 6:1183–1186. 41. McGuigan C, Sheeka HM, Mahmood N, Hay A. Phosphate derivatives of d4T as inhibitors of HIV. Bioorg Med Chem Lett 1993; 3:1203–1206. 42. Balzarini J, Kruining J, Wedgwood O, et al. Conversion of 2′,3′-dideoxyadenosine (ddA) and 2′,3′-didehydro2′,3′-dideoxyadenosine (d4A) to their corresponding aryloxyphosphoramidate derivatives markedly potentiates their activity against human immunodeficiency virus and hepatitis B virus. FEBS Lett 1997; 410:324–328. 43. Carroll SS, Olsen DB. Nucleoside analog inhibitors of hepatitis C virus replication. Infect Disord Drug Targets 2006; 6:17–29. 44. Pierra C, Benzaria S, Amador A, et al. NM 283, an efficient prodrug of the potent anti-HCV agent 2′-C-methylcytidine. Nucleosides Nucleotides Nucleic Acids 2005; 24:767–770. 45. Gardelli C, Attenni B, Donghi M, et al. Phosphoramidate prodrugs of 2′-C-methylcytidine for therapy of hepatitis C virus infection. J Med Chem 2009; 52:5394–5407. 46. Donghi M, Attenni B, Gardelli C, et al. Synthesis and evaluation of novel phosphoramidate prodrugs of 2′-methyl cytidine as inhibitors of hepatitis C virus NS5B polymerase. Bioorg Med Chem Lett 2009; 19:1392–1395. ©2011 International Medical Press Nucleotide prodrugs for HCV therapy 47. Eldrup AB, Allerson CR, Bennett CF, et al. Structure– activity relationship of purine ribonucleosides for inhibition of hepatitis C virus RNA-dependent RNA polymerase. J Med Chem 2004; 47:2283–2295. 48. Carroll SS, Ludmerer S, Handt L, et al. Robust antiviral efficacy upon administration of a nucleoside analog to hepatitis C virus-infected chimpanzees. Antimicrob Agents Chemother 2009; 53:926–934. 49. McGuigan C, Perrone P, Madela K, et al. The phosphoramidate ProTide approach greatly enhances the activity of beta-2′-C-methylguanosine against hepatitis C virus. Bioorg Med Chem Lett 2009; 19:4316–4320. 50. McGuigan C, Gilles A, Madela K, et al. Phosphoramidate ProTides of 2′-C-methylguanosine as highly potent inhibitors of hepatitis C virus. Study of their in vitro and in vivo properties. J Med Chem 2010; 53:4949–4957. 51. McGuigan C, Madela K, Aljarah M, et al. Design, synthesis and evaluation of a novel double prodrug: INX-08189. A new clinical candidate for hepatitis C virus. Bioorg Med Chem Lett 2010; 20:4850–4854. 52. Kolykhalov A, Chamberlain S, Gorovits E, et al. In vitro Characterization of INX-189, a Highly Potent Phosphoramidate Nucleoside Analogue Inhibitor of HCV. HEP DART 2009 Frontiers in Drug Development for Viral Hepatitis. 6-10 December, 2009, Kona, HI, USA. 53. Patti JM, Matson M, Goehlecke B, et al. A study of the safety and pharmacokinetics of single ascending oral doses of INX08189, a nucleotide polymerase inhibitor, in healthy subjects. 46th Annual Meeting of the European Association for the Study of the Liver. 30 March - 3 April 2011, Berlin, Germany. 54. Vernachio JH, Bleiman B, Bryant KD, et al. INX08189, a phosphoramidate prodrug of 6-O-methyl2′-C-methyl guanosine, is a potent inhibitor of hepatitis C virus replication with excellent pharmacokinetic and pharmacodynamic properties. Antimicrob Agents Chemother 2011; 55:1843–1851. 55. Hutchins JT, McGuigan C, Chamberlain SD. DMPK and metabolism studies of nucleoside phosphoramidates including INX-08189, a novel double pro-drug and clinical candidate for hepatitis C virus therapy. Antiviral Research 2011; 90:A22. 56. Zhou XJ, Pietropaolo K, Chen J, et al. Safety and pharmacokinetics of IDX184, a liver-targeted nucleotide polymerase inhibitor of hepatitis C virus, in healthy subjects. Antimicrob Agents Chemother 2011; 55:76–81. 57. Perigaud C, Gosselin G, Imbach J-L. Minireview: from the pronucleotide concept to the SATE phosphate protecting groups. In Alexander JC (Editor). Current Topics in Medicinal Chemistry. Vol. 2. Oxford: Blackwell 1997; pp. 15–29. 58. Peyrottes S, Egron D, Lefebvre I, et al. SATE pronucleotide approaches: an overview. Mini Rev Med Chem 2004; 4:395–408. 59. Standring D, Lanford R, Cretton-Scott E, et al. Potent antiviral activity of 2nd generation HCV nucleotide inhibitors, IDX102 and IDX184, in HCV-infected chimpanzees. 43rd Annual Meeting of the European Society for the Study of the Liver. 25 April 2008, Milan, Italy. Abstract 67. 60. Lallos L, LaColla M, Serra I, et al. Combination of IDX184, a nucleotide prodrug polymerase inhibitor with other classes of HCV inhibitors is additive to synergistic in the HCV peplicon in vitro. 60th Annual Meeting of the American Association for the Study of Liver Diseases. 30 October – 3 November 2009, Boston, MA, USA. Abstract 1602. 61. Standring D, Lanford R, Li B, et al. Antiviral activity of the liver-targeted nucleotide HCV polymerase inhibitor IDX184 correlates with trough serum levels of the nucleoside metabolite of HCV-infected chimpanzees. 44th Annual Meeting of the European Association for the Study of the Liver. 22–26 April 2009, Copenhagen, Denmark. Abstract 91. 62. Lalezari J, Asmuth D, Casiro A, et al. Antiviral activity, safety, and pharmacokinetics of IDX184, a liver-targeted nucleotide HCV polymerase inhibitor, in patients with chronic hepatitis C. 60th Annual Meeting of the American Association for the Study of Liver Diseases. 30 October – 3 November 2009, Boston, MA, USA. Abstract LB18. Antiviral Chemistry & Chemotherapy 22.1 63. Lalezari J, O’Riordan W, Poordad F, et al. A Phase IIa study of IDX184 in combination with pegylated interferon (pegIFN) and ribavirin (RBV) in treatmentnaive HCV genotype 1-infected subjects. 61st Annual Meeting of the American Association for the Study of Liver Diseases. 29 October – 2 November 2010, Boston, MA, USA. Abstract 34. 64. McCarville JF, Dubuc G, Donovan E, et al. No resistance to IDX184 was detected in 3-day and 14-day clinical studies of IDX184 in genotype 1-infected HCV subjects. 46th Annual Meeting of the European Association for the Study of the Liver. 30 March – 3 April 2011, Berlin, Germany. Abstract 1237. 65. HIVandHepatits.com. FDA suspends trials of experimental HCV regimen IDX184 plus IDX320 due to liver toxicity concerns. (Updated 7 September 2010. Accessed 4 October 2010.) Available from http://www.hivandhepatitis.com/ hep_c/news/2010/0910_2010_b.html 66. Index clinical hold on two Hep C programs may be resolved by year end. (Updated 7 September 2010. Accessed 13 July 2011.) Available from http://thepinksheetdaily.elsevierbi.com 67. Index: Phase IIb Start. (Updated 14 July 2011. Accessed 13 July 2011.) Available from http://www.biocentury.com/ weekinreview/clinicalstatus/2011-02-14/idx184-phase-iibstart-235641 68. Klumpp K, Leveque V, Le Pogam S, et al. The novel nucleoside analog R1479 (4′-azidocytidine) is a potent inhibitor of NS5B-dependent RNA synthesis and hepatitis C virus replication in cell culture. J Biol Chem 2006; 281:3793–3799. 69. Zeuzem S, Nelson D, Andreone P, et al. Phase IIB study of balapiravir (RG1626; nucleoside analogue inhibitor of HCV polymerase) plus peginterferon alfa-2A (40KD) and ribavirin for chronic hepatitis C genotype 1: final results. 45th Annual Meeting of the European Association for the Study of the Liver. 14–18 April 2010, Vienna, Austria. Abstract P-778. 70. McGuigan C, Kelleher MR, Perrone P, et al. The application of phosphoramidate ProTide technology to the potent anti-HCV compound 4′-azidocytidine (R1479). Bioorg Med Chem Lett 2009; 19:4250–4254. 71. Perrone P, Luoni GM, Kelleher MR, et al. Application of the phosphoramidate ProTide approach to 4′-azidouridine confers sub-micromolar potency versus hepatitis C virus on an inactive nucleoside. J Med Chem 2007; 50:1840–1849. 72. Perrone P, Daverio F, Valente R, et al. First example of phosphoramidate approach applied to a 4′-substituted purine nucleoside (4′-azidoadenosine): conversion of an inactive nucleoside to a submicromolar compound versus hepatitis C virus. J Med Chem 2007; 50:5463–5470. 73. Clark JL, Hollecker L, Mason JC, et al. Design, synthesis, and antiviral activity of 2′-deoxy-2′-fluoro-2′-C-methylcytidine, a potent inhibitor of hepatitis C virus replication. J Med Chem 2005; 48:5504–5508. 74. Stuyver LJ, McBrayer TR, Tharnish PM, et al. Inhibition of hepatitis C replicon RNA synthesis by β-D-2′-deoxy-2′fluoro-2′-C-methylcytidine: a specific inhibitor of hepatitis C virus replication. Antivir Chem Chemother 2006; 17:79–87. 75. Le Pogam S, Seshaadri A, Kosaka A, et al. Existence of hepatitis C virus NS5B variants naturally resistant to nonnucleoside, but not to nucleoside, polymerase inhibitors among untreated patients. J Antimicrob Chemother 2008; 61:1205–1216. 76. Jensen DM, Wedemeyer H, Herring RW, et al. High rates of early viral response, promising safety profile and lack of resistance-related breakthrough in HCV GT 1/4 patients treated with RG7128 plus PEGIFN alfa-2a (40KD) RBV: planned week 12 interim analysis from the PROPEL study. 61st Annual Meeting of the American Association for the Study of Liver Diseases. 30 October – 2 November 2010, Boston, MA, USA. Abstract 81. 77. Gane EJ, Roberts SK, Stedman CA, et al. Oral combination therapy with a nucleoside polymerase inhibitor (RG7128) and danoprevir for chronic hepatitis C genotype 1 infection (INFORM-1): a randomised, doubleblind, placebo-controlled, dose-escalation trial. Lancet 2010; 376:1467–1475. 47 MJ Sofia 78. Murakami E, Niu C, Bao H, et al. The mechanism of action of β-D-2′-deoxy-2′-fluoro-2′-C-methylcytidine involves a second metabolic pathway leading to β-D-2′-deoxy-2′fluoro-2′-C-methyluridine 5′-triphosphate, a potent inhibitor of the hepatitis C virus RNA-dependent RNA polymerase. Antimicrob Agents Chemother 2008; 52:458–464. 79. Murakami E, Bao H, Ramesh M, et al. Mechanism of activation of β-D-2′-deoxy-2′-fluoro-2′-C-methylcytidine and inhibition of hepatitis C virus NS5B RNA polymerase. Antimicrob Agents Chemother 2007; 51:503–509. 80. Ma H, Jiang WR, Robledo N, et al. Characterization of the metabolic activation of hepatitis C virus nucleoside inhibitor beta-D-2′-Deoxy-2′-fluoro-2′-C-methylcytidine (PSI-6130) and identification of a novel active 5′-triphosphate species. J Biol Chem 2007; 282:29812–29820. 81. Sofia MJ, Bao D, Chang W, et al. Discovery of a β-D-2′deoxy-2′-α-fluoro-2′-β-C-methyluridine nucleotide prodrug (PSI-7977) for the treatment of hepatitis C virus. J Med Chem 2010; 53:7202–7218. 82. Murakami E, Tolstykh T, Bao H, et al. Mechanism of activation of PSI-7851 and its diastereoisomer PSI-7977. J Biol Chem 2010; 285:34337–34347. 83. Lam AM, Murakami E, Espiritu C, et al. PSI-7851, a pronucleotide of β-D-2′-deoxy-2′-fluoro-2′-C-methyluridine monophosphate, is a potent and pan-genotype inhibitor of hepatitis C virus replication. Antimicrob Agents Chemother 2010; 54:3187–3196. 84. Cheng G, Fenzux M, Mabery E, et al. Nucleotide inhibitor resistance selections using GT2A infectious virus: PSI7851 and GS-6620 select for a novel resistance pathway including substitutions of M289V/L followed by S282T. 46th Annual Meeting of the European Association for the Study of the Liver. 30 March–3 April 2011, Berlin, Germany. Abstract 1198. 85. Lawitz E, Lalezari J, Rodriguez-Torres M, et al. High rapid virologic response (RVR) with PSI-7977 daily dosing plus PEG-INF/RBV in a 28-day Phase 2a trial. 61st Annual Meeting of the American Association of the Study of Liver Diseases. 29 October – 2 November 2010, Boston, MA, USA. Abstract 806. 86. Lawitz E, Lalezari J, Rodriguez-Torres M, et al. Clinical synergy of an anti-HCV nucleotide analog with SOC: viral kinetics of PSI-7977 with SOC. 61st Annual Meeting of the American Association for the Study of Liver Diseases. 29 October – 2 November 2010, Boston, MA, USA. Abstract 1861. 87. Lawitz E, Rodriguez-Torres M, Denning JM, et al. Once daily dual-nucleotide combination of PSI-938 and PSI-7977 provides 94% HCV RNA<LOD at day 14: first purine/ pyrimidine clinical combination data (The Nuclear Study). 46th Annual Meeting of the European Association for the Sudy of the Liver. 30 March – 3 April 2011, Berlin, Germany. Abstract 1370. 88. Nelson DR, Lalezari J, Lawitz E, et al. PSI-7977 QD plus PEG/RBV in GT1: 98% rapid virological response, complete early virological response: the PROTON study. 46th Annual Meeting of the European Association for the Study of the Liver. 30 March–3 April 2011, Berlin, Germany. Abstract 1371. 89. Bristol, Pharmasset. To study oral HCV regimen together. (Updated 10 January 2011. Accessed 13 July 2011.) Available from http://thepinksheetdaily.elsevierbi.com 90. Clark JL, Mason JC, Hollecker L, et al. Synthesis and antiviral activity of 2′-deoxy-2′-fluoro-2′-C-methyl purine nucleosides as inhibitors of hepatitis C virus RNA replication. Bioorg Med Chem Lett 2006; 16:1712–1715. 91. Chang W, Bao D, Chun B-K, et al. Discovery of PSI353661, a novel purine nucleotide prodrug for the treatment of HCV infection. ACS Med Chem Lett 2011; 2:130–135. 92. Lam AM, Espiritu C, Micolochick Steuer H, et al. Novel 2′-F-2′-C-methylpurine nucleotide analogs are active inhibitors of HCV replication and lack cross-resistance with other nucleos(t)ide analogs. 60th Annual Meeting of the American Association for the Study of Liver Disease. 30 October – 3 November 2010, Boston, MA, USA. Abstract 1605. 48 93. Dyatkina N, Prhavc M, Keicher J, et al. 5′-triphosphate of 7-deaza-7-ethynyl-2′-deoxy-2′-fluoro-2′-C-methyladenosine: new potent chain terminator of hepatitis C virus rna polymerase. In Institute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic (Editor). Collected Symposium Series Edited. Vol. 10. Prague: Academy of Sciences of the Czech Republic 2008; pp. 341–342. 94. Prhavc M, Dyatkina N, Keicher J, et al. Synthesis and biological activity of 7-deaza-7-ethynyl-2′-deoxy-2′-fluoro2′-C-methyladenosine and its 2′-C-methyl-ribo analogue. Nucleic Acids Symp Ser (Oxf) 2008; 52: 643–644. 95. Beres J, Bentrude WG, Kruppa G, et al. Synthesis and antitumor and antiviral activities of a series of 1-β-Dribofuranosyl-5-halocytosine (5-halocytidine) cyclic 3′,5′monophosphates. J Med Chem 1985; 28:418–422. 96. Béres J, Sági G, Bentrude WG, et al. Synthesis and antitumor and antiviral properties of 5-halo- and 5-(trifluoromethyl)-2′-deoxyuridine 3′,5′-cyclic monophosphates and neutral triesters. J Med Chem 1986; 29:1243–1249. 97. Girardet J-L, Gosselin G, Perigaud C, et al. Synthesis and antitumor properties of some neutral triesters of 5-fluoro-2′-deoxyuridine-5′-monophosphate and 3′,5′cyclic monophosphates. Nucleosides Nucleotides 1995; 14:645–647. 98. Rodriguez-Torres M, Lawitz E, Denning JM, et al. PSI352938, a novel purine nucleotide analog, exhibits potent antiviral activity and no evidence of resistance in patients with HCV genotype 1 over 7 days. 46th Annual Meeting of the European Association for the Study of the Liver. 30 March – 3 April 2011, Berlin, Germany. Abstract 1235. 99. Gunic E, Girardet J-L, Ramasamy K, et al. Cyclic monophosphate prodrugs of base-modified 2′-C-methyl ribonucleosides as potent inhibitors of hepatitis C virus RNA replication. Bioorg Med Chem Lett 2007; 17:2452–2455. 100.Meppen M, Pacini B, Bazzo R, et al. Cyclic phosphoramidates as prodrugs of 2′-C-methylcytidine. Eur J Med Chem 2009; 44:3765–3770. 101.Reddy PG, Bao D, Chang W, et al. 2′-Deoxy-2′-α-fluoro2′-β-C-methyl 3′,5′-cyclic phosphate nucleotide prodrug analogs as inhibitors of HCV NS5B polymerase: discovery of PSI-352938. Bioorg Med Chem Lett 2010; 20:7376–7380. 102. Lam AM, Espiritu C, Murakami E, et al. Inhibition of hepatitis C virus replicon RNA synthesis by PSI-352938, a cyclic phosphate prodrug of {beta}-D-2′-deoxy-2′-{alpha}fluoro-2’-{beta}-C-methylguanosine. Antimicrob Agents Chemother 2011; 55:2566–2575. 103.Zennou V, Lam AM, Keilman M, et al. Combination of two complementary nucleotide analogues, PSI-7977 and PSI-938, effectively clears wild type and NS5b:S282T HCV replicons-comparison with combinations of other antiviral compounds. 61st Annual Meeting of the European Association for the Study of the Liver. 14–18 April 2010, Vienna, Austria. Abstract 1034. 104.Symonds WT, Denning JM, Albanis E, et al. Pharmacokinetics, safety, and tolerability of PSI-352938, a novel nucleotide polymerase inhibitor for HCV, following single ascending oral doses in healthy subjects. 61st Annual Meeting of the American Association for the Study of Liver Diseases. 29 October – 2 November 2010, Boston, MA, USA. Abstract 1890. 105. Erion MD, Bullough DA, Lin C-C, et al. HepDirect prodrugs for targeting nucleotide-based antiviral drugs to the liver. Curr Opin Invest Drugs (Thomson Sci) 2006; 7:109–117. 106.Carroll SS, Tomassini JE, Bosserman M, et al. Inhibition of hepatitis C virus RNA replication by 2′-modified nucleoside analogs. J Biol Chem 2003; 278:11979–11984. 107.Eldrup AB, Prhavc M, Brooks J, et al. Structure–activity relationship of heterobase-modified 2′-C-methyl ribonucleosides as inhibitors of hepatitis C virus RNA replication. J Med Chem 2004; 47:5284–5297. ©2011 International Medical Press Nucleotide prodrugs for HCV therapy 108.Hecker SJ, Reddy KR, van Poelje PD, et al. Liver-targeted prodrugs of 2′-C-methyladenosine for therapy of hepatitis C virus infection. J Med Chem 2007; 50:3891–3896. 109.Jones RJ, Bischofberger N. Minireview: nucleotide prodrugs. Antiviral Res 1995; 27:1–17. 110.Cassidy MK, Houston JB. In vivo capacity of hepatic and extrahepatic enzymes to conjugate phenol. Drug Metab Dispos 1984; 12:619–624. 111.Pepelko WE, Gaylor DW, Mukerjee D. Comparative toxic potency ranking of chlorophenols. Toxicol Ind Health 2005; 21:93–111. Received 16 March 2011; accepted 9 May 2011; published online 16 May 2011 Antiviral Chemistry & Chemotherapy 22.1 49