* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Dabigatran
Compounding wikipedia , lookup
Drug design wikipedia , lookup
Metalloprotease inhibitor wikipedia , lookup
Plateau principle wikipedia , lookup
Orphan drug wikipedia , lookup
Polysubstance dependence wikipedia , lookup
Drug discovery wikipedia , lookup
Psychopharmacology wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Neuropharmacology wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Pharmacognosy wikipedia , lookup
Prescription drug prices in the United States wikipedia , lookup
Prescription costs wikipedia , lookup
Drug interaction wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Dydrogesterone wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Discovery and development of direct Xa inhibitors wikipedia , lookup
Discovery and development of direct thrombin inhibitors wikipedia , lookup
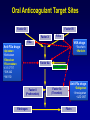
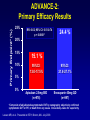
Presented at Grand Rounds Department of Neurology Loyola University Health System Maywood, Illinois 60153 September 30, 2011 LOYOLA UNIVERSITY HEALTH SYSTEM Loyola University Chicago Dabigatran and other New Oral Anticoagulants Jeanine M. Walenga, PhD Professor, Thoracic-CV Surgery and Pathology Co-Director, Hemostasis and Thrombosis Research Laboratories Loyola University Chicago Currently Available Anticoagulants DRUG • Warfarin • • • • • Heparin LMW heparins Lepirudin (DTI) Bivalirudin (DTI) Argatroban (DTI) CLINICAL USE Long-term AC, stroke prevention in AF VTE, ACS, CPB, PCI VTE prevention HIT HIT, PCI HIT, PCI Some of the New Anticoagulants Anti-FXa – – – – – – – – Anti-Flla (anti-thrombin) Rivaroxaban (o) Apixaban (o) Edoxaban (o) Otamixaban (p) LY-517717 (o) DX-9065a (p) Betrixiban (o) TK-442 (o) – – – – – – O:Oral, P:Parenteral Dabigatran (o) Odiparcil (o) Flovagatran (p) Pegmusirudin (p) Peg-hirudin (p) Desirudin (p) Oral Anticoagulant Target Sites Factor IX Factor VII Factor X FVIIa VKA drugs FIXa Anti-FXa drugs •Tecarfarin •Warfarin •Apixaban •Betrixaban •Edoxaban •Rivaroxaban •LY 517717 •TAK 442 •YM 150 Factor Xa Antithrombin Anti-FIIa drugs Factor II (Prothrombin) Fibrinogen •Dabigatran •Ximelagatran •AZD 0837 Factor IIa (Thrombin) Fibrin Comparison of NEW ORAL ANTICOAGULANTS with WARFARIN Features Onset Dosing Indications Food effect Drug interactions Monitoring Half-life Antidote Warfarin Slow Variable Same Yes Yes Yes Long Yes New Agents Rapid Fixed Same No Yes No Short No Rivaroxaban • Direct, specific, competitive factor Xa inhibitor Xarelto® • Rapid onset within 2-4 hours • High bioavailability of >80% • Metabolized via the CYP3A4, CYP211, and P-gp transport mechanisms • See interactions with drugs using the same metabolic pathways Perzborn et al., J Thromb Haemost 2005. • Renal and fecal elimination Kubitza et al., J Clin Pharmacol 2007. Rivaroxaban: Clinical Development Postsurgical prophylaxis of DVT DVT Treatment Stroke Prevention in AF ACS ODIXa-KNEE EINSTEIN-DVT ROCKET-AF ATLAS ODIXa-HIP EINSTEIN-EXT ROCKET-J RECORD-1 EINSTEIN-PE RECORD-2 RECORD-3 RECORD-4 ACS-TIMI 46 ATLAS ACS-TIMI 51 RECORD 1-4 Summary Total Treatment Duration Pool Incidence (%) 2.0 Enoxaparin regimens ARD=–0.8% p<0.001 Rivaroxaban regimens 1.5 1.0 Primary population for analysis 1.3% 0.5 0 ARD=0.2% p=0.076 0.6% 0.2% 82/6,200 35/6,183 Symptomatic VTE and all-cause mortality 13/6,200 24/6,183 Major bleeding p-values analyzed using a Cox regression model; safety population, n=12,383 Turpie AGG, et al. Presented at ASH 2008 0.4% ROCKET-AF: Stroke, Non-CNS Embolism Study Outcome: Rivaroxaban vs Warfarin On Treatment N = 14,143 1.70% vs 2.15% stroke rate 21% reduction in stroke rate w/ rivaroxaban p = 0.015 ITT N = 14,171 2.12% vs 2.42% stroke rate 12% reduction in stroke rate w/ rivaroxaban p = 0.117 ROCKET-AF: Clinical Trial Outcomes • Efficacy – Rivaroxaban: non-inferior to warfarin for SPAF – Rivaroxaban: superior to warfarin while patients were taking study drug • Safety – Similar rates of bleeding and adverse events – Less ICH and fatal bleeding with rivaroxaban • Conclusion – Rivaroxaban is a potential alternative to warfarin for moderate or high risk AF patients Rivaroxaban: FDA Status • Xarelto trade name • For the prevention of DVT/PE after orthopedic surgery: – Rivaroxaban 10 mg OD is FDA approved (July 2011) – Approved in the EU and Canada • For the prevention of stroke in patients with atrial fibrillation: – FDA advisory committee voted to approve (Sept. 2011) • Yes=9, No=2, Abstain=1 – Recommended approval in the EU (Sept. 23, 2011) Apixaban • Direct, reversible FXa inhibitor • Rapid onset, peak within 3 hrs • Bioavailability of 51-85% • Long half life, slightly longer in elderly (15 hrs) • Multiple elimination pathways Eliquis – 25% renal – 75% biliary • Metabolism via CYP3A4, SULT1AA pathways Perzborn et al., J Thromb Haemost 2005. Kubitza et al., J Clin Pharmacol 2007. Apixaban: Clinical Development Postsurgical prophylaxis of DVT APROPOS DVT Treatment Stroke Prevention in AF ACS Botticelli DVT ARISTOTLE APPRAISE-1 AVERROES APPRAISE-2 dose-ranging study ADVANCE-1 ADVANCE-2 AMPLIFY (ongoing) (study terminated because of bleeding) AMPLIFY-EXT APPRAISE Japan (ongoing) (study terminated) ADVANCE-3 Primary Endpoint* (%) ADVANCE-2: Primary Efficacy Results 25% RR: 0.62; 95% CI: 0.51-0.74 p < 0.0001* 24.4 % 20% 15% 10% 15.1 % 95%CI: 13.0-17.5% 95%CI: 21.8-27.1% Apixaban 2.5mg BID (n=976) Enoxaparin 40mg QD (n=997) 5% 0% *Composite of adjudicated asymptomatic DVT by venography; objectively confirmed symptomatic DVT or PE; or death from any cause. One-sided p-value for superiority. Lassen MR, et al. Presented at ISTH, Boston, MA. July 2009. ARISTOTLE: Study Outcomes • Treatment with apixaban as compared to warfarin in patients with AF (n>18,000) and at least one additional risk factor for stroke: – Reduces stroke and systemic embolism by 21% (p=0.01) – Reduces major bleeding by 31% (p<0.001) – Reduces mortality by 11% (p=0.047) – Consistent effects across all major subgroups – Fewer drug discontinuations on apixaban than on warfarin, consistent with good tolerability Granger CB, et al. NEJM 2011;365: Aug 28 Apixaban: FDA Status • Eliquis trade name • For the prevention of DVT/PE after orthopedic surgery: – No FDA approval yet – Apixaban approved in the EU • For the prevention of stroke in patients with atrial fibrillation: – No approvals yet Edoxaban: Clinical Development Postsurgical prophylaxis of DVT Oral direct FXa inhibition with E for thrombophylaxis after elective THR: a randomized double-blind dose-response study DVT Treatment The Edoxaban Hokusai-VTE Study (ongoing) Stroke Prevention in AF Randomized, parallel-group, multicenter, multinational Phase II study comparing E, an oral factor Xa inhibitor, with warfarin for SPAF STARS J-1 ENGAGE AF-TIMI 48 STARS J-2 Safety of edoxaban, an oral factor Xa inhibitor, in Asian patients with (completed June 2011; unpublished) NVAF STARS E-3 (completed Feb. 2010; unpublished) STARS J-4 (completed Feb. 2010; unpublished) STARS J-5 (completed March 2010; unpublished) Edoxaban: FDA Status • Lixiana trade name • For the prevention of DVT/PE after orthopedic surgery: – No FDA approval yet – Edoxaban approved in Japan • For the prevention of stroke in patients with atrial fibrillation: – No approvals Dabigatran Etexilate • Specific, competitive, reversible univalent thrombin inhibitor Pradaxa® • Pro-drug converted to active form • Rapid onset within 2 hours • Low bioavailability, 3.5-5% • Low protein binding • Half life 12-17 hours • Renal clearance as glucuronic acid conjugate: 85% • Metabolized by esterase catalyzed hydrolysis and P-gp transport mechanisms Perzborn et al., J Thromb Haemost 2005. Kubitza et al., J Clin Pharmacol 2007. Dabigatran: Clinical Development Postsurgical prophylaxis of DVT DVT Treatment RE-MODEL RE-COVER RE-MOBILIZE RE-MEDY RE-NOVATE RE-SONATE RE-NOVATE 2 (unpublished) (ongoing) (unpublished) Stroke Prevention in AF RE-LY ACS RE-DEEM (unpublished) RE-LY A Non-Inferiority Trial Atrial fibrillation with ≥ 1 Risk Factor Absence of contraindications 951 centers in 44 countries Blinded Event Adjudication R Open Warfarin Adjusted INR 2.0 – 3.0 N=6,000 Blinded Dabigatran 110 mg BID N=6,000 Connolly SJ, et al. NEJM 2009; 361:1139-1151 Dabigatran 150 mg BID N=6,000 RE-LY: Stroke Prevention 150 mg - 34% Fewer Strokes Non-inferiority Superiority p-value p-value Dabigatran 110 vs. Warfarin <0.001 0.34 Margin = 1.46 Dabigatran 150 vs. warfarin <0.001 0.50 0.75 Dabigatran better Connolly SJ, et al. NEJM 2009;361:1139-51 1.00 1.25 HR (95% CI) 1.50 Warfarin better <0.001 RE-LY: Study Outcomes Efficacy • Both doses of dabigatran were non-inferior to warfarin on the primary endpoint – reduction of the incidence of stroke including hemorrhagic and systemic embolism; p<0.001 • Dabigatran 150 mg BID was superior to warfarin on the primary endpoint by 34% – RR 0.66, 95% CI, 0.53-0.82; p<0.001 Safety • No significant difference in the rate of major bleeding for dabigatran 150 mg BID compared to warfarin – 3.11 vs 3.36 %/yr; p=0.31 • Rate of major bleeding with dabigatran 110 mg BID (2.71%/yr) was 20% lower compared to warfarin; p=0.003 RE-LY: MORTALITY REDUCTION DABIGATRAN VS WARFARIN • Lower death rate with dabigatran: – Vascular death 2.69% vs 2.43 and 2.28%* (p=0.04 for 150 mg) – All cause death 4.13% vs 3.75 and 3.64% (p=0.05 for 150 mg) *Dabigatran 110 mg and 150 mg Connolly SJ, et al. NEJM 2009;361: 1139-1151 RE-LY: BLEEDING DABIGATRAN VS WARFARIN • More bleeding with warfarin: – Life-threatening bleeding 1.8% vs 1.2 and 1.5%* (p<0.001, 0.04) – Intracranial bleeding 0.7% vs 0.2 and 0.3% (p<0.001, <0.001) – Major plus minor bleeding 18.2% vs 14.6 and 16.4% (p<0.001, 0.002) *Dabigatran 110 mg and 150 mg Connolly SJ, et al. NEJM 2009; 361:1139-1151 Dabigatran: FDA Status • Pradaxa trade name • For the prevention of DVT/PE after orthopedic surgery: – FDA approval pending – Pradaxa approved in EU and Canada • For the prevention of stroke in patients with non-valvular atrial fibrillation (SPAF): – Pradaxa 150 mg BID FDA approved (Oct. 2010) – Pradaxa approved in EU, Canada and Japan Dabigatran: FDA Approved Dosing • 150 mg BID for SPAF – 150 mg for CrCl >30 mL / min – 75 mg for CrCl 15-30 mL / min Stroke from Atrial Fibrillation • AF is the most preventable cause of stroke: – 12-16 million will be on warfarin treatment by 2050 in the US – Clinical trials have shown stroke can be reduced: • • • • Placebo vs ASA = 19% ASA vs warfarin = 30% Placebo vs warfarin = 62% Dabigatran vs warfarin = 34% Dabigatran: Not Without Issues 1. No anticoagulant effect if missed dose • 2% discontinuation rate due to GI distress • Cost of drug ($240/mo vs $4/mo for warfarin) 2. 3. 4. No test to assess anticoagulation Difficult to modulate dose Bleeding in the elderly and renal impaired patients (5 dabigatran related deaths in Japan) 5. ‘Real world’ untested populations 6. Drug interactions 7. Limited data on bridging between anticoagulants 8. No specific antidote 9. 0.2% increase in myocardial infarction 10. Off-label use New OACs: Immediate Practical Issues • Is there a specific antidote for dabigatran associated bleeding? – No, still in development • How do we manage bleeding complications associated with dabigatran? • How can we test a patient for the presence of dabigatran? • Are there drug interactions with dabigatran? – tPA (stroke, AMI) – Heparins (ACS, stroke) – Antiplatelet drugs New OACs: ‘Real Life’ Experience • Populations not studied in the clinical trials: – Elderly, pediatrics, pregnant – Heparin compromised (e.g., HIT) – Acute vs non-acute VTE – Chronically ill • Common patient characteristics that influence the drug PKs and safety/efficacy of NOACs: – Co-morbid illnesses – Reduce renal function – Reduced hepatic function – Altered metabolism, gastric pH, intestinal motility, protein binding – Extremes in body weight – Co-administered Rx drugs, dietary supplements, herbs New OACs: ‘Real Life’ Experience • Drug interactions with NOACs: – Potential adverse effects • Alter the pharmacokinetics of the NOAC • Increase or decrease exposure to the NOAC • Increase bleeding risk – Known ‘cautioned’ drugs • Proton pump inhibitors • Inhibitors or inducers of the CYP3A4 or P-gp transport mechanisms • NSAIDs, ASA, anti-platelet drugs – At risk patients • • • • • Cardiovascular disease Depression Pain Epilepsy Requiring antibiotics, anti-fungals, anti-malaria drugs Dabigatran: Bleeding Management • Short half-life • Maintain adequate diuresis • Usual supportive measures: compression, surgical hemostasis • Dialysis (low protein binding)1 • Activated charcoal w/ subsequent charcoal filtration (in vitro data)1 • Blood products – May not be effective: replace factor deficiency (warfarin) vs overcome an inhibitor (dabigatran) • PCC not effective (effective against rivaroxaban)2 • rFVIIa 1vanRyn 2 J, et al. Thromb Haemost 2010;103(6):1116-27 Eerenberg ES, et al. Circulation 2011;124:Sept 6 Dabigatran: Laboratory Testing • Monitoring vs anticoagulant assessment – PT and aPTT • • • • Differing reagent sensitivities Not a linear association between assay values and drug level Not validated for association to bleeding aPTT may be applicable for qualitative assessment – INR: not sensitive; not validated – TT: Super-sensitive; can identify if any drug onboard – Ecarin clotting time: results can vary depending on plasma factors; research use only (RUO) – PiCT: RUO – Chromogenic anti-FIIa – Hemoclot: quantitative using dabigatran calibrators; FDA approved yet? Anticoagulant Effects of Direct Thrombin and Factor Xa Inhibitors in Retrieved Plasma (Assay: PT) 140.0 120.0 Time (secs) 100.0 DABIGATRAN 80.0 MELAGATRAN OTAMIXABAN 60.0 RIVAROXABAN 40.0 20.0 0.0 0 0.5 1 1.5 Conc. (ug/ml) 2 2.5 3 2 case reports of inaccurate INR values w/ dabigatran. Baruch L, Sherman O. Ann Pharmacother 2011 Jun 28 Anticoagulant Effects of Direct Thrombin and Factor Xa Inhibitors in Retrieved Plasma (Assay: APTT) 180.0 160.0 140.0 Time (secs) 120.0 DABIGATRAN 100.0 MELAGATRAN OTAMIXABAN 80.0 RIVAROXABAN 60.0 40.0 20.0 0.0 0 0.5 1 1.5 Conc. (ug/ml) 2 2.5 3 Anticoagulant Effects of Direct Thrombin and Factor Xa Inhibitors in Human Whole Blood (Assay: TT5UCa++) 350.0 300.0 Time (secs) 250.0 DABIGATRAN 200.0 MELAGATRAN OTAMIXABAN 150.0 RIVAROXABAN 100.0 50.0 0.0 0 0.5 1 1.5 Conc. (ug/ml) 2 2.5 3 Anticoagulant Effects of Direct Thrombin Inhibitors in Retrieved Plasma (Assay: Anti-IIa) 90 80 70 % Inhibition 60 50 DABIGATRAN MELAGATRAN 40 30 20 10 0 0 0.5 1 1.5 Conc. (ug/ml) 2 2.5 3 Anticoagulant Effects of Direct Factor Xa Inhibitors in Retrieved Plasma (Assay: Anti-Xa) 120.0 100.0 % Inhibtion 80.0 OTAMIXABAN 60.0 RIVAROXABAN 40.0 20.0 0.0 0 0.5 1 1.5 Conc. (ug/ml) 2 2.5 3 Hemoclot Assay for Dabigatran Dabigatran Calibration Curve Dabigatran Treated Patients Dabigatran vs Hemoclot TT (220 mg D) 90 250 200 70 150 Hemoclot TT Hemoclot thrombin clotting time [s] 80 100 60 50 Linear fit (31.44 +0.1437x) Linear fit (30.85 +0.04948x) 50 40 95% CI 95% CI 30 95% Prediction interval 95% Prediction interval 0 0 1000 2000 3000 4000 Dabigatran concentration [nM] (Stangier et al. ISTH 2009, Boston) 20 0 50 100 150 200 Dabigatran [ng/mL] 250 300 The New Oral Anticoagulants: Similar Yet Different • Thrombin Inhibitors: 1. Dabigatran: pro-drug, renal clearance - twice daily • FXa Inhibitors: 1. Rivaroxaban: renal clearance - once daily 2. Apixaban: hepatic clearance - twice daily 3. Edoxaban: hepatic clearance - once daily Circulation 2010;121:1523-1532 The New Oral Anticoagulants: Similar Yet Different Rivaroxaban Apixaban Dabigatran Etexilate Target Xa Xa IIa Molecular Weight 436 460 628 Prodrug No No Yes Bioavailability (%) 80 50 6 Time to peak (h) 3 3 2 Half-life (h) 9 9-14 12-17 Renal excretion (%) 65 25 80 None None None Features Antidote • Each drug has its own effect in lab tests. • The metabolic mechanisms of each drug differ; see drug interactions. • Each drug has to be managed individually. The Future of Warfarin 1. Excellent efficacy 2. Low cost ($0.75 per day!) 3. Long track record (since 1954) 4. Centralized Anticoagulation Clinics that maintain TTRs > 60% 5. PT assay available: fast TAT, inexpensive 6. INR validated for warfarin relation to bleeding risk 7. Point-of-care testing 8. Rapid turnaround genetic testing Conclusions 1. New anticoagulants are now clinically available and additional drugs are being developed. 2. The new oral anticoagulants rivaroxaban, dabigatran and apixaban, though costly, will provide more options for the management of VTE; however, these drugs are not superior to the standard of care. 3. For VTE prevention, heparins and warfarin will remain the standard of care for some time, especially in medical patients. Conclusions 4. For atrial fibrillation (SPAF) the new oral anticoagulants appear to have a superior safety and efficacy profile. 5. Additional clinical trials are needed to determine the merit of these drugs beyond the ‘clinical trial’ populations, and to address unanswered questions. 6. Warfarin’s low cost, efficacy, and track record will prolong its life. Its use may decrease but it will remain a for years to come. Regulatory Approvals • Orthopedic surgery (THR/TKR/Hip fracture): – – – – Dabigatran: EU, Canada Rivaroxaban: EU, Canada, US Apixaban: EU Edoxaban: Japan • Non-ortho surgery, medical patients: – No approvals • SPAF: – Dabigatran: US, EU, Canada, Japan – Rivaroxaban: US and EU approval recommended