* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Alcohol Worksheet Key
Survey
Document related concepts
Elias James Corey wikipedia , lookup
Woodward–Hoffmann rules wikipedia , lookup
Marcus theory wikipedia , lookup
Asymmetric induction wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Hydroformylation wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
Homoaromaticity wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Ene reaction wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
George S. Hammond wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Transcript
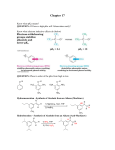
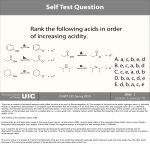
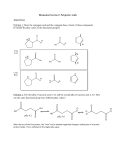
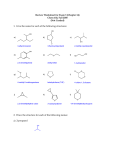
CHE 261 Alcohol Worksheet Name ___________________ 1. Name the following compounds: OH OH trans-3-propyl-4-hexen-2-ol 2. (1S)-2-cyclohexen-1-ol Draw the following compounds: 3-mercapto-2,5-pentandiol sec-butyl alcohol OH OH OH SH 3. Rank the following compounds in order of increasing acidity and justify your answer. Cl 1 OH SH OH 3 2 1 < 3 < 2, Thiols are more acidic than alcohols. The chlorine on 3 increases the acidity of 3 through the inductive effect. 4. Rank the following compounds in order of increasing solubility in water and justify your answer. OH OH OH 1 2 OH 3 2 < 1 < 3, 3 has 4 carbons and therefore is most soluble in water. 1 and 2 have the same number of carbons, but 1 has 2 alcohol groups and is therefore more soluble. 5. Rank the following compounds in order of increasing boiling point and justify your answer. Cl OH SH OH 1 3 2 2 < 1 < 3, 1 and 3 exhibit H-bonding, but 3 has a higher molecular weight. It has the highest boiling point. 2 has the lowest boiling point because it doesn’t exhibit H-bonding. 6. Draw the resonance structures of the conjugate base of 4-chlorophenol. Would you expect 4-chlorophenol to be more or less acidic than 3-chlorophenol? Explain. Why is the positioning of the chloro group important (whether it is at position 2, 3, or 4)? O O O O Cl Cl Cl Cl 4-chlorophenol is more acidic than 3-chlorophenol due to the inductive effect of the chlorine at position 4. The negative charge is not delocalized onto postions 3 or 5, so electron withdrawing groups at position 3 and 5 will have very little effect on the acidity of the phenol. 7. For the following acid-base reactions, predict the products. Label the acid (A), base (B), conjugate acid (CA), and conjugate base (CB). Using equilibrium constant data, indicate whether the reaction will proceed as written. (Which side of the reaction is favored?) phenol + ammonia O OH + NH3 pKa = 10.0 + NH4+ pKa = 9.25 The reaction will not proceed, as written, to any significant extent. The reactants are weaker acids and bases. ethyl sulfide + acetic acid O O + + OH S SH O pKa = 10.5 pKa = 4.74 The reaction will proceed as written. The products are weaker acids and bases. 8. Predict the product(s) of the following reactions: sodium ethoxide 1-iodohexane Æ + + I O 2-hexene + H3O+ Æ OH + + H3O+ OH ethanol + CH3CH2COOH Æ OH OH H + O O NO REACTION! H The reactants are much weaker acids and bases. 2-methylcyclopentanol OH H2SO4 + H2SO4 Æ O + O 9. For the following reduction reactions, predict the product(s), and classify the alcohols as primary, secondary or tertiary. OH LAH OH OH NH Raney Ni O O NaBH4 OH H N OH 10. O Describe a synthetic pathway for the following synthesis. (It is a multi-step problem.) O OH NaBH4 + H3O Br H2SO4 Br2 Br