* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Introduction To Endocrinology: The Hypothalamic
Survey
Document related concepts
Human chorionic gonadotropin wikipedia , lookup
History of catecholamine research wikipedia , lookup
Bovine somatotropin wikipedia , lookup
Hormonal contraception wikipedia , lookup
Mammary gland wikipedia , lookup
Triclocarban wikipedia , lookup
Neuroendocrine tumor wikipedia , lookup
Menstrual cycle wikipedia , lookup
Hyperthyroidism wikipedia , lookup
Hormone replacement therapy (menopause) wikipedia , lookup
Xenoestrogen wikipedia , lookup
Hormone replacement therapy (male-to-female) wikipedia , lookup
Hyperandrogenism wikipedia , lookup
Breast development wikipedia , lookup
Adrenal gland wikipedia , lookup
Endocrine disruptor wikipedia , lookup
Transcript
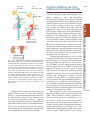
Introduction To Endocrinology: The Hypothalamic-Pituitary Axis Keith L. Parker and Bernard P. Schimmer ENDOCRINOLOGY AND HORMONES: GENERAL CONCEPTS Endocrinology analyzes the biosynthesis of hormones, their sites of production, and the sites and mechanisms of their action and interaction. The term hormone is of Greek origin and classically refers to chemical messengers that circulate in body fluids and produce specific effects on cells distant from their point of origin. The major functions of hormones include the regulation of energy storage, production, and utilization; the adaptation to new environments or conditions of stress; the facilitation of growth and development; and the maturation and function of the reproductive system. Although hormones were originally defined as products of ductless glands, we now appreciate that many organs that were not classically considered as “endocrine” (e.g., the heart, kidneys, GI tract, adipocytes, and brain) synthesize and secrete hormones that play key physiological roles; many of these hormones are now employed either diagnostically or therapeutically in clinical medicine. In addition, the field of endocrinology has expanded to include the actions of growth factors acting by means of autocrine and paracrine mechanisms, the influence of neurons—particularly those in the hypothalamus—that regulate endocrine function, and the reciprocal interactions of cytokines and other components of the immune system with the endocrine system. As discussed in Chapter 3, hormones generally exert their actions on target cells via a plenitude of receptors, including heptaspanning GPCRs, monospanning membrane tyrosine kinases and guanylyl cyclases, cytokine receptors, ligand-activated ion channels, and nuclear transcription factors. Conceptually, it is useful to divide hormones into two classes: those that act predominantly via nuclear receptors to modulate transcription in target cells and those that typically act via membrane receptors to exert rapid effects on signal transduction pathways. Steroid hormones, thyroid hormone, and vitamin D belong to the first class, whereas peptide and amino acid hormones are generally assigned to the second class. The receptors for both classes of hormones provide tractable targets for a diverse group of compounds that are among the most widely used drugs in clinical medicine. DISORDERS OF ENDOCRINE REGULATION Because of their potent effects, circulating levels of hormones generally are tightly regulated within a normal range. The physiological strategies used to maintain the appropriate levels of hormones range from relatively simple ones involving direct feedback or feed-forward mechanisms (e.g., the secretion of parathyroid hormone by the parathyroid glands is inversely related to the serum Ca2+ concentration, which is sensed by a GPCR termed the Ca2+-sensing receptor; Chapter 44) to more complex ones involving reciprocal interactions among the hypothalamus, anterior pituitary, and endocrine glands (see the section “The HypothalamicPituitary-Endocrine Axis”). Regardless of the mechanism, normal regulation can be perturbed in disease states when a given hormone is either over or underproduced or when its signaling mechanisms are impaired. Understanding the normal regulation and actions of the various hormones is critical to both diagnosis and treatment of these endocrine disorders. Chapters 38 through 44 describe the different endocrine organs and the drugs that are employed to affect their function or to mimic or block their hormonal products. THE HYPOTHALAMICPITUITARY-ENDOCRINE AXIS Many of the classic endocrine hormones (e.g., cortisol, thyroid hormone, sex steroids, growth hormone) are regulated by complex reciprocal interactions among the hypothalamus, anterior pituitary, and endocrine glands (Table 38–1). These interactions permit precise control over the levels of circulating hormones and also provide a means to alter hormone levels under special physiological or pathological circumstances. The basic organization of the hypothalamicpituitary-endocrine axis is summarized in Figure 38–1. Discrete sets of hypothalamic neurons produce different releasing hormones, which are axonally transported to the median eminence. Upon stimulation, these neurons secrete their respective hypothalamic releasing hormones into the hypothalamic-adenohypophyseal plexus, which flows to the anterior pituitary gland. The hypothalamic releasing hormones bind to membrane receptors on specific subsets of pituitary cells and stimulate the secretion of the corresponding pituitary hormones. The pituitary hormones, which can be thought of as the master signals, then circulate to the target endocrine glands, where they again activate specific receptors to stimulate the synthesis and secretion of the target endocrine hormones. These interactions thus represent feed-forward regulation in which the master (signal) hormones stimulate the production of target hormones by the endocrine organs. Superimposed on this positive feed-forward regulation is negative feedback regulation, which permits precise control of hormone levels. Figures 38–2 and 38–7 show examples of this negative feedback regulation. Typically, the endocrine target hormone circulates to both the hypothalamus and pituitary, where it acts via specific receptors to inhibit the production and secretion of both its hypothalamic releasing hormone and the regulatory pituitary hormone, thereby tightly regulating target hormone levels. In addition, other brain regions have inputs to the hypothalamic releasing neurons, further integrating the regulation of hormone levels in response to diverse stimuli. An understanding of this regulation facilitates the diagnosis and management of a number of endocrine diseases. Endocrine deficiency states can be divided into those with impaired function at the level of the target endocrine gland (primary disease; as is the case in the autoimmune destruction of the adrenal or thyroid glands) and those with defects at the level of the pituitary gland and/or hypothalamus that impair delivery of the pituitary trophic hormone to its target gland (secondary/tertiary disease). In primary hypofunction, the production of the target endocrine hormone will be impaired; however, the hypothalamus and pituitary will sense the diminished feedback inhibition and the anterior pituitary gland will secrete higher than normal levels of the signal hormone. In secondary hypofunction, both the signal hormone and the target hormone will be below the normal range. Table 38–1 Hormones that Integrate the Hypothalamic-Pituitary-Endocrine Axis HYPOTHALAMIC RELEASING HORMONE PITUITARY TROPHIC (SIGNAL) HORMONE TARGET HORMONE(S) Growth hormone-releasing hormone (GHRH) Somatostatin (SST)a Dopamine (DA)b Corticotropin-releasing hormone (CRH) Thyrotropin-releasing hormone (TRH) Gonadotropin-releasing hormone (GnRH) Growth hormone (GH) IGF-1 a Growth hormone Prolactin Corticotropin Thyroid-stimulating hormone (TSH) Follicle-stimulating hormone (FSH) Luteinizing hormone (LH) Somatostatin inhibits growth hormone release. Dopamine inhibits prolactin release. IGF-1, insulin-like growth factor-1; DHEA, dehydroepiandrosterone; f, female; m, male. b — Cortisol/DHEA Thyroid hormone Estrogen Progesterone/Estrogen (f) Testosterone (m) PVN (TRH, CRH, SST) SON, PVN (AVP, OXY) Hypothalamus ARC (GHRH, GnRH, DA) Posterior lobe Releasing factors Portal system AVP, OXY Trophic hormones (ACTH, TSH, GH, LH, FSH, prolactin) Kidney, uterus, mammary gland Figure 38–1. Organization of the anterior and posterior pituitary gland. Hypothalamic neurons in the supraoptic (SON) and paraventricular (PVN) nuclei synthesize arginine vasopressin (AVP) or oxytocin (OXY). Most of their axons project directly to the posterior pituitary, from which AVP and OXY are secreted into the systemic circulation to regulate their target tissues. Neurons that regulate the anterior lobe cluster in the mediobasal hypothalamus, including the PVH and the arcuate (ARC) nuclei. They secrete hypothalamic releasing hormones, which reach the anterior pituitary via the hypothalamic-adenohypophyseal portal system and stimulate distinct populations of pituitary cells. These cells, in turn, secrete the trophic (signal) hormones, which regulate endocrine organs and other tissues. See Table 38-1 for abbreviations. Hormone excess similarly can result either from primary disorders at the level of the target endocrine glands (e.g., a hyperfunctioning tumor of the adrenal cortex that oversecretes cortisol) or from secondary disorders at the level of the pituitary gland (e.g., a pituitary corticotrope adenoma that oversecretes corticotropin, the predominant stimulator of adrenal glucocorticoid biosynthesis). Again, knowledge of the levels of the pituitary signal hormone and the target hormone allows the clinician to identify the site of the endocrine disorder. The peptide hormones of the anterior pituitary are essential for the regulation of growth and development, reproduction, response to stress, and intermediary metabolism. Their synthesis and secretion are controlled by hypothalamic hormones and by hormones from the peripheral endocrine organs. A large number of disease states, as well as a diverse group of drugs, also affect their secretion. The complex interactions among the hypothalamus, pituitary, and target endocrine glands provide elegant examples of the integrated feedback regulation described earlier. Clinically, an improved understanding of the mechanisms that underlie these interactions provides the rationale for diagnosing and treating endocrine disorders and for predicting certain side effects of drugs that affect the endocrine system. Moreover, the elucidation of the structures of the anterior pituitary hormones and hypothalamic releasing hormones and advances in protein chemistry and molecular biology have made it possible to produce synthetic peptide agonists and antagonists that have important diagnostic and therapeutic applications. The anterior pituitary hormones can be classified into three different groups based on their structural features (Table 38–2). Corticotropin (adrenocorticotrophic hormone, ACTH) and α-melanocyte-stimulating hormone (α-MSH) are part of a family of peptides derived from pro-opiomelanocortin (POMC) by proteolytic processing (Chapters 18 and 42). Growth hormone (GH) and prolactin belong to the somatotropic family of hormones, which in humans also includes placental lactogen. The glycoprotein hormones—thyroidstimulating hormone (TSH; also called thyrotropin), luteinizing hormone (LH; also called lutropin), and folliclestimulating hormone (FSH; also called follitropin)— share a common α-subunit but have different β-subunits that determine their distinct biological activities. In humans, the glycoprotein hormone family also includes human chorionic gonadotropin (hCG). The synthesis and release of anterior pituitary hormones are influenced by the central nervous system (CNS). Their secretion is positively regulated by a group of peptides referred to as hypothalamic releasing hormones, which are released from hypothalamic neurons in the region of the median eminence and reach the anterior pituitary through the hypothalamicadenohypophyseal portal system (Figure 38–1). The hypothalamic- releasing hormones include corticotropinreleasing hormone (CRH), growth hormone–releasing 1105 CHAPTER 38 INTRODUCTION TO ENDOCRINOLOGY: THE HYPOTHALAMIC-PITUITARY AXIS Anterior lobe PITUITARY HORMONES AND THEIR HYPOTHALAMIC RELEASING FACTORS Table 38–2 Properties of the Protein Hormones of the Human Adenohypophysis and Placenta HORMONE MASS (daltons) PEPTIDE CHAINS AMINO ACID RESIDUES CHROMOSOMAL LOCATION Somatotropic hormones Growth hormone (GH) Prolactin (PRL) Placental lactogen (PL) 22,000 23,000 22,125 1 1 1 191 199 190 17q22-24 6p22.2-21.3 17q22-24 Glycoprotein hormones Luteinizing hormone (LH) 29,400 2 32,600 2 38,600 2 2 6q12.q21 19q13.3 6q12.q21 11p13 6q12.q21 19q13.3 6q12.q21 1p13 Heterodimeric glycoproteins with a common α-subunit and unique β-subunits that determine biological specificity 28,000 α-92 β-121 α-92 β-111 α-92 β-145 α-92 β-118 4500 1650 1 1 39 13 2p22.3 These peptides are derived by proteolytic hormone processing of the common precursor, pro-opiomelanocortin (POMC) Follicle-stimulating hormone (FSH) Human chorionic gonadotropin (hCG) Thyroid-stimulating hormone (TSH) POMC-derived hormones* Corticotropin (ACTH) α-Melanocyte-stimulating (α-MSH) COMMENTS *See Chapter 42 for further discussion of POMC-derived peptides, including ACTH and α-MSH. hormone (GHRH), gonadotropin-releasing hormone (GnRH), and thyrotropin-releasing hormone (TRH). Somatostatin (SST), another hypothalamic peptide, negatively regulates secretion of pituitary GH and TSH. The neurotransmitter dopamine inhibits the secretion of prolactin by lactotropes. The posterior pituitary gland, also known as the neurohypophysis, contains the endings of nerve axons arising from distinct populations of neurons in the supraoptic and paraventicular nuclei of the hypothalamus that synthesize either arginine vasopressin or oxytocin (Figure 38–1). Arginine vasopressin plays an important role in water homeostasis (Chapter 25); oxytocin plays important roles in labor and parturition and in milk letdown, as discussed in the following sections and in Chapter 66. biological features, thus providing a rationale for discussing them together. The somatotropes and lactotropes, the pituitary cells that produce GH and prolactin, respectively, derive during pituitary development from a common precursor and are eosinophilic in histological sections. Consistent with their common origin, defects in certain transcription factors affect both cell lineages. In addition to their structural similarities, GH and prolactin act via membrane receptors that belong to the cytokine receptor family and modulate target cell function via very similar signal transduction pathways (Chapter 3). The secretion of both hormones is subject to strong inhibitory input from hypothalamic neurons; for prolactin, this negative dopaminergic input clearly is the predominant regulator of secretion. Finally, several drugs that are used to treat excessive secretion of these hormones are effective to varying degrees for both GH and prolactin. SOMATOTROPIC HORMONES: GROWTH HORMONE AND PROLACTIN Physiology of the Somatotropic Hormones GH and prolactin are structurally related members of the somatotropic hormone family and share many Structures of the Somatotropic Hormones. The gene encoding human GH resides on the long arm of