* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download chem – mixtures elements compounds for ib 1 10-10
Chemical plant wikipedia , lookup
Chemical potential wikipedia , lookup
Ceramic engineering wikipedia , lookup
Colloidal crystal wikipedia , lookup
Particle-size distribution wikipedia , lookup
Liquid–liquid extraction wikipedia , lookup
Spinodal decomposition wikipedia , lookup
Thermomechanical analysis wikipedia , lookup
Gas chromatography wikipedia , lookup
Registration, Evaluation, Authorisation and Restriction of Chemicals wikipedia , lookup
History of molecular theory wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Matter wave wikipedia , lookup
Photopolymer wikipedia , lookup
Gas chromatography–mass spectrometry wikipedia , lookup
Periodic table wikipedia , lookup
Organic chemistry wikipedia , lookup
Extended periodic table wikipedia , lookup
Organosulfur compounds wikipedia , lookup
Inorganic chemistry wikipedia , lookup
Safety data sheet wikipedia , lookup
Chemical element wikipedia , lookup
Abundance of the chemical elements wikipedia , lookup
Drug discovery wikipedia , lookup
Condensed matter physics wikipedia , lookup
History of chemistry wikipedia , lookup
Vapor–liquid equilibrium wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Atomic theory wikipedia , lookup
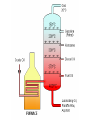
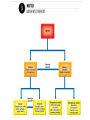
Warm-Up 9/9 1. 2. Define the term matter. Name something in this room that is not matter. Warm-Up 9/16 1. 2. List the three most important rules of lab safety. Would you classify jello as a solid or a liquid? Justify your choice. Today’s Learning Targets Power Standard: Students understand that physical and chemical properties depend on the ways in which different atoms combine. Today’s Learning Targets 1. 2. 3. I can list examples of physical properties. I can describe the physical characteristics and particle arrangement in solids, liquids, and gases. I can explain observable changes in physical properties and temperature during changes of state. A. Properties of Matter 1. 2. 3. 4. Matter- anything that has mass and volume. Mass - a measure of the amount of matter in a substance Substance (pure substance) - matter with uniform and definite composition Physical Change – A change that does not alter the chemical composition of the substance B. Physical Properties Include— Mass Weight Color Density Melting point Etc. B. Physical Properties Can be observed without changing the chemical composition of the substance. Example— List 3 physical properties of water. B. States of Matter 1. Solid - has definite shape and volume; particles are tightly packed and held in fixed positions; incompressible 2. Liquid - has an indefinite shape, definite volume; particles are tightly packed, but not in fixed positions (slide past one another -flow); incompressible B. States of Matter 3. Gas - indefinite shape and volume; particles are far apart and moving rapidly; compressible Note: A vapor is a gaseous form of a substance that is normally liquid at room temperature vapor and gas should not be used as interchangeable terms. Animation States of Matter Animation Are there other states of matter? D. Phase Changes 1. Temperature— Measures the KE of particles of matter. Always constant during a phase change. (Yes, really!) 2. Names of Phase Changes a. Solid/Liquid b. Liquid/Gas c. Melting or Freezing Boiling or Condensing Solid/Gas Sublimation or Deposition D. Heating/Cooling Curve Animation 1 Animation 2 D. Heating/Cooling Curve Copy this heating curve onto your notes. D. Heating/Cooling Curve Assume the heating curve represents substance X that starts as a solid below its melting point and is heated uniformly. 1. Identify the process that takes place during line segment DE of the heating curve 2. Identify a line segment in which the average kinetic energy is increasing. 3. Using "o" to represent particles of substance X, draw at least five particles as they would appear in the substance at point F. 4. Describe, in terms of particle behavior or energy, what is happening to substance X during line segment BC. Warm-Up 9/11 1. 2. State 2 physical properties of sucrose (table sugar). Give an example of a physical change that water can undergo. Today’s Learning Targets Power Standard: Students understand that physical and chemical properties depend on the ways in which different atoms combine. Today’s Learning Targets 1. 2. 3. 4. 5. I can state and explain common methods for separating mixtures. I can state that atoms of different elements combine in fixed ratios to form compounds, which have different properties from their component elements. I can state that mixtures contain more than one element and/or compound that are not chemically bonded together and so retain their individual properties. I can classify mixtures as homogeneous or heterogeneous. I can explain that elements are the basic building blocks of matter and discuss how they can physically mix or chemically combine. E. Physical Changes Alter the material without changing the chemical composition of it. Examples - cutting, bending, melting, freezing 2. Terms - tear, smash, crush, boil, freeze, dissolve, melt, condense, grind, etc. 1. II. Mixtures Key Definitions Mixture--two or more substances physically, not chemically, combined. Heterogeneous mixture Contains more than one phase Ex - oil & water, chicken noodle soup Key Definitions Homogeneous mixture Only has one phase Ex - salt water, soda Solution--a homogeneous mixture; dissolved substance CANNOT be filtered out Key Definitions Phase Any part of a mixture with uniform composition and properties Question How do you know if a sample is a mixture or a substance? Answer: Mixtures have variable composition— they come in different formulations Substances have definite composition—they only come in one chemical form Classifying mixtures 1. Can mixtures always be identified by sight? Chicken noodle soup? Coin jar? (yes) Air? Gasoline? Soda? (no) 2. Variable Composition Different samples have different ratios of ingredients (i.e. - iced tea) Indication of a mixture A. Examples of Mixtures Air Tap water Blood Soap Apple Juice Brine (salt water) Question How might you separate a mixture of sulfur and iron? Examine properties and look for a difference Magnetism B. Separating Mixtures 1. 2. Can be done by physical means, without chemistry. Common ways mixtures are separated include… Separating Mixtures 1. Distillation Boiling a solution where the water (vapor) is collected and then condensed Fractional distillation - collecting many different parts of a mixture as they cool and condense at different temperatures Separating Mixtures 2. Filtering 3. Evaporation 4. Removes solid phase from liquid phase Liquid phase is not collected; solid left Other methods? Decanting, chromatography, crystallization, melting point, solubility, density, separating funnel, centrifuging III. Elements & Compounds Key Definitions Element The simplest form of matter that exists under normal laboratory conditions Ex - hydrogen, sulfur, gold Another element song Key Definitions Compounds Chemically bonded combinations of elements in specific ratios Chemical Symbol One or two letter designation for an element. Chemical Formula Shows the ratio of atoms in the simplest unit of a substance Elements & Symbols There are 90 naturally occurring elements Element symbols come from the Latin names for elements Examples: Carbon (C), Iron (Fe) Forging Elements The Elements Song Warm-Up 9/15 1. 2. 3. What are the 2 classes of mixtures? Which has only 1 phase? What is a physical property you could use to separate a mixture of sand and salt? Today’s Learning Targets Power Standard: Students understand that physical and chemical properties depend on the ways in which different atoms combine. Today’s Learning Targets 1. 2. 3. 4. 5. I can state that atoms of different elements combine in fixed ratios to form compounds, which have different properties from their component elements. I can write and recognize a chemical symbol for an element using the periodic table. I can give examples of chemical compounds. I can explain that elements are the basic building blocks of matter and discuss how they can physically mix or chemically combine. I can suggest experimental techniques to distinguish between mixtures, elements, and compounds. Compounds Definite and Uniform composition Examples– Sodium chloride Sucrose Sodium hydrogen carbonate Representing (formulas) NaCl C12H22O11 NaHCO3 Distinguishing between compounds & elements Elements cannot be broken down by chemical means Compounds can be separated into simpler substances by chemical change Properties of compounds differ from the properties of their elements. Distinguishing between Mixtures & Compounds Can be difficult for homogeneous mixtures Ask yourself if it can have variable composition from sample to sample. If so, it’s a mixture. 1. Examples: Gasoline? Milk? Exists in different grades, may contain ethanol = mixture Contains different amounts of fat = mixture Water? Always H2O = compound Today’s Learning Target I can compare and contrast the different forms of matter, including elements, compounds, and mixtures.