* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Hydroxyl Group of a Phosphorylated Ribose
Physical organic chemistry wikipedia , lookup
George S. Hammond wikipedia , lookup
Reaction progress kinetic analysis wikipedia , lookup
Ionic compound wikipedia , lookup
Acid dissociation constant wikipedia , lookup
Metastable inner-shell molecular state wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Transition state theory wikipedia , lookup
Determination of equilibrium constants wikipedia , lookup
Surface properties of transition metal oxides wikipedia , lookup
Supramolecular catalysis wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Equilibrium chemistry wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Electrochemistry wikipedia , lookup
Acid–base reaction wikipedia , lookup
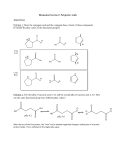
166 J. Am. Chem. Soc. 2000, 122, 166-167 Determination of the pKa of the 2′-Hydroxyl Group of a Phosphorylated Ribose: Implications for the Mechanism of Hammerhead Ribozyme Catalysis Paul D. Lyne†,‡ and Martin Karplus*,† Department of Chemistry and Chemical Biology HarVard UniVersity, 12 Oxford Street Cambridge, Massachusetts 02138 Physical and Theoretical Chemistry Laboratory Department of Chemistry, Oxford UniVersity South Parks Road, Oxford OX1 3QZ, England ReceiVed June 1, 1999 ReVised Manuscript ReceiVed September 8, 1999 Hammerhead ribozymes1 are small catalytic domains found in some viral satellite RNAs and are involved in RNA selfcleavage.2-4 The reaction catalyzed by hammerhead ribozymes is a phosphate ester hydrolysis that is thought to proceed via activation of a specific 2′-OH group by proton abstraction. The resultant 2′-alkoxide acts as a nucleophile, attacking the electropositive phosphorus in an intramolecular fashion and displacing the 5′-oxygen of the cleaved nucleotide (Figure 1). Hammerhead ribozymes are metalloenzymes5,6 with an absolute requirement for divalent metal ions for catalysis of the cleavage reaction.7 Magnesium ions are the natural cofactors for the hammerhead ribozyme, but other divalent cations can replace magnesium. They include Mn2+, Cd2+, and Co2+, which actually catalyze the reaction at higher rates than Mg2+ under the same conditions, and Ca2+, which catalyzes the reaction at a slower rate; a recent study reports that very high concentrations of Li+ ions can replace the Mg2+ ions.8 Cleavage rates are correlated with the pKa of the solvated metal ion, with the rate increasing as the pKa of the solvated ion decreases. This has traditionally been interpreted9 as indicating that a metal-coordinated hydroxide acts as the general base in the reaction. An alternative interpretation of the data10 suggests that the mechanism involves two metal ions but that a metal hydroxide ion is not the base in the reaction. The exact number of metal ions involved in the reaction and the nature of their interaction with the hammerhead ribozyme remains an area of discussion.11 A knowledge of the pKa values of ionizable groups at the active site of the ribozyme may aid in indicating possible mechanistic pathways and in inferring the nature of the chemically active species.12,13 Since deprotonation of the 2′-hydroxyl group is involved in generating the attacking nucleophile in the hammerhead ribozyme reaction, it is important to know the pKa of this group. The experimental determination of pKa values in complex systems, particularly the direct measurement of titrating groups of catalyti† Harvard University. Oxford University. (1) Birikh, K. R.; Heaton, P. A.; Eckstein, F. Eur. J. Biochem. 1997, 245, 1-16. (2) Buzayan, J. M.; Gerlach, W. L.; Bruening, G. Nature 1986, 323, 349353. (3) Hutchins, C. J.; Rathjen, P. D.; Forster, A. C.; Symons, R. H. Nucleic Acids Res. 1986, 14, 3627-3640. (4) Prody, G. A.; Bakos, J. T.; Buzayan, J. M.; Schneider, I. R.; Bruening, G. Science 1986, 231, 1577-1580. (5) Pyle, A. M. Science 1993, 261, 709-714. (6) Wilcox, D. E. Chem. ReV. 1996, 96, 2435-2458. (7) Dahm, S. C.; Uhlenbeck, O. C. Biochemistry 1991, 30, 9464-9469. (8) Murray, J. B.; Seyhan, A. A.; Walter, N. G.; Burke, J. M.; Scott, W. G. Chem. Biol. 1998, 5, 587-595. (9) Dahm, S. C.; Derrick, W. B.; Uhlenbeck, O. C. Biochemistry 1993, 32, 13040-13045. (10) Pontius, B. W.; Lott, W. B.; von Hippel, P. H. Proc. Natl. Acad. Sci., U.S.A. 1997, 94, 2290-2294. (11) Zhou, D.-M.; Taira, K. Chem. ReV. 1998, 98, 991-1026. (12) Jencks, W. P. Catalysis in Chemistry and Enzymology; Dover: New York, 1987. (13) Fersht, A. Enzyme Structure and Mechanism, 2nd ed.; Freeman: New York, 1985. ‡ Figure 1. (A) Schematic of the proposed mechanism for the first step of phosphate ester hydrolysis in the hammerhead ribozyme (divalent metals not shown). (B) Model phosphorylated ribose used in this study. cally important residues in enzymes, is not a straightforward task. Kinetic assignments of pKa values can be complicated by uncertainties in interpreting the pH dependence of the measured parameters.14 Moreover, different values are often obtained with different techniques.15 An estimate16,17 of the pKa of the 2′-OH group of a nucleotide is based on a value of 12.5 for the nucleoside 2′-anhydro-1-R-D-xylofuranosyluracil. However, the presence of the phosphate group could modify the pKa value. Usher et al. have reported18 a value of 13.9 for the pKa of the 2′-OH group in cistetrahydrofuran-4-ol 3-phenyl phosphate, while Guthrie estimated19 the pKa of the 2′-OH group of nucleotides to be approximately 16 based on thermodynamic arguments. Kinetic pKa values of 13 (at 333 K) have also been reported for dinucleotides.20 Since the value for the pKa of the 2′-OH group of a nucleotide is not well established, a theoretical estimate of the pKa of the 2′-OH at the active site of hammerhead ribozymes is presented here based on quantum chemistry calculations of a phosphorylated ribose (Figure 1). The pKa of a given acid HA in aqueous solution is related to the standard Gibbs free energy change of the ionization reaction by the thermodynamic relationship ∆Gaq0 1 ) × pKa ) -log Ka ) 2.303RT 2.303RT (∆Gg0 + ∆Gsolv0(H+) + ∆Gsolv0(A-) - ∆Gsolv0(HA)) (1) corresponding to the thermodynamic cycle in eq 1.21 In eq 1, ∆Gg0 and ∆Gaq0 are the standard free energies of ionization in the gas and in solution, respectively, and the ∆Gsolv0 values are the free energies of solvation. All gas-phase free energies were calculated using the Gaussian 94 program.22 Solvation energies were calcu(14) Knowles, J. R. CRC Crit. ReV. Biochem. 1975, 3, 165. (15) Marcus, Y. Ion SolVation; Wiley: New York, 1985. (16) Birnbaum, G. I.; Giziewicz, J.; Huber, C. P.; Shugar, D. J. Am. Chem. Soc. 1976, 98, 4640-4644. (17) Izatt, R. M.; Hassen, L. D.; Rytting, J. H.; Christensen, J. J. J. Am. Chem. Soc. 1965, 87, 2760-2761. (18) Usher, D. A.; Richardson, D. I., Jr.; Oakenfull, D. G. J. Am. Chem. Soc. 1970, 92, 4699-4712. (19) Guthrie, J. P. J. Am. Chem. Soc. 1977, 99, 3991. (20) Jarvinen, P.; Oivanen, M.; Lonnberg, J. J. Org. Chem. 1991, 56, 5396. (21) Kollman, P. Chem. ReV. 1993, 93, 2395. (22) Gaussian 94 (revision D.1) Gaussian Inc.: Pittsburgh, 1995. 10.1021/ja991820i CCC: $19.00 © 2000 American Chemical Society Published on Web 12/18/1999 Communications to the Editor J. Am. Chem. Soc., Vol. 122, No. 1, 2000 167 Table 1. Solvation Free Energy Changes Involved in the Ionization of MeOH and H3PO4a ∆Gaq experiment36,37 B3LYP/6-31+G** a MeOH MeO- H3PO4 H2PO4- -5.08 -5.50 -95.00 -93.03 -26.0 -20.8 -76.00 -79.83 All values in kcal/mol. Table 2. Electronic and Solvation Energies for the Ribose (RH) and Phosphorylated Ribose (pRH) and Their Conjugate Bases (Rand pR-) Calculated at the B3LYP/6-31+G** Levela RH RpRH pRa EElec ZPE G°(g) ∆Gs -382.9107 -382.3388 -989.4385 -988.7286 0.1259 0.1107 0.16718 0.15209 -382.8161 -382.2584 -989.3113 -988.6156 -0.0213 -0.1205 -0.1089 -0.3423 G°(s) pKa -382.8374 13.1 -382.3789 -989.4202 14.9 -988.9579 Units are hartrees. lated using the Poisson-Boltzmann method as implemented in the Jaguar program.23,24 The value of the solvation free energy of the proton was set equal to -262.23 kcal/mol. Since pKa calculations depend strongly on solvation free energies, accurate values for them are required. To address this issue, certain of the parameters used in the standard Jaguar model23 were optimized for systems related to the one of interest here. In particular, it is known that anions often pose problems for the determination of accurate solvation energies with continuum methods.25,26 The values of solvation energies obtained by using a continuum dielectric method depend on the van der Waals radii employed in the calculation. Several studies have used adjusted van der Waals radii to obtain agreement with experiment.26-28 To determine the optimal set of radii for the molecules being studied here, in particular the radii for the 2′-alkoxide oxygen and the pro-R and pro-S oxygens of the phosphate group, the solvation free energies of methanol and H3PO4 and their conjugate bases were determined and compared with experimental data. The calculated thermodynamic values are given in Table 1. It was found that values of 1.42 and 1.26 Å for the phosphate oxygens and methoxide oxygen, respectively, gave good agreement with the available experimental data; the default radii in Jaguar for these atoms are somewhat larger (1.60 and 1.45 Å, respectively). The adjusted radii were used for the solvation calculations on the model nucleotides. As a test of the method we have calculated the pKa of the 2′hydroxyl of ribose. The relevant gas phase and solvation energies are presented in Table 2. The pKa of a ribose using the optimized radius for the alkoxide oxygen of the conjugate base is calculated to be 13.14, which is rather close to the experimental value of 12.5; the value of the pKa calculated with the default Jaguar radii is 18.45, showing the importance of consistent van der Waals radii for continuum electrostatic calculations.26-28 The various model calculations suggest that the basis set, the form of the nonlocal functional, and the optimized radii for the PoissonBoltzmann calculations are appropriate for determining the pKa of the phosphorylated ribose. The gas-phase electronic energies and corresponding thermochemical data for the phosphorylated ribose and its conjugate base are shown in Table 2. The pKa calculated for the phosphorylated ribose (14.9) is considerably higher than the only available experimental pKa (12.5) of a (23) Jaguar, 3.5 ed.; Schrodinger, Inc.: Portland, OR, 1998. (24) Tannor, D. J.; Marten, B.; Murphy, R.; Friesner, R. A.; Sitkoff, D.; Nicholls, A.; Rignalda, M.; Goddard, W. A., III; Honig, B. J. Am. Chem. Soc. 1994, 116, 11875-11882. (25) Honig, B.; Sharp, K.; Yang, A.-S. J. Phys. Chem. 1993, 97, 1101. (26) Stefanovich, E.; Truong, T. Chem. Phys. Lett. 1995, 244, 65-74. (27) Lim, C.; Bashford, D.; Karplus, M. J. Phys. Chem. 1991, 95, 56105620. (28) Sitkoff, D.; Sharp, K. A.; Honig, B. J. Phys. Chem. 1994, 98, 19781988. nucleoside (see above).16,17,29 This is not surprising since the conjugate base of that nucleoside is expected to be stabilized by a hydrogen bond between the adjacent 3′-OH group and the deprotonated 2′-O-. The pKa of a nucleoside that has no such stabilization of the conjugate base should have a higher pKa. Additionally, the molecule studied here has a negatively charged phosphate group in close proximity to the 2′-OH group which should make it thermodynamically less favorable for the 2′-OH group to ionize than for the same group in the unphosphorylated molecule. Thus, the upward shift by nearly 2 pKa units is reasonable. The large value for the pKa of the 2′-OH group found here (pKa ) 14.9) has implications for the mechanism of hammerhead ribozyme catalysis and more specifically for the role played by metal ions in the reaction. It has been established, based on atomic substitution of the pro-R and pro-S oxygens by sulfurs (the thioeffect30) and by spectroscopic studies,31 that a metal ion coordinates directly with the pro-R oxygen of the phosphate at the active site.31 Kinetic studies of hammerhead cleavage have found that there is an inverse relationship between the pKa values of solvated metal ions and the rate of the cleavage reaction.9 This has been interpreted to indicate that a metal hydroxide ion acts as the general base in the reaction since the concentration of metal hydroxide ions increases as the pKa of the hydrated metal ion decreases. However, increasing the acidity of the hydrated metal ion lowers the basicity of the metal hydroxide. This is expected to lower its ability to deprotonate the 2′-OH group at the active site of the hammerhead. The pKa calculated here for a 2′-OH group in a nucleotide model is several units higher than the pKa of several metal ions (e.g., Pb2+(7.7), Zn2+(9.0), and Mg2+(11.4)32) that readily catalyze the reaction. It therefore seems unlikely that metal hydroxide ions act as the general base in the reaction.10 Since a metal ion is known to bind directly to the adjacent pro-R oxygen of the phosphate, it is conceivable that it promotes the reaction by stabilizing the transition state. It is also possible that it could bridge the 2′-oxygen and pro-R oxygen, which is consistent with the. two metal ion model proposed for a number of phosphoryl transfer reactions10,33,34 If so, it is expected that the hydroxide bond at 2′-OH would be polarized and that the pKa of the group would be reduced. The magnitude of the polarization of the bond would be directly related to the identity of the metal ion. Since other ribozymes5 and ribonucleases35 employ mechanisms that involve deprotonation of a 2′-OH at the active site, the result presented here has implications for phosphate hydrolysis reactions in many biological systems. Acknowledgment. This work was supported in part by the Human Frontiers in Science Program (P.D.L.), the Wellcome Trust (P.D.L.), and National Institute of Health (M.K.). Calculations were performed at the Pittsburgh Supercomputing Center, the Goddard Space Flight Center (NASA), and the Oxford University Supercomputer Centre. Supporting Information Available: Details are given of the methods used for the calculation of the results in the text and tables (PDF). This material is available free of charge via the Internet at http://pubs.acs.org. JA991820I (29) Saenger, W. Principles of nucleic acid structure; Springer-Verlag: New York, 1984. (30) Scott, E. C.; Uhlenbeck, O. C. Nucleic Acids Res. 1999, 27, 479-484. (31) Cunningham, L. A.; Li, J.; Lu, Y. J. Am. Chem. Soc. 1998, 120, 45184519. (32) Pan, T.; Long, D. M.; Uhlenbeck, O. C. DiValent Metal Ions in RNA Folding and Catalysis; Pan, T., Long, D. M., Uhlenbeck, O. C., Eds.; Cold Spring Harbor Press: Plainview, NY, 1993; pp 271-302. (33) Steitz, T. A.; Steitz, J. A. Proc. Natl. Acad. Sci. 1993, 90, 6498-6502. (34) Sawata, S.; Komiyama, M.; Taira, K. J. Am. Chem. Soc. 1995, 117, 2357-2358. (35) Fersht, A. Structure and Mechanism in Protein Science; W. H. Freeman and Company: New York, 1999. (36) Cabani, S.; Gianni, P.; Mollica, V.; Lepori, L. J. Solution Chem. 1981, 10, 563-595. (37) George, P.; Witonsky, R. J.; Trachtman, M.; Wu, C.; Dorwart, W.; Richman, L.; Richman, W.; Shurayh, F.; Lentz, B. Biochim. Biophys. Acta 1981, 223, 1-15.