* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download ANPS 020 Black 03-16
Survey
Document related concepts
Cryobiology wikipedia , lookup
Biochemical cascade wikipedia , lookup
Proteolysis wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Endocannabinoid system wikipedia , lookup
Paracrine signalling wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Lipid signaling wikipedia , lookup
Signal transduction wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Blood sugar level wikipedia , lookup
Calciseptine wikipedia , lookup
Transcript
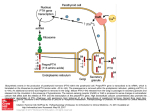
ANPS 020 ENDOCRINOLOGY III MARCH 16, 2012 (Cont’d from endo. II) PARATHYROID HORMONE/CALICUM HOMEOSTASIS Calcium importance -bone maintenance -nervous signaling -muscle contraction, skeletal, smooth, cardiac -blood clotting -others… All cells need calcium Three regulators: -parathyroid hormone -vitamin D -calcitonin (important during development held in equilibrium) Here integrated sites of action: -bone: largest reservoir of body calcium -GI calcium absorption -kidney calcium excretion PARATHYROID HORMONE (PTH) -Produced by parathyroid gland – located on the posterior thyroid -activates G protein coupled receptors on target tissues (bone, kidney) VITAMIN D -not one but class of compounds -synthesis in 3 tissues (skin, liver, kidney) -PTH important in final synthesis of active vitamin D in kidney Slide 33 chart is important BODY CALCIUM HOMEOSTASIS (does not have to go to hypothalamus) Free (ionized) calcium is active (50% free and 50% bound) PTH and blood calcium levels are tightly balanced Drop in blood calcium leads to increased PTH releases PTH FUNCTIONS PTH increases kidney calcium reabsorption (fast) PTH increases bone resporption to free calcium (slow) PTH stimulates kidney vit D synthesis – 1, 25 – (OH) 2D 1, 25 (OH)2D binds to GI steroid – type receptors to stimulate calcium absorption All mechanisms will increase calcium in blood CLACITONIN Peptide produced by thyroid C cells Decreases blood calcium, opposite effects of PTH and vitamin D Blood calcium so tightly coupled with PTH/vit D that calcitonin is more important during development than in adults ENDOCRINOLOGY III Hypothalamic – Pituitary – Adrenal Axis (HPA) Stress, pain, circadian drive activate hypothalamic corticotropin releasing hormone (CRH) release CRH binds to G protein coupled receptors on anterior pituitary corticotroph cells Cortciotrophs release POMC product ACTH ACTH binds to adrenal cortex G protein coupled receptors for cortisol release for adaptive responses Cortisol binds to steroid receptors Cortisol has long feedback to hypothalamus and pituitary gland ADRENAL GLAND Cortex – 3 contiguous layers Medulla – sympathetic in nature CORTISOL Cortisol is lipophilic and enters cells Cortisol binds to cytosolic glucocorticoid (steroid) receptors (GR) associated with chaperone heat-shock protein (HSP90) Bound to GR complex translocate in nucleus Complex acts as transcription factor to activate or repress genes on a variety of tissues HPA axis hormone response CRH-ACTH- Cortisol response is tightly coupled Peak cortisol response lags slightly but more persistent CORTISOL ACTIONS: PERMISSIVE MAKES THINGS WORK -metabolic -anti-inflammatory -immunosuppressive -vascular Metabolic responses: “gluco” in glucocorticoids implies increased blood glucose levels Liver – stimulates gluconeogenesis Muscle- stimulates protein catabolism for amino acids as substrates for gluconeogenesis, inhibits glucose uptake Fat- stimulate lipolysis, inhibits glucose uptake Net effect- disbetogenic In excess- cortisol leads to muscle wasting and thin skin from connective tissue loss Bone resorption/loss – osteoporosis Centripetal (trunk) obesity Sodium retention and potassium loss from binding to mineralocorticoid receptors ANTI-INFLAMMATRY/IMMUNOSUPPRESIVE RESPONSES Cortisol inhibits inflammatory mediator production in arachidonic pathway (prostaglandins, interleukins, thromboxane) Decreases other inflammatory molecules – TNF Reduces T lymphocytes and interferon production in immune responses Decrease antibody production (long term) Vascular: Facilitates vascular adrenergic function to maintain tone and blood pressure GLUCOCORTICOIDS: THE GOOD AND BAD The good: The anti-inflammatory/immunosuppressive effects of synthetic glucocorticoids (dexamethasone) are used therapeutically to blunt sever inflammation, allergic reactions, autoimmune responses and transplant rejections The bad: Long term use can lead to immunosuppression (bad for infections), muscle wasting, osteoporosis, hyperglycemia, obesity, neuronal/psychiatric disorders, adrenal insufficiency (from HPA negative feedback) PANCREAS, ISLETS, AND GLUCOSE HOMEOSTASIS Exocrine/endocrine pancreas Endocrine islets of Langerhans: Beta islets cells – insulin (green) Alpha islet cells – glucagon (red) Sigma islets cells – somatostatin (not shown) INSULINAND INSULIN SIGNALING: are key regulators of blood glucose (after dinner hormone) -insulin actions are opposed and balanced by glucagon -both insulin and glucagon are modulated in inhibitory somatostatin actions INSULIN: TRIGGERING ITS RELEASE Increase in blood glucose (after a meal) result in glucose entry into beta cells via GLT2 Cellular glucose metabolism result in increased ATP Increase in ATP inhibit intracellular K+ efflux Increase in cellular K+ results in cell depolarization and calcium entry Increased calcium stimulates insulin release from secretory granules ISLET BETA CELL INSULIN PRODUCTION Synthesized as 21 amino acid chain 3 disulfide bonds Intervening connecting peptide (also called C-peptide) is removed by dibasic RR/KR cleavage INSULIN REPCEPTOR SIGNALING Insulin binds to tyrosine receptors kinases in target cells Different signaling pathways from scaffold increase cell survival/proliferation, decrease glucose synthesis, and increase GLUT transporter insertion to enhance cell glucose entry Slide 53-important chart IN BRIEF, ELEVATED GLUCOSE LEVELS WILL: Increase GLUT4 insertion into tissues for glucose entry Increase tissue glycogen production and storage (from excess glucose) in liver and muscle Increase amino acid into tissues for protein synthesis Inhibit glycogenesis (glycogen breakdown) Inhibit gluconeogenesis (glucose synthesis) Inhibit lipolysis and reduce circulating free fatty acids Inhibit free fatty acid oxidation ( esp. in liver) and keto acid formation Net result is glycogen and triglyceride storage (i.e. fuel storage) FROM DECREASED IN BLOOD GLUCOSE LEVELS 9BETWEEN MEALS, FASTING) Glucagon I released from islet alpha cells Glucagon binds to target tissue G protein – coupled receptors Receptor activation of cAMP/PKA pathways result in enzyme phosphorylation and activity GLUCAGON EFFECTS ARE (OPPOSITE TO INSULIN) Increased glycogenolysis (breakdown of glycogen) to release glucose Increased gluconeogenesis in liver Increased protein breakdown to amino acids Increased lipid catabolism to free fatty acids and keto acids Net effect is fuel mobilization to serve metabolic demands DIABETES – 2 TYPES INSULIN DEPENDENT DIABETES MELLUTUS (IDDM) Type 1 About 5% of all cases Genetic predisposition Pancreatic beta cells fail Environmental factors NON INSULIN DEPENDENT DIABETES MELLITUS (NIDDM) Type II About 95% of all cases Genetic predisposition Body responds poorly to insulin Pancreatic beta cells can’t keep up The biggest culprit: overeating/obesity