* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Power Point Slides P..
Geometrical frustration wikipedia , lookup
Density of states wikipedia , lookup
Creep (deformation) wikipedia , lookup
Pseudo Jahn–Teller effect wikipedia , lookup
X-ray crystallography wikipedia , lookup
State of matter wikipedia , lookup
Heat transfer physics wikipedia , lookup
Bose–Einstein condensate wikipedia , lookup
Electronic band structure wikipedia , lookup
Colloidal crystal wikipedia , lookup
Low-energy electron diffraction wikipedia , lookup
Energy applications of nanotechnology wikipedia , lookup
Spinodal decomposition wikipedia , lookup
Crystallographic defects in diamond wikipedia , lookup
Tight binding wikipedia , lookup
Paleostress inversion wikipedia , lookup
Electromigration wikipedia , lookup
Radiation damage wikipedia , lookup
Crystal structure wikipedia , lookup
Work hardening wikipedia , lookup
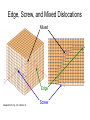
Dislocations – Linear Defects – Two-dimensional or line defect – Line around which atoms are misaligned – related to slip • Edge dislocation: – extra half-plane of atoms inserted in a crystal structure – Or – think of it as a partially slipped crystal – b to dislocation line • Screw dislocation: – spiral planar ramp resulting from shear deformation – b to dislocation line Burger’s vector, b: measure of lattice distortion or the amount of displacement. Burger’s vector is equal in magnitude to interatomic spacing. Edge Dislocation • This is a crystal that is slipping • Slip has occurred in the direction of slip vector over the area ABCD • Boundary between portion that has slipped and not slipped is AD • AD is the edge dislocation • The Burger’s vector b is = magnitude to the amount of slip Is acting in the direction of slip Note that b is ┴ dislocation line Source: G. Dieter, Mechanical Metallurgy, McGraw Hill, 1986. Dislocations – Linear Defects Edge Dislocation Fig. 4.3, Callister 7e. Motion of Edge Dislocation • Dislocation motion requires the successive bumping of a half plane of atoms (from left to right here). • Bonds across the slipping planes are broken and remade in succession. Atomic view of edge dislocation motion from left to right as a crystal is sheared. (Courtesy P.M. Anderson) Dislocations – Linear Defects Screw Dislocation b Dislocation line Burgers vector b (b) (a) Adapted from Fig. 4.4, Callister 7e. Edge, Screw, and Mixed Dislocations Mixed Edge Adapted from Fig. 4.5, Callister 7e. Screw Dislocations – Linear Defects Dislocations are visible in electron micrographs Transmission Electron Micrograph of Titanium Alloy. Dark lines are dislocations. 51450X Adapted from Fig. 4.6, Callister 7e. Interfacial - Planar Defects Surfaces – – – – Atoms do not have the same coordination number Therefore are in higher energy state Surface energy, g [=] J/m2 Materials always try to reduce surface energy – tendency towards spherical shapes Grain Boundaries – Interfacial Defects Solidification- result of casting of molten material – 2 steps • Nuclei form • Nuclei grow to form crystals – grain structure • Start with a molten material – all liquid nuclei liquid crystals growing grain structure • Crystals grow until they meet each other Grain Boundaries Grain Boundaries • regions between crystals • transition from lattice of one region to that of the other • slightly disordered • low density in grain boundaries – high mobility – high diffusivity – high chemical reactivity High energy locations where impurities tend to segregate to Planar Defects in Solids • One case is a twin boundary (plane) – Special kind of grain boundary – Mirror lattice symmetry – Essentially a reflection of atom positions across the twin plane. Brass at 60X Figure4.13c Adapted from Fig. 4.9, Callister 7e. • Stacking faults – For FCC metals an error in ABCABC packing sequence – Ex: ABCABABC Diffusion Diffusion - Mass transport by atomic motion Mechanisms • Gases & Liquids – random (Brownian) motion • Solids – vacancy diffusion or interstitial diffusion Diffusion • Interdiffusion: In an alloy, atoms tend to migrate from regions of high conc. to regions of low conc. Initially After some time Adapted from Figs. 5.1 and 5.2, Callister 7e. Diffusion • Self-diffusion: In an elemental solid, atoms also migrate. Label some atoms After some time C C A D B D A B Diffusion Mechanisms Vacancy Diffusion: • atoms exchange with vacancies • applies to substitutional impurity atoms • rate depends on: --number of vacancies --activation energy to exchange. increasing elapsed time Diffusion Simulation • Simulation of interdiffusion across an interface: • Rate of substitutional diffusion depends on: --vacancy concentration --frequency of jumping. (Courtesy P.M. Anderson) Diffusion Mechanisms Interstitial diffusion – smaller atoms can diffuse between atoms in lattice positions. Adapted from Fig. 5.3 (b), Callister 7e. Which will be faster – vacancy diffusion or interstitial diffusion? Processing Using Diffusion Case Hardening: • Diffuse carbon atoms into the host iron atoms at the surface. • Use a controlled atmosphere with a specific carbon potential (effective concentration) • Elevated Temperature • Example of interstitial diffusion is a case hardened gear. Result: The higher concentration of C atoms near the surface increases the local hardness of steel.