* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chapter 6
Renormalization group wikipedia , lookup
History of quantum field theory wikipedia , lookup
Canonical quantization wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Hidden variable theory wikipedia , lookup
Renormalization wikipedia , lookup
EPR paradox wikipedia , lookup
Quantum state wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
Quantum electrodynamics wikipedia , lookup
Atomic theory wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Symmetry in quantum mechanics wikipedia , lookup
Particle in a box wikipedia , lookup
Electron scattering wikipedia , lookup
Tight binding wikipedia , lookup
Atomic orbital wikipedia , lookup
Wave–particle duality wikipedia , lookup
Matter wave wikipedia , lookup
Hydrogen atom wikipedia , lookup
Electron configuration wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
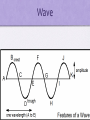
Chapter 6 Electronic Structure and the Periodic Table c= λf •λ = c/f •f = c/λ c = speed of light, 2.998 x 108m/s λ = wavelength, m f = frequency, Hertz, Hz Problem Microwaves have a wavelength of 1.000 cm. Calculate their frequency in Hz. 1.000 cm = 1.000 x 10-2m f = (2.998 x 108m/s)/1.000 x 10-2m = 2.998 x 1010Hz Problem Calculate the wavelength of a band on your AM radio with a frequency of 4.56 x 105Hz. λ = (2.998 x 108m/s)/(4.56 x 105Hz) = 657 m Wave Energy E = hc/λ E = hf E = energy, Joules, J c = speed of sound, 2.998 x 108m/s h = Planck’s constant, 6.626 x 10-34J*s Problem Calculate the the energy of microwaves in Joules, using the frequency 2.998 x 1010Hz. E = hf E = (6.626 x 10-34J*s)(2.998 x 1010Hz) = 1.986 x 10-23J Problem Calculate the energy in Joules of an AM radio wave having a wavelength of 657 m. E = hc/λ E = (6.626 x 10-34J*s)(2.998 x 108m/s)/657 m = 3.0 x 10-28J Quantum Numbers Principle Quantum Number Symbolized by n Indicates the main energy level occupied by the electron Values are integers starting with 1 Angular Momentum Quantum Number Symbolized by l Indicates shape Values are 0, 1, 2, 3 s p d f 0 1 2 3 Magnetic Quantum Number Symbolized by ml Indicates the orientation of the orbital around the nucleus Values are l,…, +1, 0, -1, ..., -l Spin Quantum Number Symbolized by ms Indicates the fundamental spin states of an electron in an orbital Values are + ½ and -1/2 Review Electron Configurations Orbital Diagrams Noble Gas Configurations Periodic trends Atomic Radius Ionization Energy Electronegativty Periodic Trends Atomic Radius Decreases across periodic table from left to right Increases down a group Ionization Energy Increases across periodic table from left to right Decreases down a group Electronegativity Increases across periodic table from left to right Decreases down a group Ionic Radius Ionic Radii increase down a group. Cations are smaller than their corresponding atoms. Anions are larger than their corresponding atoms. Chapter 6 Assignment Summary problem page 158 Chapter Review p. 159 2, 5, 10, 30, 32, 34, 34, 38, 40