* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Modern Theory of the Atom: Quantum Mechanical Model
Survey
Document related concepts
Nuclear structure wikipedia , lookup
Wave packet wikipedia , lookup
Photon polarization wikipedia , lookup
Quantum tunnelling wikipedia , lookup
Double-slit experiment wikipedia , lookup
Eigenstate thermalization hypothesis wikipedia , lookup
Quantum electrodynamics wikipedia , lookup
Density of states wikipedia , lookup
Old quantum theory wikipedia , lookup
Photoelectric effect wikipedia , lookup
Matter wave wikipedia , lookup
Heat transfer physics wikipedia , lookup
Atomic theory wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
Transcript
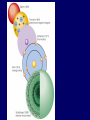
Modern Theory of the Atom: Quantum Mechanical Model Recap of Bohr Model • Electrons are particles moving in circular orbits – Specific speed, position, energy • Quantization of energy levels is imposed • Ground state: electrons closest to nucleus • Electrons can move between energy levels – higher energy levels farther from nucleus – moving up to higher E level: electron absorbs energy – moving down to lower E level: electron emits light energy 1924: De Broglie • Proposed: – if light can show both particle and wave behavior, maybe matter can too 2 kinds of waves Traveling wave Standing wave • Wave not confined to • Confined to given space given space (ends are pinned) • Travels from one location to another • Interference between incident & reflected waves • Interrupted by boundary or another wave • At certain frequencies: – certain points seem to be standing still – Other points - displacement changes in regular way Traveling Wave #1 • Traveling Wave #2 Guitar string • Standing wave #1 DeBroglie Electron-Wave wavelength describing electron depends on energy of electron At certain energies, electron waves make standing waves in atom wave does not represent electron path Modern Theory • Electron treated as wave – Cannot specify both position & speed of electron – Can determine probability of locating electron in given region of space • Quantized energy levels arise naturally out of wave treatment Bohr Model vs. Modern Theory • • • • • Electron = particle Orbit Holds 2n2 electrons Circular Each orbit has specific energy • Can find exact position/ speed • • • • • Electron = Wave Orbital Holds 2 electrons Not necessarily circular Each orbit has specific energy • Probable location Orbital – Modern Theory • Orbital = term used to describe region where electron might be • Each orbital has specific energy and specific shape • Described by 4 parameters of wave function (like an address): – quantum numbers = n, l, m, s What can orbitals do for us? • Physical structure of orbitals explain: – Bonding – Magnetism – Size of atoms – Structure of crystals Heisenberg uncertainty principle • Fundamentally impossible to know velocity and position of particle at same time • Impossible to make observation without influencing system n: principal quantum number • Specifies atom’s principal energy levels • whole number values: 1, 2, 3, 4, … • Maximum # electrons in any principal energy level = 2n2 l = Describes sublevels • Principal energy levels have sublevels • # sublevels depends on principal energy level – – – – 1st principal energy level has 2nd “ “ “ “ 3rd “ “ “ “ 4Th “ “ “ “ 1 sublevel 2 “ 3 “ 4 “ , etc. Naming sublevels • Sublevels are labeled by shapes: – s, p, d, f • s orbitals: spherical • p orbitals: dumbbell shaped • d & f orbitals: more complex shapes m = 3rd quantum number • Sublevels made up of orbitals • Each sublevel has specific # of orbitals Sublevel # of orbitals s 1 p 3 d 5 f 7 s orbitals p orbitals d orbitals 4th quantum number = s • Electron spin: 2 possible values • 4 quantum numbers = address for each electron • No 2 electrons in atom can have same 4 quantum numbers – only 2 electrons per orbital = Pauli exclusion principle Prin.E Level Sublevels 1 s 2 s p 3 s p d 4 s p d f # orbitals 1 1 3 1 3 5 1 3 5 7 Total # elec 2 2 6 2 6 10 2 6 10 14 3rd principal energy level, 3 sublevels 2nd principal energy level, 2 sublevels – s&p 1st principal energy level, 1 sublevel – s Each box represents an orbital and holds 2 electrons Order of fill: Aufbau principle • Each electron occupies lowest energy orbital available • Learn sequence of orbitals from lowest to highest energy • Some overlap between sublevels of different principal energy levels 1s 2s 3s 4s 5s 6s 7s 2p 3p 4p 5p 6p 7p 3d 4d 5d 6d Sequence of orbitals: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, … 4f 5f 6f Follow arrows Exceptions do occur: - half-filled orbitals have extra stability Electron Configurations Compare Bohr & Schrodinger Frequencies in Chemistry Electron Configuration & PT Principle Energy Levels n = 1,2,3,4 Sublevels hold 2 Orbitals electrons max 1st E level has 1 sublevel : 2nd “ “ 2 sublevels : 3rd “ “ 3 “ : 4th “ “ 4 “ : holds 2n2 electrons max s sublevel holds 1 orbital p sublevel holds 3 orbitals d sublevel holds 5 orbital f sublevel holds 7 orbitals s s and p s, p, and d s, p, d, and f