* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Angiotensin Receptor Blockers - Advantages of the
Survey
Document related concepts
Discovery and development of direct thrombin inhibitors wikipedia , lookup
Prescription costs wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Psychopharmacology wikipedia , lookup
Discovery and development of beta-blockers wikipedia , lookup
Adherence (medicine) wikipedia , lookup
Discovery and development of ACE inhibitors wikipedia , lookup
NK1 receptor antagonist wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Theralizumab wikipedia , lookup
Neuropharmacology wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Discovery and development of angiotensin receptor blockers wikipedia , lookup
Transcript
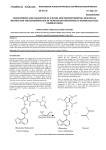
28 Journal of the association of physicians of india • july 2013 • VOL. 61 Drug Corner Angiotensin Receptor Blockers - Advantages of the New Sartans Zia Al Sabbah*, Aijaz Mansoor**, Upendra Kaul** Abstract Advantages of the new angiotensin receptor blockers (ARBs) include once daily dosing, an absence of significant adverse reactions, well tolerated side effect profile and cost effectiveness. A growing realization is their beneficial pleotropic effects. Antihypertensive agents are widely used to reduce the risk of cardiovascular events partly beyond that of blood pressure-lowering. The RAAS, and its primary mediator Ang II, also have a direct influence on the progression of the atherosclerotic process via effects on endothelial function, inflammation, fibrinolytic balance, and plaque stability. For patients at high cardiovascular risk based on the results of the ONTARGET and TRANSCEND studies, telmisartan is indicated for cardiovascular prevention. Studies have shown that olmesartan medoxomil treatment may slow the progression of atherosclerosis and postpone albuminuria thereby potentially improving CV outcomes. T he renin–angiotensin system (RAS) participates significantly in the pathophysiology of hypertension, congestive heart failure, myocardial infarction, and diabetic nephropathy. 1 Angiotensin (Ang) II, induces not only acute vasoconstriction by binding mainly to the ang II type 1 receptor (AT 1) but also promotes vascular growth and proliferation, acts as a proinflammatory mediator and causes endothelial dysfunction, leading to cardiovascular disease. 2 Research focused on blocking the RAS led to the discovery of angiotensin-converting-enzyme (ACE) inhibitors, which are effective in the treatment of hypertension and heart failure but are associated with a high frequency of cough and other adverse effects. AT-II-receptor blockers (ARBs) were developed as agents that would more completely block the RAS and decrease the adverse effects seen with ACE inhibitors. Although both classes of drugs (ACE inhibitors and ARBs) block the RAS, they differ in several important aspects: • ACE inhibitors reduce the biosynthesis of Ang II by the action of ACE, but do not inhibit alternative non-ACE Ang II-generating pathways. ARBs block the actions of Ang II via the AT1 receptor regardless of the biochemical pathway leading to Ang II formation. • Unlike ACE inhibitors, ARBs allow activation of AT 2 receptors. 3 ARBs cause a several-fold increase in circulating levels of Ang II. Because ARBs block AT1 receptors, this increased level of Ang II is available to activate AT2 receptors. AT2 receptor activation is thought to have the opposite effect of those mediated by the AT1 receptor, which are beneficial to the cardiovascular system and help protect target organs from damage. • ACE inhibitors increase the levels of a number of ACE substrates, including bradykinin. • ACE inhibitors may increase Ang (1–7) levels more than do ARBs. ACE is involved in the clearance of Ang (1–7), so College of Medicine, King Khalid University, Abha Kingdom of Saudi Arabia; **Fortis Escorts Heart Institute and Research Center, New Delhi -110025 Received: 02.12.2011; Revised: 27.04.2012; Accepted: 28.05.2012 * 464 inhibition of ACE may increase Ang (1–7) levels more so than do ARBs. ACE is a relatively nonspecific enzyme that has substrates in addition to angio I, including bradykinin and other tachykinins and thus inhibition of ACE may result in accumulation of these substrates. Production of angiotensin II can occur through non-ACE pathways as well as through primary ACE pathway, and these alternative pathways are unaffected by ACE inhibition. (Figure1). Conversion of AT-I to AT-II is not the only pathway for AT-II generation. AT-II is also formed via pathways involving cathepsin G, elastase, tissue plasminogen activator, chymostatinsensitive AT-II-generating enzyme, and chymase.4 Thus, ACE inhibition only partially reduces the formation of AT-II. Agents that can specifically and selectively inhibit the action of AT-II could completely block the RAS. Currently, two classes of drugs have the mechanistic potential to completely block the RAS: renin inhibitors and AT-II-receptor antagonists. ARBs displace angiotensin II from the angiotensin I receptor and produce their blood pressure lowering effects by antagonizing angiotensin II actions (vasoconstriction, aldosterone release, catecholamine release, arginine vasopressin release, water intake and hypertrophic response. 5 ARBs down regulate sympathetic adrenergic activity by blocking the effects of AT-II on sympathetic nerve release and re-uptake of norepinephrine. Although the ARBs have some structural and pharmacokinetic differences, few pharmacological differences separate these agents from one another. Two subtypes of AT-II receptors have been identified. Type 1 receptors are predominantly found on vascular endothelium and are linked to all the known physiological and pharmacologic actions of AT-II. Stimulation of type 1 receptors by AT-II induces vasoconstriction, renal tubular sodium reabsorption, aldosterone release, vascular smooth muscle remodeling, and stimulation of central and peripheral sympathetic activity, thus leading to increases in blood volume and blood pressure. Antagonism of type 1 receptors lowers blood pressure by inhibiting these actions. Type II receptors are predominantly found in the adrenal medulla, uterus, and fetal tissue and may play a role in fetal growth and differentiation, although the exact function of these receptors has not been identified.6 © JAPI • july 2013 • VOL. 61 29 Journal of the association of physicians of india • july 2013 • VOL. 61 There are eight ARBs currently on the market for hypertension and in different cardiovascular indications, ie, losartan, valsartan, candesartan, eprosartan, irbesartan telmisartan, olmesartan, and azilsartan, All ARBs are approved for the treatment of hypertension. In addition, irbesartan and losartan are approved for diabetic nephropathy, losartan is approved for stroke prophylaxis, and valsartan and candesartan are approved for heart failure and to reduce cardiovascular mortality in clinically stable patients with left ventricular failure or left ventricular dysfunction following myocardial infarction. ARBs also demonstrated effectiveness in preventing atheromas, decreasing endothelial dysfunction, increasing fibrinolysis, reducing proteinuria, and preserving kidney function in diabetic patients.7 Pharmacokinetics of various ARBs are depicted in Table 1. A succinct discussion of the newer ARBs follows. Telmisartan Telmisartan is licensed for the treatment of essential hypertension. Telmisartan, a nonpeptide AT-II-receptor antagonist, gained FDA approval for use in the treatment of hypertension in 1998. Peak plasma levels are obtained 0.5-1 hour after oral administration, and the plasma t1/2 is ~24 hours. Oral bioavailability of ARBs generally is low (<50%, except for irbesartan, with 70% available), and protein binding is high (>90%). Telmisartan is cleared from the circulation mainly by biliary secretion of intact drug. The plasma clearance of telmisartan is affected by hepatic but not renal insufficiency.8 The recommended oral dosage of telmisartan is 40-80 mg once daily. When further blood pressure reduction is needed (beyond that achieved with 80 mg/day), the addition of hydrochlorothiazide has been found to produce incremental reductions. 9 Telmisartan lowers systolic/diastolic blood pressure in patients with hypertension by up to 12/9 mm Hg at 40 mg once daily, and up to 13/10 mm Hg at 80 mg once daily. It is at least as effective Fig. 1 : Renin-angiotensin-aldosterone system Table 1 : Pharmacokinetic properties of the angiotensin II receptor blockers F (%) (bioavailability Active metabolite Tmax (hr) t ½ (hr) Metabolism (primary pathway) Elimination (%) Food interactions Losartan Valsartan Irbesartan Candesartan Eprosartan Telmisartan 33 10-35 60-80 15 13 42-58 Olmesartn Medosomil 26 Yes No No Yes No No Yes I (metabolite,3-4) I (metabolite, 6-9) 2-4 6 1.5-2 11-15 3-4 3.5-4 (metabolite, 3-11) 1-2 5-9 No Only 11% biotransformed CYP-2C9 and 3A4 Unknown CYP 2C9 O-demethylation Glucoronide conjugation Conjugation 1-3 12-18 (metabolite, 8-13) De-eserifictation 20 renal, 80 biliary No 33 renal, 67 biliary No 35 renal, 10 renal, 60 bilary >80 bilary 10%decrease in ~50% decrease in bioavailability AUC (NS) 7 renal, >97 biliary 90 biliary Delayed 6%-20% decrease absorption (NS) in Bioavailability None Digoxin 8-12 renal, biliary No Drug Rifampin, None None None None Interactions fluconazole (significant) No change in No change in No change in Use with caution No change in Dose in hepatic Initial dosage No change in dose* does* does does impairment dose* Dose in renal No change in No change in No change in No change in No change in No change in No change in impairment does does does does does does does * No change in dosage for mild to moderate hepatic dysfunction; exercise care in severe disease (no data available); No dosage adjustment necessary unless the patient is volume-depleted. No change in dosage for mild to moderate renal dysfunction; exercise care in severe disease (no data available). AUC= area under the curve; CYP = cytochrome P-450; F= bioavailability; t½ =elimination half-life; T max=time of maximum plasma concentration. © JAPI • july 2013 • VOL. 61 465 30 Journal of the association of physicians of india • july 2013 • VOL. 61 Table 2 : Important clinical trials of Telmisartan Clinical Trial Patient population Design Outcomes Patients aged 35–80 years, with T2DM, Telmisartan or enalapril on a background Equivalent reduction in the primary DETAIL38 mild to moderate hypertension and early of antihypertensive treatment; mean/ endpoint of the change in the GFR from nephropathy; n = 250 median follow-up not available baseline during 5 years of treatment with telmisartan vs enalapril Patients aged 21–80 years, with T2DM, Telmisartan or losartan on a background Significant reduction in the primary AMADEO46 hypertension, or on antihypertensive of antihypertensive treatment mean endpoint of the difference in the urinary drugs and overt nephropathy; n 860 follow-up 0.89 years albumin to creatinine ratio from baseline to week 52 with telmisartan vs losartan despite similar BP reductions in the 2 groups Patients aged 30–80 years, with T2DM, Telmisartan or valsartan on a background Equivalent reductions in the primary VIVALDI47 hypertension and overt nephropathy; n of antihypertensive treatment mean/ endpoint of the change from baseline in = 885 median follow-up not available the 24-hour proteinuria after 12 months for telmisartan and valsartan. Greater renoprotection was seen in those patients with better BP control High-risk patients aged $55 years, with Telmisartan-, ramipril- or telmisartan plus Telmisartan was equivalent to ramipril ONTARGET39 coronary, peripheral, or cerebrovascular ramipril-based antihypertensive regimens; for the primary composite endpoint of disease or diabetes with end-organ median follow-up 4.7 years CV death, MI, stroke, or hospitalization damage; n 25,620 due to HF, but it was associated with less angioedema and better treatment adherence. The combination was associated with more adverse events without an increase in efficacy Telmisartan regimen or placebo based Equivalent reduction in the primary TRANSCEND40 High-risk patients aged $55 years, with coronary, peripheral, or cerebrovascular regimen (on a background of other composite endpoint of CV death, MI, disease or diabetes with end-organ antihypertensive agents); median stroke, or hospitalization for HF with damage, and intolerant to ACE inhibitors; follow-up 4.7 years telmisartan vs placebo. Significant n 5926 reduction in the secondary composite endpoint of CV death, MI, or stroke with telmisartan (13%; HR 0.87) vs placebo (14.8%) as enalapril, lisinopril, and losartan in the treatment of mild to moderate hypertension. Important clinical studies of telmisartan are shown in Table 2. Drug interactions : No interactions with drugs that inhibit or are metabolized by CYP isoenzymes would be expected, given that CYP isoenzymes are not involved in telmisartan’s metabolism, with the possible exception of interference with the metabolism of drugs metabolized by CYP2C19. When telmisartan is administered with digoxin, peak and trough plasma concentrations of digoxin are increased 49% and 20%, respectively. When telmisartan is given with warfarin there is no evidence of any change in the International Normalized Ratio. Adverse events : The overall frequency of adverse events with telmisartan 20-160 mg/ day was reported to be similar to that with placebo. Rates of upper-respiratory-tract infection (7%), dizziness (5%), back pain (3%), sinusitis (3%), and diarrhea (3%) were similar to the rates for placebo (6%, 6%, 1%, 3%, and 2%, respectively). The rate of cough with telmisartan (15.6%) was comparable to that with placebo (9.6%) and significantly less than with lisinopril (60%).9 Olmesartan Medoximil In April 2002 the FDA approved Olmesartan medoxomil for the treatment of hypertension. Olmesartan medoxomil, which is administered as a prodrug, is rapidly and completely desterified to the active metabolite olmesartan during absorption from the gastrointestinal tract. Following the conversion of olmesartan medoxomil to olmesartan, virtually no further metabolism occurs. The bioavailability of olmesartan is approximately 26%, similar to that of losartan and valsartan. Following oral 466 administration, the peak plasma concentration (Cmax) of olmesartan is reached after one to two hours. The bioavailability of olmesartan is not affected by food.10,11 The plasma t1/2 is between 10 and 15 hours. Plasma clearance of olmesartan is due to both renal elimination and biliary excretion. Although renal impairment and hepatic disease decrease the plasma clearance of olmesartan, no dose adjustment is required in patients with mild-to-moderate renal or hepatic impairment. The oral dosage of olmesartan medoxomil is 20-40 mg once daily. If BP is not controlled by olmesartan medoxomil alone, a diuretic may be added. Adverse Effects : Based on data from a number of studies, patients have tolerated the drug well, and the incidence of adverse events was similar to that for placebo (42.2% and 42.7%, respectively.12-15 The most commonly reported side effects were headache, upper respiratory tract infections, and influenza-like symptoms. Dizziness was also frequently noted. Oparil et al16 found that the rate of dizziness associated with olmesartan medoxomil (1.4%) was similar to the rates for losartan (0.7%), valsartan (1.4%), and irbesartan (3.4%).. In clinical trials, the incidence of cough was similar for olmesartan medoxomil (0.9%) and placebo (0.7%).17 This rate is much lower than that reported in users of the ACE inhibitors; in those patients, cough has been noted to occur in up to 39% of cases.18 Angioedema has rarely occurred with ARB therapy, although facial edema has been reported in five patients receiving olmesartan medoxomil.17 Drug Interactions : Olmesartan medoxomil does not appear to have any clinically relevant drug interactions. The co-administration of antacids does not significantly alter its bioavailability19 and no clinically significant drug interactions have been reported with the co-administration of digoxin or © JAPI • july 2013 • VOL. 61 31 Journal of the association of physicians of india • july 2013 • VOL. 61 to 8 mm Hg. A higher dose of azilsartan (80 mg) was superior to valsartan 320 mg or olmesartan 40 mg in lowering systolic blood pressure in short-term studies. Additional blood pressure reduction is expected when Azilsartan is used adjunctively with a diuretic. Findings from recent studies suggest that azilsartan medoxomil can lower 24-hour blood pressure more effectively than maximally recommended doses of other ARBs. Experimental studies in animals have revealed the pleotropic effects of Azilsartan. like improvement in insulin sensitivity and activation of PPAR gamma resulting in a favourable metabolic profile. Azilsartan is well tolerated; the most common side effects are headache and diarrhea. No cases of hyperkalemia have been reported in 6-week clinical trials. Worsening of renal function and hypotension should be monitored, particularly in those with baseline risk factors. There is a lack of human data supporting the use of Azilsartan for improvement in cardiovascular outcomes; therefore, Azilsartan is not approved for indications other than the treatment of hypertension. warfarin.20 Because olmesartan medoxomil is not metabolized by the cytochrome P-450 system, drugs that induce, inhibit, or are metabolized by this enzyme do not appear to interact with it. Clinical Efficacy : Seven placebo-controlled studies involved 2,693 patients with essential hypertension; 2,145 patients received olmesartan medoxomil, and 548 patients received placebo. Doses ranged from 2.5 to 80 mg once daily for six to12 weeks. Patient responses to olmesartan medoxomil were doserelated; doses of 20 mg daily produced an overall reduction of sitting trough BP (the lowest measured BP with the patient sitting) of about –10/6 mm Hg over placebo, and doses of 40 mg daily produced an overall sitting trough BP reduction of about –12/7 mm Hg over placebo. Doses greater than 40 mg did not offer any additional effects. Various published and unpublished comparative studies have also reported on the antihypertensive efficacy of olmesartan medoxomil.21-25 Clinical trials of Olmesartan Results from clinical trials suggest that olmesartan medoxomil can be as effective as atenolol (Van Mieghem et al,26 Püchler et al)27 and more effective than captopril (Williams et al),28 losartan (Ball et al),29 valsartan and irbesartan (Oparil et al)30 in reducing systolic or diastolic BP. Olmesartan for the Delay or Prevention of Microalbuminuria in Type 2 Diabetes. (ROADMAP) is the first large outcomes trial with olmesartan;31 it was conducted in 4447 patients with type 2 diabetes and one or more additional cardiovascular risk factors, but no evidence of microalbuminuria. Participants could have normal blood pressure or well-controlled hypertension. The primary end point was a renal one, time to onset of albuminuria. Patients were randomized to either 40 mg of olmesartan (n=2232) or placebo (n=2215) daily and were all allowed to take additional non-renin-angiotensin system (RAS) antihypertensive medications to reach target BP (≤130/80 mm Hg), until the predefined number of adjudicated microalbuminuria events occurred at a median follow-up of 3.2 years. The average baseline BP of participants was 137/80 mm Hg. The results show there was a cumulative incidence of microalbuminuria of 8.2% with olmesartan and 9.8% with placebo; the primary end point, time to onset of microalbuminuria, was delayed by 23% with olmesartan (hazard ratio 0.77, p=0.01), with the majority of this effect being BP. The higher rate of fatal cardiovascular events with olmesartan among patients with preexisting coronary heart disease was noted (15 patients (0.7%) in the olmesartan group as compared with 3 patients (0.1%) (P=0.01) in the placebo group. This excess mortality in the olmesartan group prompted an FDA safety review which is ongoing, and so far, FDA has determined that the benefits of olmesartan continue to outweigh its potential risks when used for the treatment of patients with high blood pressure according to the drug label. Advantages of the Newer ‘Sartans’ Advantages of these drugs include once daily dosing, an absence of significant adverse reactions, well tolerated side effect profile and cost effectiveness. A growing realization is their beneficial pleotropic effects. ARBs are better tolerated than ACE inhibitors and other antihypertensive agents in both the short term and the long term. This is an important benefit, because hypertension is often asymptomatic, making long-term treatment adherence a challenge. None of the ARBs interact with food, which makes oral administration very easy. The newer agents, Telmisartan, and Olmesartan have longer half-lives and durations of action than the older agents losartan and Valsartan. Twenty-four–hour blood pressure control could be more readily achievable with the newer agents. ARBs are often used in patients who are intolerant of ACE inhibitors due to the development of cough or angioedema, or in those who are at a high risk of developing either of these side effects. Patients over 60 years, females, those of east-Asian ethnicity and smokers are at increased risk of cough, and patients of AfricanAmerican ethnicity, smokers, or those patients with a history of ACE inhibitor cough are at increased risk of angioedema.33 Studies in hypertensive patients have shown consistently that Telmisartan improves insulin sensitivity and lipid profiles.34 Telmisartan has been demonstrated to improve markers of glycemic control, such as glycosylated hemoglobin, in patients with type 2 diabetes.35 Telmisartan is the only ARB shown to be able to activate PPAR (peroxisome proliferator-activated receptor gamma) at therapeutic dosages,36 and in general, Telmisartan produces greater beneficial effects on glucose metabolism than the other ARBs. Azilsartan Clinical evidence for improvements in endothelial function with Telmisartan is provided by the Telmisartan versus Ramipril in renal ENdothelial DYsfunction (TRENDY) study.37 In the TRENDY® study, Telmisartan not only improved renal endothelial function in patients with type 2 diabetes but also preserved renal function. In comparison with Ramipril, Telmisartan significantly improved resting renal plasma flow, renal vascular resistance, and lowered albuminuria. The Diabetics Exposed to Telmisartan And enalaprIL (DETAIL) study38 showed the long-term benefit of Telmisartan in patients with type 2 diabetes and either micro- or macroalbuminuria. GR rate of decline was markedly reduced. It is the latest ARB to be approved for hypertension.32 The key points of Azilsartan are its potency, its ability of sustained blood pressure control over a 24- hour period, and experimental evidence of favorable pleotropic cardioprotective effects. azilsartan medoxomil at a dose of 40 mg or 80 mg once daily showed greater efficacy (about 10% in absolute rate) than a 320 mg dose of valsartan, the highest approved dose for this drug. Azilsartan is a prodrug that is quickly hydrolyzed to the active moiety azilsartan, a potent and highly selective ARB with estimated bioavailability of 60% and elimination half-life of 12 hours. At the approved dosage, it reduces systolic blood pressure by 12 to 15 mm Hg and diastolic blood pressure by 7 © JAPI • july 2013 • VOL. 61 On the basis of the ONTARGET results, 39 Telmisartan 467 32 Journal of the association of physicians of india • july 2013 • VOL. 61 is proven to have cardiovascular protective effects. The ONTARGET study in patients at high risk of cardiovascular events showed that Telmisartan was non-inferior, as defined by pre-specified boundaries, to the ACEi ramipril given at the same dose as had been proven to be beneficial in the HOPE study. It is as effective as ramipril but is associated with less angioedema and cough. In the TRANSCEND40 study, Telmisartan was well tolerated among patients who were unable to tolerate ACE inhibitors and their was a significant reduction in the risk of the composite outcomes of cardiovascular death, myocardial infarction, or stroke by 13%. There is substantial evidence supporting the hypothesis that Ang II plays a significant role in the initiation and progression of atherogenesis,41 with endothelial dysfunction a hallmark of early event in atherogenesis. Data from the Olmesartan medoxomil clinical trials OLIVUS,42 EUTOPIA,43 VIOS,44 and MORE45 have demonstrated the specific utility of RAAS suppression in reducing atherosclerotic plaque volume, improving plaque composition and stability, and in improving endothelial dysfunction. These studies have shown that Olmesartan medoxomil treatment may slow the progression of atherosclerosis, thereby potentially improving CV outcomes. The currently available ARBs have demonstrated their differential efficacy along the cardiovascular and renal disease continua. For patients at high cardiovascular risk based on the results of the ONTARGET and TRANSCEND studies, Telmisartan is indicated for cardiovascular prevention beyond that of blood pressure-lowering alone. Combination Therapy Combination therapy is an effective strategy to increase antihypertensive efficacy in those patients with poor blood pressure (BP) control. A calcium channel blocker (CCB)/ angiotensin receptor blocker (ARB) combination is a rational approach for such an antihypertensive strategy. The ARBs confer stroke protection, renal protection, and tolerability similar to placebo, without dose-related symptomatic and metabolic AEs, while CCBs are beneficial in reducing stroke and treating angina and cardiac ischemia. The antihypertensive efficacy of combinations of once-daily oral amlodipine and valsartan (administered as separate agents or as amlodipine/valsartan) has been demonstrated in several large, randomized, doubleblind clinical trials of 8-16 weeks’ duration; BP reductions were maintained for approximately 1 year in open-label extensions of some of these studies. Combination therapy was more effective than amlodipine or valsartan monotherapy in reducing BP in patients with mild to moderate hypertension, and more effective than amlodipine monotherapy in reducing BP in patients with moderate to severe (stage 2) hypertension. CREATE (Heart Institute of Japan Candesartan Randomized Trial for Evaluation in Coronary Artery Disease) was a randomized, double-blind, placebo-controlled study; data from 1025 patients with CAD and hypertension and an eCrCl less than 60 ml/min were included in the subanalysis.46 Patients were randomized to candesartan 4 mg to 12 mg daily or placebo and followed for an average of 4.2 years. The primary CV outcome was the composite of CV death, nonfatal MI, unstable angina, heart failure, stroke, and CV events requiring hospitalization. An 11% lower rate in the composite CV outcome was observed with candesartan versus placebo. There was also a lower rate of hospitalization for unstable angina in patients with CKD given candesartan compared with those given placebo. In IDNT study, irbesartan was renoprotective independently of its BP lowering effect in patients with NIDDM and microalbuminuria.47 In RENAAL study 48 (losartan vs placebo) losartan conferred significant renal benefits in patients with NIDDM and nephropathy, benefit not attributable to changes in BP. Telmisartan has also demonstrated renal protective effects. The beneficial effects on vascular growth and fibrosis in the kidney via blockade of the AT1 receptor as well as the stimulation of PPAR-γ make telmisartan useful in the prevention of hypertensive renal disease. In the VIVALDI49 (inVestIgate the efficacy of telmisartan versus VALsartan in hypertensive type 2 DIabetic) trial, Telmisartan 80 mg/day and Valsartan 160 mg/day produced similar reductions in 24-hour urinary protein excretion rates by 33% after 12 months of treatment in diabetic patients with hypertension and overt nephropathy.10 It is thought that their renoprotective benefit in VIVALDI, was solely due to the antihypertensive effect. However, in the AMADEO50 (A comparison of telMisartan versus losArtan in hypertensive type 2 DiabEtic patients with Overt nephropathy) trial, Telmisartan 80 mg/day was superior to losartan 100 mg/day in terms of renoprotective properties. JNC 8 guidelines are anticipated this year and it is expected that there will be specific recommendations on hypertension in the CKD population. Central Aortic Pressure Activation of the RAAS plays an important role in the progression of CKD regardless of the initial nephropathy, and its blockade remains the most important goal in achieving preservation of renal function. The level of augmentation of central systolic blood pressure (SBP) or the central augmentation index (cAI) is caused by reflection of pulse waves in the periphery. Increased pulse wave velocity from stiff large arteries and increased peripheral vascular resistance are two major causes for an earlier return, and higher amplitude of the reflected pulse wave, respectively. Earlier return of the reflected pulse wave shifts central BP augmentation from diastole into late systole, and therefore leads to augmentation of central SBP and hence cardiac after load. In addition to detrimental effects on the heart, elevated central SBP is also thought to be a major determinant of the risk for stroke. Studies51 have shown that ARBs (irbisartan) reduce cAI whereas atenolol increases it. Furthermore, central to peripheral pulse pressure (PP) amplification is unaffected by treatment with irbesartan, but decreases with atenolol. This could partly explain the reported differential effects of ARB versus β-blocker treatment on cardiovascular mortality in patients with essential hypertension. In 2007, the AHA developed a scientific statement on treating hypertension and designated the CKD population as a high coronary artery disease (CAD) risk group and recommended a blood pressure goal of less than 130/80 mm Hg. Angiotensinconverting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) are preferred antihypertensive agents in patients with CKD as per the NKF KDOQI and JNC 7 guidelines. HIJ- A meta-analysis published in Lancet Oncology (2010)52 raised the possibility that angiotensin receptor blockers (ARBs) might increase the risk of malignancy. This generated a significant debate until the publication of two further meta-analyses,53,54 neither of which demonstrated an increased risk of new cancer ARBs and CKD 468 ARBs and Cancer Risk © JAPI • july 2013 • VOL. 61 33 Journal of the association of physicians of india • july 2013 • VOL. 61 occurrence or cancer-related death with the use of ARBs in patients with hypertension, heart failure, and/or nephropathy. Overall, the bulk of evidence today indicates that ARBs are not associated with increased cancer risk, as endorsed by the FDA.54 14. Püchler K, Laeis P, Stumpe KO. A comparison of the efficacy and safety of the oral angiotensin II antagonist olmesartan medoxomil with those of atenolol in patients with moderate to severe hypertension under continuous treatment with hydrochlorothiazide [Abstract No. P2.175]. J Hypertens 2001;19(Suppl 2):153. Individual Differences Among ARBs 15. Neutel JM. Clinical studies of CS-866, the newest angiotensin II receptor antagonist. Am J Cardiol 2001;87:37–43. Telmisartan vs. others 16. Oparil S, Williams D, Chrysant SG, et al. Comparative efficacy of olmesartan, losartan, valsartan, and irbesartan in the control of essential hypertension. J Clin Hypertens 2001;3:283–291. Ambulatory blood pressure monitoring (ABPM) has shown that Telmisartan 80 mg confers significantly greater blood pressure lowering than several other ARBs. When compared with Valsartan 160 mg, Telmisartan provided sustained antihypertensive efficacy and superior control of blood pressure during the early morning period.55 3 ABPM studies comparing Telmisartan 40 or 80 mg with losartan 50 or 100 mg demonstrated that Telmisartan provided greater reductions than losartan in both the 24-hour mean SBP and DBP and in the in last 6 hours of the dosing interval.56 17. Benicar® package insert. Sankyo Pharma, Inc, New York. 18. Israili ZH, Hall WD. Cough and angioneurotic edema associated with angiotensin-converting enzyme inhibitor therapy: A review of the literature and pathophysiology. Ann Intern Med 1992;117:234– 242. 19. Kawaratani T, Laeis P, Püchler K, et al. The effect of an antacid (aluminum magnesium hydroxide) on the pharmacokinetics and safety of the oral angiotensin II antagonist CS-866 in healthy male subjects [Abstract No. 145]. J Hypertens 1999;17(Suppl 3):S243. ABPM provides more clinical data and is a better predictor of target- organ damage than other BP monitoring methods. Considerable focus was placed on the use of ABPM in the clinical program of Telmisartan, providing the largest ABPM database for a single ARB. Studies using ABPM showed that differences in efficacy and duration of action exist within the ARB class. A meta-analysis of trials using ABPM showed that Telmisartan was the most efficacious and exhibited the longest duration of action.57 In clinical practice, these qualities of ARBs may optimize 24-hour BP control, including the critical early morning period. 20. Püchler K, Laeis P, Kawaratani T, et al. The effect of the combination of the oral angiotensin II antagonist CS-866 and warfarin on pharmacodynamics, pharmacokinetics and safety in healthy male subjects [Abstract No. 271]. J Hypertens 1999;17(Suppl 3):275. 21. Van Mieghem W. A multicentre, double-blind, efficacy, tolerability and safety study of the oral angiotensin II antagonist olmesartan medoxomil versus atenolol in patients with mild to moderate essential hypertension [Abstract No. P2.174]. J Hypertens 2001;19(Suppl 2):152. 22. Williams PA. A multicentre, double-blind, efficacy, tolerability and safety study of the oral angiotensin II antagonist olmesartan medoxomil versus captopril in patients with mild to moderate essential hypertension. J Hypertens 2001;19(Suppl 2):300. References 1. The renin-angiotensin aldosterone system: Pathophysiological role and pharmacologic inhibition. J Manag Care Pharm. 2007;13 Suppl B:9–20. 2. Paul M, Poyan Mehr A, Kreutz R. Physiology of local reninangiotensin systems. Physiol Rev 2006;86:747–803. 3. Stelings UM, Kaschina E, Unger T. The AT2 receptor – a matter of love and hate. Peptides. 2005;26:1401–1409. 4. Paul M, Poyan Mehr A, Kreutz R. Physiology of local reninangiotensin systems. Physiol Rev 2006;86:747–803. 5. Burneir M, Brunner HR. Angiotensin 11 receptor antagonists. Lancet 2000;355:637-645 6. Rodgers JE, Patterson JH.Angiotensin 11 receptor blockers. Clinical relevance and theraupeutic role. Am J Health Syst Pharma 2001;58:671-683 7. Norwood D, Branch E, Smith B et al. Olmesartan Medoxomil for Hypertension: A Clinical Review. Drug Forecast 2002;27:12. 8. Wienen W, Entzeroth M, van Meel JCA, et al. A review on telmisartan: a novel, long-acting angiotensin II-receptor antagonist. Cardiovasc Drug Rev 2000;18:127–156. 9. Meredith P. Optimal dosing characteristics of the angiotensin II receptor antagonist telmisartan. Am J Cardiol 1999;84:7K-12K. 23. Ball K. A multicentre, double-blind, efficacy, tolerability and safety study of the oral angiotensin II antagonist olmesartan medoxomil versus losartan in patients with mild to moderate essential hypertension [Abstract No. P2.176]. J Hypertens 2001;19(Suppl 2):153. 24. Oparil S, Williams D, Chrysant SG, et al. Comparative efficacy of olmesartan, losartan, valsartan and irbesartan in the control of essential hypertension. J Clin Hypertens 2001;3:283–291. 25. Cozaar® package insert. Merck, West Point, PA, 1999. 26. Van Mieghem W. A multicentre, double-blind, efficacy, tolerability and safety study of the oral angiotensin II antagonist olmesartan medoxomil versus atenolol in patients with mild to moderate essential hypertension [Abstract No. P2.174]. J Hypertens 2001;19(Suppl 2:152 27. 28. Williams PA. A multicentre, double-blind, efficacy, tolerability and safety study of the oral angiotensin II antagonist olmesartan medoxomil versus captopril in patients with mild to moderate essential hypertension. J Hypertens 2001;19(Suppl 2):300. 10. Neutel JM. Clinical studies of CS-866, the newest angiotensin II receptor antagonist. Am J Cardiol 2001;87(Suppl):37C–43C. 29. Ball K. A multicentre, double-blind, efficacy, tolerability and safety study of the oral angiotensin II antagonist olmesartan medoxomil versus losartan in patients with mild to moderate essential hypertension [Abstract No. P2.176]. J Hypertens 2001;19(Suppl 2):153. 11. Schwocho LR, Masonson HN. Pharmacokinetics of CS-866, a new angiotensin II receptor blocker, in healthy subjects. J Clin Pharmacol 2001;41:515–527. 12. Püchler K, Laeis P, Gunther A, et al. Safety, tolerability and efficacy of the new oral angiotensin II (AT1)-receptor antagonist CS-866 in patients with mild to moderate hypertension [Abstract No. P.11]. J Hum Hypertens 1999;13(Suppl 3):4. 30. Oparil S, Williams D, Chrysant SG, et al. Comparative efficacy of olmesartan, losartan, valsartan and irbesartan in the control of essential hypertension. J Clin Hypertens 2001;3:283–291. 31. Haller H, Ito S, Izzo JL, et al; ROADMAP Trial Investigators. Olmesartan for the delay or prevention of microalbuminuria in Type 2 diabetes. N Engl J Med 2011;364:907–917. 13. Masonson HN, Punzi HA, Neutel JM, et al. CS-866 (angiotensin II receptor antagonist): A double-blind study using ambulatory blood pressure monitoring in hypertensive patients [Abstract No. D035]. Am J Hypertens 1998;11(4 Pt 2):77. © JAPI • july 2013 • VOL. 61 Safety of the oral angiotensin II antagonist olmesartan medoxomil with those of atenolol in patients with moderate to severe hypertension under continuous treatment with hydrochlorothiazide [Abstract No. P2.175]. J Hypertens 2001;19(Suppl 2):153. 469 34 Journal of the association of physicians of india • july 2013 • VOL. 61 32.Lam, Sum. Azilsartan: A Newly Approved Angiotensin II Receptor Blocker. Cardiology in Review: November/December 2011 Volume 19 - Issue 6 - pp 300-304 33. Morimoto T, Gandhi TK, Fiskio JM, et al. An evaluation of risk factors for adverse drug events associated with angiotensinconverting enzyme inhibitors. J Eval Clin Pract 2004;10:499–509 34. Derosa G, Ragonesi PD, Mugellini A, et al. Effects of telmisartan compared with eprosartan on blood pressure control, glucose metabolism and lipid profile in hypertensive, type 2 diabetic patients. a randomized, double-blind, placebo-controlled 12-month study. Hypertens Res 2004;27:457–464. 35. Food and Drug Administration. FDA drug safety communication: ongoing safety review of Benicar and cardiovascular events. June 11, 2010. http://www.fda.gov/Drugs/DrugSafety/Postma rketDrugSafetyInformationforPatientsandProviders/ucm215222. htm. 36. Benson SC, Pershadsingh HA, Ho CI, et al. Identification of Telmisartan as a unique angiotensin II receptor antagonist with selective PPAR gamma modulating activity. Hypertension 2004;43:993–1002. 37. Schmieder RE, Delles C, Mimran A, et al. Impact of telmisartan versus ramipril on renal endothelial function in patients with hypertension and type 2 diabetes. Diabetes Care 2007;30:1351–1356. 38. Barnett AH, Bain SC, Bouter P, et al. Angiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathy. N Engl J Med 2004;351:1952–1961 39. ONTARGET Investigators, Yusuf S, Teo KK, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008;358:1547–1559. 40. TRANSCEND Investigators, Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trial. Lancet 2008;372:1174–1183. 45. Shiga T, Kasanuki H, Hagiwara N, et al. Angiotensin receptor blocker-based therapy and cardiovascular events in hypertensive patients with coronary artery disease and impaired renal function. Blood Pressure 2010;19:359-365. 46. Berl T, Hunsicker LG, Lewis JB, et al. Cardiovascular outcomes in the Irbesartan Diabetic Nephropathy Trial of patients with type 2 diabetes and overt nephropathy. Ann Intern Med 2003;138:542-549. 47. Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001;345:861-869 48. Galle J, Schwedhelm E, Pinnetti S, Böger RH, Wanner C; VIVALDI investigators. Antiproteinuric effects of angiotensin receptor blockers: telmisartan versus valsartan in hypertensive patients with type 2 diabetes mellitus and overt nephropathy. Nephrol Dial Transplant 2008;23:3174–3183. 49. Bakris G, Burgess E, Weir M, Davidai G, Koval S; AMADEO Study Investigators. Telmisartan is more effective than losartan in reducing proteinuria in patients with diabetic nephropathy. Kidney Int 2008;74:364–369. 50. Schneider MP, Delles C* Klingbel AU. Effect of angiotensin receptor blockade on central haemodynamics in essential hypertension: results of a randomized trial. JRAAS 2008;9: 49–55 51. Sipahi I, Debanne SM, Rowland DY, Simon DI, Fang JC. Angiotensin receptor blockade and risk of cancer: meta analysis of randomized controlled trials. Lancet Oncol. 2010;11:627–636. 52. Bangalore S, Kumar S, Kjeldsen SE, et al. Antihypertensive drugs and risk of cancer: network meta analyses and trial sequential analyses of 324,168 participants from randomised trials. Lancet Oncol. 2011;12:65–82. 53. Teo KK, Sleight P, Gao P, et al. Effects of Telmisartan, irbesartan, valsartan, candesartan, and losartan on cancers in 15 trials enrolling 138,769 individuals. J Hypertens 2011;29:623–635. 41. Hirohata A, Yamamoto K, Miyoshi T, et al. Impact of Olmesartan on progression of coronary atherosclerosis: a serial volumetric intravascular ultrasound analysis from the OLIVUS (impact of olmesarten on progression of coronary atherosclerosis: evaluation by intravascular ultrasound) trial. J Am Coll Cardiol 2010;55:976–982 54.US Food and Drug Administration (FDA). FDA drug safety communication: ongoing safety review of the angiotensin receptor blockers and cancer [web page on the Internet]. Rockville, MD: 2010 [updated February 6, 2011]. Available from: http://www.fda.gov/ Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatients and/ucm218845.htm. 42. Fliser D, Buchholz K, Haller H. Antiinflammatory effects of angiotensin II subtype 1 receptor blockade in hypertensive patients with microinflammation. Circulation 2004;110:1103–1107. 55.Lacourcière Y, Krzesinski JM, White WB, et al. Sustained antihypertensive activity of telmisartan compared with valsartan. Blood Press Monit 2004;9:203–210. 43. 56. Mallion JM, Siché JP, Lacourcière Y; The Telmisartan Blood Pressure Monitoring Group. ABPM comparison of the antihypertensive profiles of the selective angiotensin II receptor antagonists’ telmisartan and losartan in patients with mild-to-moderate hypertension. J Hum Hypertens 1999;13:657–664. Smith RD, Yokoyama H, Averill DB, Schiffrin EL, Ferrario CM. Reversal of vascular hypertrophy in hypertensive patients through blockade of angiotensin II receptors. J Am Soc Hypertens 2008;2:165– 172. 44. Stumpe KO, Agabiti-Rosei E, Zielinski T, et al. Carotid intima-media thickness and plaque volume changes following 2-year angiotensin IIreceptor blockade. The Multicentre Olmesartan atherosclerosis Regression Evaluation (MORE) study. Ther Adv Cardiovasc Dis 2007;1:97–106. 470 57. Neutel J, Smith DH. Evaluation of angiotensin II receptor blockers for 24 h blood pressure control: meta-analysis of a clinical database. J Clin Hypertens 2003;5:58-63. © JAPI • july 2013 • VOL. 61