* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download HBV DNA - Scioto County Medical Society
Survey
Document related concepts
Human cytomegalovirus wikipedia , lookup
Middle East respiratory syndrome wikipedia , lookup
Eradication of infectious diseases wikipedia , lookup
Herpes simplex virus wikipedia , lookup
Oesophagostomum wikipedia , lookup
Chagas disease wikipedia , lookup
Marburg virus disease wikipedia , lookup
Sexually transmitted infection wikipedia , lookup
Schistosomiasis wikipedia , lookup
Visceral leishmaniasis wikipedia , lookup
Leptospirosis wikipedia , lookup
African trypanosomiasis wikipedia , lookup
Antiviral drug wikipedia , lookup
Transcript
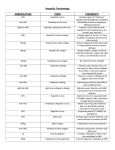
S 1 S 2 S 3 S Hepatitis A virus(HAV) VIRUS Classical infectious hepatitis 27nm RNA picornavirus Single strand RNA 4 S 5 EPIDEMIOLOGY HEPATITIS A 10-20% of Americans Ab by age 20 50% Ab age 50 and 75% no hx of illness 90% underdeveloped countries age 10 Fecal-oral transmission S Geographic Distribution of HAV Centers for Disease Control and Prevention. Health Information for International Travel, 2003-2004.;2003. 6 S Risk Factors for Hepatitis A United States, 1990–2000 CDC. Epidemiology and Prevention of Vaccine-Preventable Diseases. 8th ed. 2004. 7 S Overview of HAV Transmission Primary route of transmission: fecal-oral HAV is spread by: Person-to-person contact Eating or drinking contaminated food or water HAV can persist in the environment for months Incubation period: 15-50 days 1.Centers for Disease Control and Prevention Hepatitis A. In: Epidemiology and Prevention of VaccinePreventable Diseases. 9th ed., 2006. 2. Centers for Disease Control and Prevention. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. 1999;48(RR-12):1-37. 8 S Clinical Characteristics of HAV Symptoms are age related and more severe in adults than children Asymptomatic in >70% of children <6 years Symptomatic in >70% of older children and adults Clinical illness generally lasts up to 2 months 10%-15% of patients have symptoms lasting up to 6 months High morbidity/low mortality More severe complications in patients with chronic liver disease CDC. Epidemiology and Prevention of Vaccine-Preventable Diseases. 9th ed. 2006:193-206 9 S 10 DIAGNOSIS-HEPATITIS A HAV IgM positive Occasionally relapsing course Chronic liver disease doesn’t occur TREATMENT -HEPATITIS A Non-specific- bedrest,good diet,no alcohol No effective antiviral therapy Prevention- good sanitation Immunization-active and passive S 11 S 12 HBV Markers HBsAg: First viral marker to appear, indicates viral DNA presence in body HBeAg: Associated with viral particles; some mutants do not produce HBV DNA: Associated with viral particles; most reliable marker of circulating infectious virus S 13 HBV Antibodies Anti-HBc: First antibody to appear, lasts for life, does not give immunity Anti-HBs: Indicates immunity; only marker after vaccination Anti-HBe: Appears with loss of infectious virus; with mutants, not reliable marker of clearance Estimated Burden of HBV Infection in the United States New HBV infections 78,000 per year Chronic HBV infections >1-1.25 million New Chronic HBV infections 5,000-8,000 per year Deaths 5,000 per year TYZEKA® (telbivudine) is not indicated to treat acute hepatitis B. TYZEKA® (telbivudine) is not indicated to prevent mortality associated with hepatitis B. http://www.cdc.gov/ncidod/diseases/hepatitis/b/bfact.pdf. Accessed 1/23/07. 14 Transmission of HBV Infection Transfusion (blood, blood products) Fluids (blood, semen) Organs and tissue transplantation Mother to baby HEPATITIS B Contaminated needles and syringes Child to child 15 16 17 18 Signs & Symptoms: Chronic Hepatitis B Usually Asymptomatic Malaise/Fatigue Extrahepatic Symptoms Signs & Symptoms of Liver Failure Hepatocellular Carcinoma and/or Death Pathophysiologic Cascade of Chronic HBV Infection HBV Replication (Measured by Serum HBV DNA) 19 Liver Inflammation ALT Elevation Worsening Histology • Necroinflammation • Fibrosis • Cirrhosis Disease Progression • Liver Failure • Liver Cancer • Transplant • Death TYZEKA® (telbivudine) is not indicated to treat or prevent liver failure or liver cancer. Adapted from: Lavanchy D. Journal of Viral Hepatitis, 2004, 11, 97–107. Chen JC, et al. JAMA. 2006;295:65-73. Iloeje U. H, et al. Gastroenterology. 2006;130:678-86. S 20 21 22 S 23 S 24 US Treatment Algorithm: Patients With Compensated Disease HBeAg-Positive HBV DNA <20,000 IU/mL HBV DNA ≥20,000 IU/mL (~5 Log10) (~5 Log10) Normal ALT No treatment Monitor every 6-12 months Monitor every 3-12 months (immune tolerant) Consider biopsy, especially if age>35-40 years, and treat if significant disease Adapted from Keeffe E et al. Clin Gastroenterol Hepatol 2006;4:936-962. ALT elevated Treat 25 US Treatment Algorithm: Patients With Compensated Disease HBeAg-Negative HBV DNA < 2,000 IU/mL HBV DNA ≥2,000 IU/mL (~4 Log10) (~4 Log10) Normal ALT ALT elevated No treatment Monitor ALT, or Treat Monitor every 6-12 months Consider biopsy, since ALT often fluctuates, and treat if significant disease Long-term treatment required Long-term treatment required Adapted from Keeffe E et al. Clin Gastroenterol Hepatol 2006;4:936-962. 26 S 27 The Twin Pillars of HBV Therapy Viral suppression Avoidance of resistance S Treatment of Established Resistance: Choose One Drug From Each Class Nucleosides Nucleotides LAM ADV LdT TDF* ETV FTC*† *off label † available in combination tablet with TDF Guidance based on clinical data and author’s experience 28 S Chronology of Events in Patients Developing Resistance Emergence of genotypic resistance Virologic breakthrough Biochemical breakthrough Time on Therapy 29 S Possible Algorithm to Assist in Determining Which Strategy to Use Low HBV DNA* at Week 24 Low genetic barrier drug Add on or switch High HBV DNA† at Week 24 (any drug) Any detectable HBV DNA at Week 48 (any drug) ‡ Add on Add on ‡ High genetic barrier drug Continue *Low HBV DNA < 2000 IU/mL †High HBV DNA ≥ 2000 IU/mL ‡If viral level very low on ETV or TDF (off label) can continue with further observation Guidance based on clinical data and author’s experience Slide courtesy of Ira Jacobson, MD. 30 S Consequences of antiviral-resistance Loss of initial virologic, biochemical and histological response Virologic and biochemical breakthrough Hepatitis flares, hepatic decompensation and death Increased risk of HBV recurrence post-liver transplant Limit future treatment options Transmission to treatment-naïve persons → public health problem 31 S 32 S 33 S 34 Hepatitis C Virus Transmission Rate (%) High Risk Profile 40 30 20 10 0 ~5 2-3 Sexual Contact With Infected Individuals Organ Transplant Recipients Prior to July 1992 2 Health Care Workers, Including NeedleStick Injury Transmission Factor Other cohorts at increased risk include immigrants from areas where where universal precautions are not practiced, Vietnam veterans, and p atients with a history of incarceration— incarceration—likely due to the overlap in the highhigh-risk profile Alter et al. N Engl J Med. 1999;341:556-562. NIH Consensus Development Conference Statement. 2002. Ohto et al. N Engl J Med. 1994:330:744-750. S 35 S 36 S 37 S 38 S 39 S 40 S Extrahepatic Manifestations of Hepatitis C Hematologic: Mixed cryoglobulinemia (10%–25% of HCV patients)* Renal: Glomerulonephritis Dermatologic: Porphyria cutanea tarda Cutaneous necrotizing vasculitis Lichen planus Management of Hepatitis C. NIH Consensus Statement, 2002. 41 Factors Associated With Disease Progression • Alcohol consumption – 30 g/day in men – 20 g/day in women ~ 2 drinks per day • Disease acquisition at >40 years • Male gender • HIV coinfection • Hepatitis B virus coinfection • Immunosuppression NIH Consensus Development Conference Statement. 2002. Poynard et al. Lancet. 1997;349:825-832. S 42 Factors Not Influencing Progression • Alanine aminotransferase level (ALT) • Viral load • Mode of transmission • Genotype NIH Consensus Development Conference Statement. 2002. Poynard et al. Lancet. 1997;349:825-832. S 43 S 44 S 45 PEG IFN Alfa-2a + RBV SVR in Genotype 1 PEG IFN alfa-2a 180 μg + RBV SVR (%) 60 40 41 51 40 29 20 0 n=101 n=118 RBV RBV 800 mg 1000-1200 mg 24 Weeks n=250 n=271 RBV RBV 800 mg 1000-1200 mg 48 Weeks PEGASYS® (peginterferon alfa-2a) [package insert]. Nutley, NJ: Hoffmann-La Roche; 2002. (Study 5). Hadziyannis et al. EASL; April 18-21, 2002; Madrid, Spain. Abstract 536. S 46 S 47 S 48 S 49 Clinical Significance of Sustained Virologic Response • > 90% maintain response during 1 - 6 years of follow-up • Liver histology improves or stabilizes Lindsay et al. Hepatology. 1997;26(suppl 1):71S-77S. S 50 S Contraindications Ribavirin PEG IFN Alfa-2a • Hypersensitivity • Hypersensitivity • Autoimmune hepatitis • • Hepatic decompensation (Child-Pugh class B and C) before or during treatment Women who are pregnant • Men whose female partners are pregnant • Patients with hemoglobinopathies (eg, thalassemia major or sickle-cell anemia) • Neonates and infants *Ribavirin must not be used alone because ribavirin monotherapy is not effective for the treatment of chronic hepatitis C. PEGASYS® (peginterferon alfa-2a) [package insert]. Nutley, NJ: Hoffmann-La Roche; 2002. 51 S PEG IFN Alfa-2a + RBV Warnings • Neuropsychiatric • Pulmonary disorders • Infections • Colitis • Bone marrow toxicity • Pancreatitis • Cardiovascular • Ophthalmologic • Hypersensitivity • Pregnancy • Endocrine disorders • Anemia • Autoimmune disorders • Renal disorders disorders COPEGUS™ (ribavirin, USP) [package insert]. Nutley, NJ: Hoffmann-La Roche; 2002. PEGASYS® (peginterferon alfa-2a) [package insert]. Nutley, NJ: Hoffmann-La Roche; 2002. 52 S 53 Side Effects of Interferon • Flu-like symptoms – Headache – Fatigue or asthenia – Myalgia, arthralgia – Fever, chills • Neuropsychiatric disorders – Depression – Mood lability Alopecia Thyroiditis Nausea • Diarrhea • Injection-site reaction • Lab alterations • • • – Neutropenia – Anemia – Thrombocytopenia PEGASYS® (peginterferon alfa-2a) [package insert]. Nutley, NJ: Hoffmann-La Roche; 2002. PEG-Intron™ (peginterferon alfa-2b) [package insert]. Kenilworth, NJ: Schering Corporation; 2001. S 54 Side Effects of Ribavirin • Hemolytic anemia • Teratogenicity • Cough and dyspnea • Rash and pruritus • Insomnia • Anorexia COPEGUS™ (ribavirin, USP) [package insert]. Nutley, NJ: Hoffmann-La Roche; 2002. S 55 S 56 S 57 S 58 S 59 ACUTE HEPATITIS Viral-A,B,C,D,E and nonA-E Alcohol induced abnormalities Autoimmune hepatitis Viral-CMV,Herpes,Rubella,EBV Drug-induced S 60 Wilson’s Ds Reye’s Syndrome Acute fatty liver of pregnancy Hypoperfusion States Hepatic Vein occlusion(Budd-Chiari) Malignancy(esp lymphoproliferative) S 61 CLINICAL PRESENTATION OF CHRONIC HEPATITIS Failure to resolve acute hepatitis(10%) Abnormal LFT’S(80%) Stigmata of complications of chronic liver disease(10%) S 62 CLINICAL SPECTRUM Abnormal transaminases may fluctuate and even return to normal May be asymptomatic to markedly symptomatic Non-specific-fatigue, malaise,or anorexia Portal hypertension and/or hepatic failurejaundice,ascites,coagulopathy, GI bleed S 63 DIFFERENTIAL DIAGNOSIS OF CHRONIC HEPATITIS Autoimmune(10%) Viral-Hepatitis B (25%) -Delta Hepatitis(10%) -Hepatitis C(50%) Drugs(5%) Wilson’s Disease S 64 SIMILAR PRESENTATION TO CHRONIC HEPATITIS Alpha 1 antitrypsin deficiency Hemochromatosis Primary biliary cirrhosis Primary Sclerosing cholangitis Alcoholic liver disease NAFLD/NASH