* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download system
Survey
Document related concepts
Thermoregulation wikipedia , lookup
Building insulation materials wikipedia , lookup
Intercooler wikipedia , lookup
Solar water heating wikipedia , lookup
Heat exchanger wikipedia , lookup
Solar air conditioning wikipedia , lookup
Copper in heat exchangers wikipedia , lookup
R-value (insulation) wikipedia , lookup
Heat equation wikipedia , lookup
Cogeneration wikipedia , lookup
Transcript
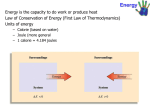
Chemistry: Atoms First Second Edition Julia Burdge & Jason Overby Chapter 10 Energy Changes in Chemical Reactions M. Stacey Thomson Pasco-Hernando State College Copyright (c) The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 10.1 Energy and Energy Changes The system is a part of the universe that is of specific interest. The surroundings constitute the rest of the universe outside the system. System Surroundings Universe = System + Surroundings The system is usually defined as the substances involved in chemical and physical changes. Energy and Energy Changes Thermochemistry is the study of heat (the transfer of thermal energy) in chemical reactions. Heat is the transfer of thermal energy. Heat is either absorbed or released during a process. heat The SI energy unit is a Joule, J. Often calories are used and 1 calorie is the amount of heat required to raiseSurroundings 1 g of water by 1°C. 1 cal = 4.184 J calorie is not the same as a nutritional calorie (Cal) 1 Cal=1000 cal Energy and Energy Changes An exothermic process occurs when heat is transferred from the system to the surroundings. “Feels hot!” 2H2(g) + O2(g) System 2H2O(l) + energy heat Surroundings Universe = System + Surroundings Energy and Energy Changes An endothermic process occurs when heat is transferred from the surroundings to the system. “Feels cold” energy + 2HgO(s) System 2Hg(l) + O2(g) heat Surroundings Universe = System + Surroundings • http://highered.mcgrawhill.com/sites/0073511161/student_view0/chapter10/animations.html# 10.2 Introduction to Thermodynamics Thermodynamics is the study of the interconversion of heat and other kinds of energy. In thermodynamics, there are three types of systems: An open system can exchange mass and energy with the surroundings. A closed system allows the transfer of energy but not mass. An isolated system does not exchange either mass or energy with its surroundings. The First Law of Thermodynamics The first law of thermodynamics states that energy can be converted from one form to another, but cannot be created or destroyed. ΔUsys + ΔUsurr = 0 ΔU is the change in the internal energy. “sys” and “surr” denote system and surroundings, respectively. ΔU = Uf – Ui; the difference in the energies of the initial and final states. ΔUsys = –ΔUsurr Work and Heat The overall change in the system’s internal energy is given by: ΔU = q + w q is heat q is positive for an endothermic process (heat absorbed by the system) q is negative for an exothermic process (heat released by the system) w is work w is positive for work done on the system w is negative for work done by the system Work and Heat ΔU = q + w Worked Example 10.1 Calculate the overall change in internal energy, ΔU, (in joules) for a system that absorbs 188 J of heat and does 141 J of work on its surroundings. Strategy Combine the two contributions to internal energy using ΔU = q + w and the sign conventions for q and w. Text Practice: 10.2 10.3 10.7 10.10 10.12 10.3 Enthalpy The thermodynamic function of a system called enthalpy (H) is defined by the equation: H = U + PV A note about SI units: Pressure: pascal; 1Pa = 1 kg/(m . s2) Volume: cubic meters; m3 PV: 1kg/(m . s2) x m3 = 1(kg . m2)/s2 = 1 J Enthalpy: joules U, P, V, and H are all state functions. Enthalpy and Enthalpy Changes The enthalpy of reaction (ΔH) is the difference between the enthalpies of the products and the enthalpies of the reactants: ΔH = H(products) – H(reactants) Assumes reactions in the lab occur at constant pressure ΔH > 0 (positive) endothermic process ΔH < 0 (negative) exothermic process Thermochemical Equations H2O(s) H2O(l) ΔH = +6.01 kJ/mol Concepts to consider: What is the system? What are the surroundings? ΔH > 0 endothermic Thermochemical Equations CH4(g) + 2O2(g) CO2(g) + 2H2O(l) ΔH = −890.4 kJ/mol Concepts to consider: What is the system? What are the surroundings? ΔH < 0 exothermic Thermochemical Equations Enthalpy is an extensive property. Extensive properties are dependent on the amount of matter involved. H2O(l) → H2O(g) Double the amount of matter 2H2O(l) → 2H2O(g) ΔH = +44 kJ/mol Double the enthalpy ΔH = +88 kJ/mol Units refer to mole of reaction as written Thermochemical Equations The following guidelines are useful when considering thermochemical equations: 1) Always specify the physical states of reactants and products because they help determine the actual enthapy changes. CH4(g) + 2O2(g) CO2(g) + 2H2O(g) different states CH4(g) + 2O2(g) CO2(g) + 2H2O(l) ΔH = −802.4 kJ/mol different enthalpies ΔH = +890.4 kJ/mol Thermochemical Equations The following guidelines are useful when considering thermochemical equations: 2) When multiplying an equation by a factor (n), multiply the ΔH value by same factor. CH4(g) + 2O2(g) CO2(g) + 2H2O(g) ΔH = − 802.4 kJ/mol 2CH4(g) + 4O2(g) 2CO2(g) + 4H2O(g) ΔH = − 1604.8 kJ/mol 3) Reversing an equation changes the sign but not the magnitude of ΔH. CH4(g) + 2O2(g) CO2(g) + 2H2O(g) ΔH = − 802.4 kJ/mol CO2(g) + 2H2O(g) CH4(g) + 2O2(g) ΔH = +802.4 kJ/mol Worked Example 10.3 Given the thermochemical equation for photosynthesis, 6H2O(l) + 6CO2(g) → C6H12O6(s) + 6O2(g) ΔH = +2803 kJ/mol calculate the solar energy required to produce 75.0 g of C6H12O6. 10.4 Calorimetry Calorimetry is the measurement of heat changes. Heat changes are measured in a device called a calorimeter. The specific heat (s) of a substance is the amount of heat required to raise the temperature of 1 g of the substance by 1°C. Specific Heat and Heat Capacity The heat capacity (C) is the amount of heat required to raise the temperature of an object by 1°C. The “object” may be a given quantity of a particular substance. heat capacity of 1 kg of water = 4.184 J 1000 g = 4184 J/C 1 g C Specific heat capacity of water Specific heat capacity has units of J/(g • °C) Heat capacity has units of J/°C heat capacity of 1 kg water Specific Heat and Heat Capacity The heat associated with a temperature change may be calculated: q = msΔT q = CΔT m is the mass. s is the specific heat. ΔT is the change in temperature (ΔT = Tfinal – Tinitial). C is the heat capacity. Worked Example 10.4 Calculate the amount of heat (in kJ) required to heat 255 g of water from 25.2°C to 90.5°C. Calorimetry A coffee-cup calorimeter may be used to measure the heat exchange for a variety of reactions at constant pressure: Calorimetry Concepts to consider for coffee-cup calorimetry: qP = ΔH System: reactants and products (the reaction) Surroundings: water in the calorimeter For an exothermic reaction: the system loses heat the surroundings gain (absorb) heat qsys = −msΔT qsurr = msΔT qsys = −qsurr The minus sign is used to keep sign conventions consistent. Worked Example 10.5 A metal pellet with a mass of 100.0 g, originally at 88.4°C, is dropped into 125 g of water originally at 25.1°C. The final temperature of both pellet and the water is 31.3°C. Calculate the heat capacity C (in J/°C) of the pellet. • http://highered.mcgrawhill.com/sites/0073511161/student_view0/chapter10/animations.html# Constant-Volume Calorimetry Constant volume calorimetry is carried out in a device known as a constant-volume bomb. A constant-volume calorimeter is an isolated system. Bomb calorimeters are typically used to determine heats of combustion. qcal = −qrxn Constant-Volume Calorimetry To calculate qcal, the heat capacity of the calorimeter must be known. qcal = CcalΔT qrxn = −qcal qrxn = −CcalΔT Worked Example 10.6 A chocolate chip cookie weighing 7.25 g is burned in a bomb calorimeter to determine its energy content. The heat capacity of the calorimeter is 39.97 kJ/°C. During the combustion, the temperature of the water in the calorimeter increases by 3.90°C. Calculate the energy content (in kJ/g) of the cookie. Study Guide for Sections 10.1-10.4 DAY 26, Terms to know: Sections 10.1-10.4 system, surroundings, thermochemistry, thermal energy, exothermic, endothermic, open system, closed system, isolated system, first law of thermodynamics (law of conservation of energy), enthalpy, calorimetry, specific heat, heat capacity, heat of reaction, coffee cup calorimeter, bomb calorimeter DAY 26, Specific outcomes and skills that may be tested on exam 4: Sections 10.1-10.4 •Be able to describe potential energy changes for the system, kinetic energy and temperature changes for the surroundings, and bond stability changes for the system in any endothermic or exothermic system. •Given either change in energy for the system or surroundings, be able to calculate the other •Given two out of three of any of the following quantities, be able to calculate the third: change in heat for the system, change in work for the system, overall change in energy for the system •Given initial heat and final heat, be able to calculate the change in enthalpy for a system •Be able to determine whether a process is endo or exothermic based on the mathematical sign for the change in enthalpy •Be able to calculate how the enthalpy change will be different if a chemical equation is reversed or multiplied by a certain stoichiometric coefficient •Given two of the following quantities, be able to calculate the third: temperature change, heat change, heat capacity •Given three of the following quantities, be able to calculate the fourth: temperature change, heat change, mass, specific heat •Be able to describe how to set up a coffee cup or bomb calorimeter to calculate changes in energy based on measured changes in temperature and energy exchanges between system and surroundings Extra Practice Problems for Sections 10.1-10.4 Complete these problems outside of class until you are confident you have learned the SKILLS in this section outlined on the study guide and we will review some of them next class period. 10.5 10.7 10.11 10.13 10.23 10.27 VC10.3 VC10.8 10.31 10.35 10.37 10.41 10.97 10.115 10.121 Prep for Day 27 Must Watch videos: https://www.youtube.com/watch?v=8m_FCe5aCqY (energy calculations, Tyler DeWitt) https://www.youtube.com/watch?v=SV7U4yAXL5I (calculations with energy, crash course chemistry) http://echem1a.cchem.berkeley.edu/#modules (Energy, UC-Berkeley, lessons 20.2-20.5 and 21.3-21.6) http://www.youtube.com/watch?v=0mLFSDId6pQ (Bond enthalpy, TheChemistrySolution) https://www.youtube.com/watch?v=9i26eK6xyNI (colligative properties, Isaacs) https://www.youtube.com/watch?v=Y-wtTUPPeww (colligative properties II, Isaacs) Other helpful videos: http://ocw.mit.edu/courses/chemistry/5-111-principles-of-chemical-science-fall-2008/videolectures/lecture-16/ (MIT, start at 29 minute mark) http://ps.uci.edu/content/general-chemistry-1b (UV-Irvine, lecture 6 and the first 30 minutes of lecture 7) https://www.youtube.com/watch?v=7ugNp7-OTC8 (Bond enthalpy, Annette Tomory) https://www.youtube.com/watch?v=CRIdLFod8uU (UC-Irvine) Read Sections 10.5-10.8, 13.5-13.6