* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download TB Review (Click Here)
Survey
Document related concepts
Psychedelic therapy wikipedia , lookup
Adherence (medicine) wikipedia , lookup
Orphan drug wikipedia , lookup
Discovery and development of non-nucleoside reverse-transcriptase inhibitors wikipedia , lookup
Drug discovery wikipedia , lookup
Polysubstance dependence wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Psychopharmacology wikipedia , lookup
Pharmacognosy wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Neuropharmacology wikipedia , lookup
Theralizumab wikipedia , lookup
Prescription drug prices in the United States wikipedia , lookup
Drug interaction wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Transcript
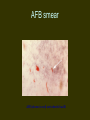
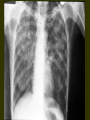
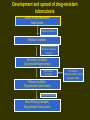
INTERNATIONAL SCENARIO • ONE-THIRD OF THE WORLD POP I.E. 1.9B IS INFECTED WITH M.TUBERCULOSIS • GLOBAL PRAVELANCE 1S 16-20M • 9M NEW CASES ADDED EVERY YEAR (INC. 136/100000) • 1.82M DEATHS FROM TB • 12% OF HIV DEATHS ATTRIBUTABLE TO TB • 95% OF NEW CASES AND DEATHS OCCUR IN DEVELOPING COUNTRIES 22 HIGHBURDEN DISEASE COUNTRIES • • • • • • • INDIA CHINA INDONESIA NIGERIA BANGLADESH ETHIOPIA PHILIPPINES • PAKISTAN • SOUTH AFRICA • CONGO • RUSSIAN FEDERATION • • • • • • • • • • • KENYA VIET NAM UR TANZANIA BRAZIL UGANDA ZIMBAVAA MOZAMBIQUE THAILAND AFGHANISTAN CAMBODIA MYANMAR Estimated TB incidence rate, 2005 Estimated new TB cases (all forms) per 100 000 population No estimate 0–24 25–49 50–99 100–299 300 or more The boundaries and names shown and the designations used on this map do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. WHO 2006. All rights reserved STATUS OF TB IN PAKISTAN • RANK: 8TH AMONGST 22 HBD COUNTRIES • PAKISTAN CONTRIBUTES 43% OF THE TUBERCULOSIS BURDEN IN THE EMRO REGION • PREVALANCE OF TB 1.5 M • INCIDENCE: 181/100000 POPULATION 250000 NEW CASES EVERY YEAR • 3 OUT OF 4 PATIENTS ARE ADULTS (15-59) ECONOMICALLY PRODUCTIVE AGE GROUP STATUS OF TB IN PUNJAB • Punjab Accounts for > 50 % of disease burden in country I.E.1.5 million cases. • Fifty percent of patients are females. • 60000 new smear positive cases every year. Tuberculosis - 2 main types 1. Mycobacterium tuberculosis - most common infection in humans. 2. Mycobacterium bovis (animal form) is responsible for an increasing proportion of human TB cases. 3. More recently, M. tuberculosis has been documented in a free-ranging animal, the banded mongoose. Banded Mongoose Possible Implications Expansion of ecotourism, excalating human populations, and changes in land-use practices have increased the possible disease threat humans pose to wildlife. AGRICULTURAL HEALTH IS NOT PREPARED TO FACE RE-EMERGING ZOONOSIS In 22/34 countries of Latin America and the Caribbean Tuberculosis Milk Aerosols Milk Aerosols Food Water Source: Zoonotic Tuberculosis in Developing Countries. EID - CDC. TRADE AND TOURISM CHALLENGE AGRICULTURAL AND PUBLIC HEALTH West Nile Virus Source: Promed 4,324 bird cases 57 equine cases New York, 1999 19 human cases 4 dead AFB smear AFB (shown in red) are tubercle bacilli Reporting on AFB Microscopy Number of bacilli seen Result reported None per 100 oil immersion fields Negative 1-9 per 100 oil immersion fields Scanty, report exact number 10-99 per 100 oil immersion fields 1+ 1-10 per oil immersion field 2+ > 10 per oil immersion field 3+ Diagnosis of Pulmonary TB Cough 3 weeks If 1 positive, X-ray and evaluation AFB X 3 If 2/3 positive: Anti-TB Rx If negative: Broad-spectrum antibiotic 10-14 days If symptoms persist, repeat AFB smears, X-ray If consistent with TB Anti-TB Treatment Chemotherapy Era Streptomycin (s) – 1940 INH (H) – 1952 PAS - 1949 Standard chemotherapy was effective but unpleasant. Initially H.S.PAS 3 months, continuation phase H + PAS – 15 months. Current Standard Chemotherapy (WHO/IUATLD RECOMMENDATIONS) Rich countries – Initial phase 2 HRZE/S – Continuation phase 4 HR Poor countries – Initial phase 2 HRZE – Continuation phase 6 H.T./H.E. Tuberculosis is a disease of great antiquity Evidence in Egyptian and precolumbian mummies. Before the availability of drugs, diagnosis of T.B. – a life time sentence. Bed Rest, good diet, sanatoria on hillsides – only available treatment. Collapse Therapy Artificial Pneumothorax Pneumoperitoneum Phrenic Crush Thoracoplasty Plombage Tuberculosis & Diabetes Mellitus BOTH T.B. & DIABETES MELLITUS COMMON CLINICAL PROBLEM IN DEVELOPING COUNTRIES LIKE PAKISTAN. DIABETICS HAVE A HIGHER RATE OF T.B. BY A FACTOR OF THREE. REASONS FOR HIGH INCIDENCE OF T.B. IN DIABETICS NOT CERTAIN MAY BE DUE TO: POOR GLYCEMIC CONTROL DUE TO DEFECT IN T. CELL ALVEOLAR MACROPHAGES ACTIVATION IN DIABETICS Anti Tuberculosis Treatment and Diabetic Control 1. 2. 3. Rifampicin - causes hyperglycemia due to:* increased metabolism of oral hypoglycemic agents - as a liver microsomal enzyme inducer. * Initial hyperglycemia - unknown mechanism. Patients needs high dose of oral hypoglycemic agents High incidence of peripheral neuropathy with INH, Ethambutol and Ethionamide. So pyridoxine must be advised with ATT. Ethionamide causes hypoglycemia so critical control of blood glucose level. Relapse of Tuberculosis Relapse should be less than 1% Resistance studies should be obtained. If previous treatment adequate: 1/3 rd patients have drug resistance. If previous treatment inadequate: Resistance in 2/3 rd of patients, initial therapy should be for presumed drug resistance. Liver Impairment Drug induced hepatotoxicity 3-5% in the population.In our population it is around 9% (Haq M.U, Rasul S*, Iqbal Z.H. Ch. MK, Bhatti A.H, Anwar N, Nasir Incidence of Hepatitis in patients taking Antituberculosis treatment. Annals KEMC, June- Dec. 1996;49 – 51) Drug induced Hepatitis – stop the regimen . Start- Ethambutol, Streptomycin and ofloxacin. Pregnancy Primary TB drugs are safe- no evidence of teratogenecity. Rifampicin, INH, Ethambutol, PZA – all safe. Streptomycin: may produce ototoxicity of fetus not recommended. Preferable :-Female married patients avoid pregnancy during treatment course. Pregnancy Rifampicin baby: Rifampicin decreases the efficacy of oral contraceptives. Pregnancy may occur while taking contraceptive pill. Hence dose of pill should be doubled or alternative methods used. If pregnancy occurs – then treat with ATT. First time diagnosis of TB during pregnancy give ATT. Dot not advise termination of pregnancy. HIV Infection The normal regimen is as effective as in HIV negative patients. Adverse reactions to thiacetazone are common. Higher relapse rates are found, so that treatment may be prolonged. Silicosis • • • • • • • More prone to active pulmonary tuberculosis Difficult to treat i) Function of alveolar macrophages is impaired. ii) Massive fibrosis may prevent penetration of drugs to the site. Forty percent of silicosis patients had active TB in Hong Kong. More relapse. Prolonged treatment required. MULTI DRUG RESISTANCE (MDR) Defined as resistance to both isoniazed and rifampicin with or without the presence of resistance to another drug. Factors contributing to MDR TB Non compliant patient – Multifactorial (Interruption, Selection of Drugs, Premature cessation of treatment) Inadequate regimen. Prolonged treatment. Exposure to an MDR TB patient (Lack of facilities to isolate the patient) Factors contributing to MDR TB (Contd.) Asian origin. Homelessness. Drug abuse. HIV. Adverse reaction to anti T.B. drugs. Development and spread of drug-resistant tuberculosis Colony of mycobacterium tuberculosis Natural mutations Resistant mutants Selection of resistant strains by inadequate treatment Secondary (multiple) Drug-resistant tuberculosis Transmission in droplets Primary (multiple) Drug-resistant tuberculosis Further transmission More Primary (multiple) Drug-resistant tuberculosis HIV Infection Inadequate infection control Diagnostic delay Drug Resistance Status in Pakistan In 1967: 87% of isolates from treated patients resistant to one or more drugs. In 1989: 31.6% in treated patients Resistance to H: Primary Secondary Resistance Resistance 1976-80 24.5% 57% 1981-82 25.8% 52.6% 1993 53% 1995 29% 53% Recommendations:- Initial phase 3 HRZE (S) Continuation phase 6 HRE Primary and secondary resistance to individual drugs Drug Case with Resistance Primary Resistance Secondary Resistance Unknown N % N % N % N % H 65 43.6 13 28.9 45 52.9 7 36. 8 <0.001 R 8 5.4 2 4.4 5 5.9 1 5.2 NS Z 6 4.0 2 4.4 4 4.7 - - NS E 6 4.0 2. 4.4. 4 4.7 - - NS S 25 16.7 1 2.2 23 27.0 1 5.2 <0.001 PAS 19 12.75 4 8.9 14 16.5 1 5.2 <.05 TH 5 3.3 1 2.2 3 3.5 1 5.2 NS ETH 8 5.3 2 4.4 5 5.9 1 5.2 NS NS = NOT SIGNIFICANT, N = NUMBER Biomedica Vo. 11 (Jul, Dec 1995) 54-57. P-Value Drug resistance in North West Frontier Province, Pakistan, 1994 Primary resistance (%) Acquired resistance (%) Streptomycin 10 46 Isoniazid 1 57 Rifampicin 3 50 Pyrazinamide 3 57 Ethambutol 11 50 Thiacetazone 2 30 Drug Percentage resistant to one drug 8.8 Percentage resistant to two to four drug 12.7 Percentage resistant to five drugs 20.0 Percentage resistant to six drugs 8.8 Source, Tuberculosis in Pakistan A. Javaid and M. Amjad in clinical tuberculosis ed.. P.D.O.Davies. 2nd ed. 1998 Resistance pattern of 228 culture positive cases to various antituberculosis drugs. DRUG All Patients N=228 Percent resistant Isoniazid + 15.78% (36) Primary Cases N=123 Acquired Cases N=105 P Value 7.31% (9) 25.71% (27) <. 001 Rifampicin (MDR) Isoniazid 25.43% (58) 21.13% (26) 30.47% (32) .10 Rifampicin 25.00% (57) 15.44% (19) 36.19% (38) <.001 Streptomycin 24.12% (55) 16.26% (20) 33.30% (35) <.01 Pyrazinamide 21.49% (49) 11.38% (14) 36.19% (38) <.001 Ethambutol 10.00% (23) 04.87% (06) 16.19% (17) <.01 Shamshad Rasul, Iffat Shabbir, Rizwan Iqbal et al: Trends in multidrug resistant tuberculosis, Pakistan Journal of Chest Medicine, Volume 7, No. 3, 2001, 1-28 Primary Drug Resistance At JPMC Karachi • • • • • INH Rifampicin Ethambutol Streptomycin MDR 16% 07% 02% 03% 01% • Rano Mal,Nadeem Rizvi,Shahina Qayyum SAARC JTB,L DIS&HIV/AIDS.2004-1(1)20-23 Pattern of drugs resistance among mycobacterium tuberculosis isolates (1998 to 2002) Year No of patients (MDR) H+R Isoniazid Rifamp icin Pyrazin amide Strepto mycin Ethamb utol 1998 204 17.08% (41) 22.91% (55) 22.5% (54) 29.16% (70) 17.5% (42) 10.41% (25) 1999 228 15.78% (36) 25.43% (58) 25% (57) 21.49% (49) 24.12% (55) 10.08% (23) 2000 238 15.96% (38) 26% (62) 28.15 % (67) 26.89% (64) 25.21% (60) 15.54% (37) 2001 212 16.50% (35) 27.35% (58) 30.18 % (64) 31.13% (66) 26.88% (57) 16.50% (35) 2002 228 15.35% (35) 25% (57) 27.63 % (63) 29.38% (67) 22.36% (51) 14.47% (33) Rizwan Iqbal, Iffat Shabbir et al: TB drug resistance alarming challenge – answer DOTS., Pakistan J. Med. Res. Vol. 42 No.3, 2003, 134-138. Gulab Devi Chest Hospital, Lahore From 01 July 2004 to 31th June, 2005. • Isolates of Mycobacterium TB • Resistant to Rif & INH(MDR) 116 27 (23.27 %) Resistant Pattern of individual drugs • • • • • • Resistant to Rifampicin Resistant to Isoniazid Resistant to Streptomycin Resistant to Ethambutol Resistant to Thiacetazone Resistant to Pyrazinamide 38.79% 42.42% 37.06% 18.96% 21.55% 58.62% Management of MDR - T.B. 1. 2. Detailed evaluation regarding history, clinical examination and previous treatment Culture and sensitivity pattern. Principles of MDR TB management At least 4 drugs to be given never used before. An injectable should be used ___ one of the aminoglycosides not used earlier. Never add a single drug to a failing regimen ____ a minimum of 2 drugs be added. DOTS Plus Must Duration of therapy 18 – 24 months. Second Line Antituberculosis drugs Drugs Daily Dose Adverse Effects Ethionamide 500-1000 mg P.O. (In divided doses if necessary) Gastrointestinal intolerance hepatitis, endocrine disturbances, hypersensitivity Cycloserine 250 – 750 mg P.O. (In divided doses adjust for renal impairment) Neurological and Psychiatric Disturbances Capreomycin 15 mg / kg i.m. Hearing loss, Vestibular Amikacin Kanamycin 5 days a week (adjust for renal impairment) Renal toxicity Electrolyte disturbances Second Line Antituberculosis drugs (Contd.) Drugs Daily Dose Adverse Effects Para – AminoSalicylic Acid 10-20 g P.O. (In divided doses) Ciprofloxacin Ofloxacin Levo Floxacin 500 – 1000 mg P.O. Gastrointestinal 400 – 800 mg p.o. intolerance headache, Restlessness, 500 mg P.O. Hypersensitivity, Drug interactions Clofazimine 100 – 300 mg q.d.s. Abdominal pain, Skin P.O. Discoloration (both dose related) photosensitivity Gastrointestinal intolerance hepatitis, Hypersensitivity Recommended Regimens for the Treatment of Tuberculosis in problem cases Initial Phase Indication Duration, Months Drugs Continuation Phase Duration Months Failure and relapse* Standard 3 HRZES** retreatment (susceptibility testing unavailable) Resistance to H + R Throughout (12-18) ZE + O + S (or another injectable agent) Resistance to all first Throughout (24) 1 injectable agent*** + 3 of these 4: ethionamide, cycloserine, PAS, O Drugs - - 5 HRE - - * Regimen is tailored according to the results of drug susceptibility results. ** Streptomycin should be discontinued after 2 months *** Amikacin, Kanamycin or Capreomycin. Treatment with all of these agents should be discontinued after 2-6 months depending on patient’s response and tolerance. Regimen for the Treatment of MDR tuberculosis Resistance to Initial phase Drugs Continuation phase Minimum duration in months Drugs Duration in months Isoniazid 1. Aminoglycoside 3 1. ethionamide 18 rifampicin and 2. Pyrazinamide 3 2. Ofloxacin; 18 streptomycin 3. Ethionamide 3 3. ethambutol; 18 4. Ofloxacin 3 5. Ethambutol 3 Isoniazid 1. aminoglycoside 3 1. ethionamide 18 rifampicin, 2. ethionamide 3 2. Ofloxacin; 18 streptomycin and 3. pyrazinamide 3 3. Cycloserine; 18 ethambutol 4. ofloxacin 3 5. cycloserine 3 Prevention for MDR TB Proper management – DOTS. Proper regimens. Adequate dosage (Fixed dose combination – A partial solution) Treatment should consider patient’s needs, constraints, preferences and confidentiality. Prevention for MDR TB (Contd.) Early case detection of primary MDR cases. Education of medical and paramedical professionals in all aspects to be maintained or reemphasized. Free treatment and other incentives for the patients. Renal Impairment •Rifampicin, INH, PZA, Eithionamide and Prothionamide eliminated almost entirely by normal routes – Hepatic metabolism or billiary excretion. •In severe renal failure – INH dose be reduced to 200 mg daily with pyridoxine supplementation. • No adjustment required, if patient on hemodialysis. Renal Impairment Streptomycin and other amino glycosides – need dose adjustment. Streptomycin injections should be spaced, so that trough levels of the drugs does not exceed 4mg/L. In patients on dialysis, streptomycin should be given 6-8 hours prior to dialysis. Ethambutol excreted predominantly by kidney. The dose needs to be adjusted (decreased). Renal Impairment Ethambutol If renal clearance 50-100 ml/min, 25mg/kg three times a week. If renal clearance 30-50 ml/min, above dose twice a week. If renal clearance <10-25 ml/min, a dose of 15 mg / Kg at 36 to 48 hours interval. Patients on thrice weekly hemodialysis, 25 mg / Kg 4 to 6 hours before the procedure. Renal Impairment Thiacetazone, PAS Partly excreted through kidney unchanged partly metabolized through liver. Therapeutic index for thiacetazone is low, generally not recommended. PRE DOTS SCENARIO Low Priority by Policy makers Reliance on specialized units not accessible to all Inappropriate diagnostic procedures and over reliance on x-ray Lack of recording ,reporting and Evaluating system Use of non standardized drug regimen Non existent supervision TB Control: The 5 components of DOTS Political commitment Diagnosis by microscopy Adequate supply of the right drugs Directly observed treatment Accountability TB Register