* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download b - Gordon State College
Spinodal decomposition wikipedia , lookup
Self-assembled monolayer wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Transition state theory wikipedia , lookup
Kinetic resolution wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Rate equation wikipedia , lookup
Click chemistry wikipedia , lookup
Computational chemistry wikipedia , lookup
Chemical reaction wikipedia , lookup
Electrochemistry wikipedia , lookup
Debye–Hückel equation wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Process chemistry wikipedia , lookup
Extended periodic table wikipedia , lookup
Stability constants of complexes wikipedia , lookup
Crystallization wikipedia , lookup
Nanofluidic circuitry wikipedia , lookup
Electrolysis of water wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Acid–base reaction wikipedia , lookup
Acid dissociation constant wikipedia , lookup
Gas chromatography–mass spectrometry wikipedia , lookup
Liquid–liquid extraction wikipedia , lookup
Implicit solvation wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
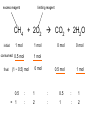
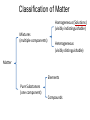
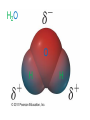
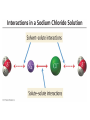
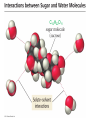
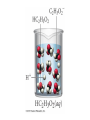
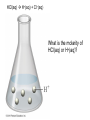
Ch 4. Chemical Quantities and Aqueous Reactions CH4 (g) + 2O2 (g) CO2 (g) + 2H2O (g) 1 mol 2 mol 1 mol 2 mol Stoichiometry of the reaction FIXED ratio for each reaction Can calculate how much other chemicals are required or produced if the amount of one chemical is known. CH4 (g) + 2O2 (g) CO2 (g) + 2H2O (g) 1 mol 2 mol 1 mol 2 mol 2 mol 4 mol 2 mol 4 mol 3 mol 6 mol 3 mol 6 mol 5.22 mol 10.44 mol 5.22 mol 10.44 mol 4 NH3 (g) + 5 O2 (g) 4 NO (g) + 6 H2O (g) 5.02 mol of NH3 is used in the above reaction. How many moles of O2 is required to react with all the NH3? How many moles of H2O will be produced? 2NH3(g) + 3CuO(s) N2(g) + 3Cu(s) + 3H2O(g) 2 mol 5.02 mol 3 mol 1 mol 3 mol 3 mol x y z u 2 mol 5.02 mol 2 3 mol x 3 x 7.53 mol 2 5.02 mol 1 y y 2.51 mol 2 5.02 mol 3 z z 7.53 mol 2 5.02 mol 3 u u 7.53 mol 2NH3(g) + 3CuO(s) N2(g) + 3Cu(s) + 3H2O(g) 2 mol 3 mol 1 mol 6.04 g mass? mass? 3 mol 3 mol 6.04 g ÷ (14.01 g/mol x 1 + 1.008 g/mol x 3) = 0.355 mol 0.355 mol 2 0.355 mol 3 x x y x 0.533 mol Mass of CuO = 0.533 mol x 79.55 g/mol = 42.4 g 2 0.355 mol 1 y y 0.178 mol Mass of N2 = 0.178 mol x 28.02 g/mol = 4.99 g Other Examples: page 131 ― 132 CH4 + 2O2 CO2 + 2H2O initial: 1 mol 2 mol 0 mol 0 mol final: 0 mol 0 mol 1 mol 2 mol The actual amount of reactants consumed and actual amount of products generated agree with the stoichiometry. initial: 1 mol 1 mol 0 mol 0 mol final: ? mol ? mol ? mol ? mol CH4 + 2O2 CO2 + 2H2O initial: consumed: final: 1 mol 1 mol x = 0.5 mol 1 mol (1 − 0.5) mol 0 mol 1 x 2 1 mol 0 mol 0 mol y = 0.5 mol z = 1 mol x = 0.5 mol 1 mol CH4 requires 2 mol O2, available O2 is 1 mol: limiting reagent. Result: 1 mol O2 will be consumed completely and CH4 will have leftover: excess reagent. The reactant of which there are fewer moles than the stoichiometry requires is the limiting reagent. The reactant of which there are more moles than the stoichiometry requires is the excess reagent. Chemical reactions always occur according to the stoichiometry, therefore the limiting reagent is consumed and the excess reagent has leftover. The amount of products is determined by the amounts of reagents that are actually consumed. excess reagent limiting reagent CH4 + 2O2 CO2 + 2H2O initial: 1 mol 1 mol consumed: 0.5 mol final: (1 − 0.5) mol 0.5 = 1 0 mol 0 mol 0.5 mol 1 mol 1 mol 0 mol : 1 : 0.5 : 1 : 2 : 1 : 2 + + + + + + + + 2 slices of bread + 1 slice if ham 1 sandwich 4 slices of bread + 1 slice if ham 1 sandwich + 2 slices of bread excess reagent limiting reagent amount of product excess reagent leftover For the following reaction, if a sample containing 18.1 g of NH3 is reacted with 90.4 g of CuO, which is the limiting reagent? How many grams of N2 will be formed? 2NH3(g) + 3CuO(s) N2(g) + 3Cu(s) + 3H2O(g) How many grams of excess reagent will be leftover? If 6.63 g of N2 is actually produced, what is the percent yield? actual yield percent yi eld 100 % theoretica l yield Procedure for limiting/excess reagent calculations aA + bB cC + dD 1) Make sure the equation is balanced. 2) Find the moles of each reactant: moles = mass in gram / molar mass 3) Pick up any reactant, say A, and use the stoichiometry to calculate the required amount of the other reactant B. 4) Compare the required amount of B with the available amount of B. a) If required > available, then B is the limiting reagent and A is the excess reagent. b) If required < available, then B is the excess reagent and A is the limiting reagent. 5) Use the amount of the limiting reagent and the stoichiometry to calculate the amount of any product and the amount of the excess reagent that has been consumed. 6) Leftover excess reagent = available − consumed 7) If actual yield is given percent yield = (actually yield / theoretical yield) x 100% 68.5 g CO reacts with 8.60 g H2 in the following reaction. What is the limiting reagent? How many grams of excess reagent is leftover? What is the theoretical yield of CH3OH? If 35.7 g CH3OH is actually produced, what is the percent yield of CH3OH? H2(g) + CO(g) CH3OH(g) Procedure for limiting/excess reagent calculations aA + bB cC + dD 1) Make sure the equation is balanced. 2) Find the moles of each reactant: moles = mass in gram / molar mass 3) Pick up any reactant, say A, and use the stoichiometry to calculate the required amount of the other reactant B. 4) Compare the required amount of B with the available amount of B. a) If required > available, then B is the limiting reagent and A is the excess reagent. b) If required < available, then B is the excess reagent and A is the limiting reagent. 5) Use the amount of the limiting reagent and the stoichiometry to calculate the amount of any product and the amount of the excess reagent that has been consumed. 6) Leftover excess reagent = available − consumed 7) If actual yield is given percent yield = (actually yield / theoretical yield) x 100% 1.50 g of ammonia reacts with 2.75 g of oxygen gas to produce nitrogen monoxide and water. NH3(g) + O2(g) NO(g) + H2O(g) a) b) c) d) e) f) g) Balance the equation. What is the mass of O2 in grams required by NH3? Which reactant is the limiting reagent? How many grams of NO will be produced in theory? How many grams of H2O will be produced in theory? How many grams of the excess reagent remain unreacted? If only 1.80 g of NO are produced, what is the percent yield? Classification of Matter Homogeneous (Solutions) (visibly indistinguishable) Mixtures (multiple components) Heterogeneous (visibly distinguishable) Matter Elements Pure Substances (one component) Compounds Solute + Solvent Solution Solvent = water, aqueous solution Water can dissolve many substances H2O O H H C12H22O11 Based on the electrical conductivity in aqueous solution salts strong electrolytes strong acids strong bases electrolytes weak acids solutes weak electrolytes weak bases nonelectrolytes strong electrolytes: dissociate 100 % into ions weak electrolytes: only a small fraction dissociate into ions nonelectrolytes: no dissociation salts: NaCl, Na2SO4, Fe(ClO4)3 …… strong acids: HCl, HNO3, H2SO4, HClO4 remember strong bases: NaOH, KOH Base: compounds that give OH− when dissolved in water. weak acids: acetic acid: HC2H3O2 remember weak bases: ammonia: NH3 HCl is Completely Ionized Aqueous Solution of NaOH Reaction of NH3 in Water concentrations mass of component mass percent 100 % mass of whole sample mass of solute mass percent 100 % mass of solution no unit 10. g of sugar is dissolved in 40. g of water. What is the mass percent of sugar in this solution? moles of solute Molarity (M) liters of solution Unit: mol/L or M 0.50 mol of KBr is dissolved in water and forms a solution of 12 L. What is the molarity of the solution? Molarity (M) Example 4.5, page 141 moles of solute liters of solution 25.5 g of KBr is dissolved in water and forms a solution of 1.75 L. What is the molarity of the solution? Example 4.6, page 142 How many liters of a 0.125 mol/L NaOH solution contains 0.255 mol of NaOH? How to prepare 1.00 L of NaCl aqueous solution with a molarity of 1.00 mol/L? 1.00 mol NaCl + 1.00 L of H2O = 1.00 mol/L NaCl (aq) Solution Dilution Concentrated solutions for storage, called stock solutions stock solution + water desired solution moles of solute before dilution = moles of solute after dilution moles of solute Molarity (M) liters of solution M1V1 = M2V2 M1: molarity of concentrated solution V1: volume of concentrated solution M2: molarity of diluted solution V2: volume of diluted solution Example on page 143 A lab procedure calls for 3.00 L of a 0.500 mol/L CaCl2 solution. How should we prepare it from a 10.0 mol/L stock solution? Example 4.7, page 144 To what volume should you dilute 0.200 L of a 15.0 mol/L NaOH solution to obtain a 3.00 mol/L NaOH solution? Types of reactions Precipitation reactions NaCl(aq) + AgNO3(aq) AgCl(s) + NaNO3(aq) formula equation Na+(aq) + Cl−(aq) + Ag+(aq) + NO3−(aq) AgCl(s) + Na+(aq) + NO3−(aq) complete ionic equation Na+(aq) + Cl−(aq) + Ag+(aq) + NO3−(aq) AgCl(s) + Na+(aq) + NO3−(aq) spectator ions Cl−(aq) + Ag+(aq) AgCl(s) net ionic equation EXAMPLE 4.9 Predicting whether an Ionic Compound Is Soluble Predict whether each compound is soluble or insoluble. (a) PbCl2 (b) CuCl2 (c)Ca(NO3)2 (d) BaSO4 BaCl2(aq) + K2SO4(aq) BaSO4(s) + 2KCl(aq) BaCl2(aq) Ba2+(aq) + 2Cl−(aq) K2SO4(aq) 2K+ (aq) + SO42− (aq) Ba2+(aq) + 2Cl−(aq) + 2K+ (aq) + SO42− (aq) BaSO4(s) + 2Cl−(aq) + 2K+(aq) Ba2+(aq) + SO42− (aq) BaSO4(s) Fe(NO3)3(aq) + KOH(aq) Fe(NO3)3(aq) + 3KOH(aq) Fe(OH)3(s) + 3KNO3(aq) Fe(NO3)3(aq) Fe3+(aq) + 3NO3−(aq) KOH(aq) K+ (aq) + OH− (aq) 3KOH(aq) 3K+ (aq) + 3OH− (aq) Fe3+(aq) + 3NO3−(aq) +3K+ (aq) +3OH− (aq) Fe(OH)3(s) + 3NO3−(aq) + 3K+(aq) Fe3+(aq) + 3OH− (aq) Fe(OH)3(s) BaCl2(aq) + KNO3(aq) BaCl2(aq) + 2KNO3(aq) Ba(NO3)2(aq) + 2KCl(aq) BaCl2(aq) Ba2+(aq) + 2Cl−(aq) KNO3(aq) K+ (aq) + NO3− (aq) 2KNO3(aq) 2K+ (aq) + 2NO3− (aq) Ba2+(aq) + 2Cl−(aq) + 2K+ (aq) + 2NO3− (aq) Ba2+(aq) + 2NO3− (aq) + 2Cl−(aq) + 2K+(aq) Types of reactions Precipitation reactions Acid-base reactions Acid: Substance that produces H+ ions in aqueous solution Base: Substance that produces OH− ions in aqueous solution HCl(aq) H+(aq) + Cl−(aq) NaOH(aq) Na+(aq) + OH−(aq) H+(aq) + OH−(aq) H2O(l) HCl(aq) + NaOH(aq) H2O(l) + NaCl(aq) H+(aq) + Cl−(aq) +Na+(aq) +OH−(aq) H2O(l) + Na+(aq) + Cl−(aq) H+(aq) + OH−(aq) H2O(l) acidic basic neutral neutralization HCl(aq) H+(aq) + Cl−(aq) What is the molarity of HCl(aq) or H+(aq)? HCl(aq) + NaOH(aq) H2O(l) + NaCl(aq) When reaction completes nNaOH = nHCl MNaOHVNaOH = MHClVHCl prepared, known measured by buret, known unknown measured by pippet, known MNaOHVNaOH = MHClVHCl Read acid-base titration starting on page 158 and the online instruction for next week’s titration Example 4.14 The titration of a 10.00-mL sample of an HCl solution of unknown concentration requires 12.54 mL of a 0.100 M NaOH solution to reach the equivalence point. What is the concentration of the unknown HCl solution in M? HCl(aq) + NaOH(aq) H2O(l) + NaCl(aq) MNaOHVNaOH = MHClVHCl Types of reactions Precipitation reactions Acid-base reactions Oxidation-Reduction reactions 2Mg(s) + O2(g) 2MgO(s) Reactions that involve electron transfer are called oxidation-reduction reactions, or redox reactions. Oxidation numbers (states) A way to keep track of the electrons gained or lost 1) For atoms in its elemental form, oxidation number = 0 Na, Ag, Ar, O2, N2, P4 2) For monatomic ion, oxidation number = charge of the ion Na+, Ca2+, Co2+, Co3+, Cl−, O2− NaCl, Na2O, CaCl2, CaO, CoCl2, CoCl3, Co2O3, CoO H2O O H H 3) In covalent compounds O: −2 H: +1 F: −1 In a neutral compound, the sum of the oxidation number = 0 CO, CO2, SF6, SF4, H2S, NH3, P2O5, N2O3 In a polyatomic ion, the sum of the oxidation number = ion charge NO3−, SO42−, NH4+, Cr2O72−, MnO4−