* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Ch04-04-alkenes-2
Survey
Document related concepts
Homoaromaticity wikipedia , lookup
Physical organic chemistry wikipedia , lookup
2-Norbornyl cation wikipedia , lookup
Cracking (chemistry) wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Asymmetric induction wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Vinylcyclopropane rearrangement wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Hydroformylation wikipedia , lookup
Transcript
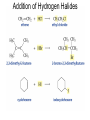
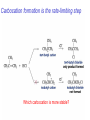
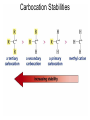
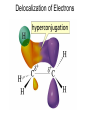
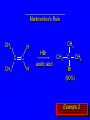
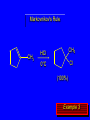
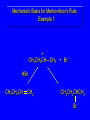
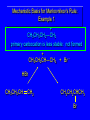
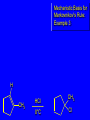
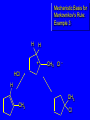
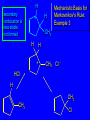
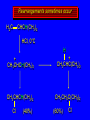
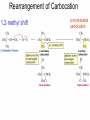
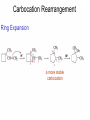
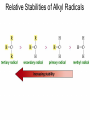
Chapter 4 Reactions of Alkenes Adapted from Profs. Turro & Breslow, Columbia University and Prof. Irene Lee, Case Western Reserve University Electrophilic Additions: Alkenes Addition of Hydrogen Halides What is the product? Carbocation formation is the rate-limiting step Which carbocation is more stable? Carbocation Stabilities Alkyl groups decrease the concentration of positive charge in the carbocation Delocalization of Electrons Molecular Orbital Diagram in a Hyperconjugation System Hammond postulate: the transition state will be more similar to the species that it is closer to energetically Exergonic reaction: early transition state resembles reactants (I). Endergonic reaction: late transition state resembles products (II). I: early transition state (Like reactants) II: mid-transition state III: later transition state (Like products) Markovnikov’s Rule The electrophile adds to the sp2 carbon that is bonded to the greater number of hydrogens In a regioselective reaction, one constitutional isomer is the major or the only product. Explained by the intermediates, for example: tert-butyl cation is formed faster and it is more stable than isobutyl. Regioselectivity of Hydrogen Halide Addition: Markovnikov's Rule Markovnikov's Rule When an unsymmetrically substituted alkene reacts with a hydrogen halide, the hydrogen adds to the carbon that has the greater number of hydrogen substituents, and the halogen adds to the carbon that has the fewer hydrogen substituents. Markovnikov's Rule CH3CH2CH CH2 HBr acetic acid CH3CH2CHCH3 Br (80%) Example 1 Markovnikov's Rule CH3 C CH3 CH3 H HBr C H acetic acid CH3 C CH3 Br (90%) Example 2 Markovnikov's Rule CH3 HCl CH3 0°C Cl (100%) Example 3 Mechanistic Basis for Markovnikov's Rule Protonation of double bond occurs in direction that gives more stable of two possible carbocations. Mechanistic Basis for Markovnikov's Rule: Example 1 CH3CH2CH CH2 HBr CH3CH2CHCH3 acetic acid Br Mechanistic Basis for Markovnikov's Rule: Example 1 + CH3CH2CH—CH3 + Br – HBr CH3CH2CH CH2 CH3CH2CHCH3 Br Mechanistic Basis for Markovnikov's Rule: Example 1 + CH3CH2CH2—CH2 primary carbocation is less stable: not formed + CH3CH2CH—CH3 + Br – HBr CH3CH2CH CH2 CH3CH2CHCH3 Br Mechanistic Basis for Markovnikov's Rule: Example 3 H CH3 HCl CH3 0°C Cl Mechanistic Basis for Markovnikov's Rule: Example 3 H H + CH3 Cl – HCl H CH3 CH3 Cl H secondary carbocation is less stable: not formed H + Mechanistic Basis for Markovnikov's Rule: Example 3 CH3 H H + CH3 Cl – HCl H CH3 CH3 Cl Carbocation Rearrangements in Hydrogen Halide Addition to Alkenes Rearrangements sometimes occur H2C CHCH(CH3)2 HCl, 0°C H + CH3CHCH(CH3)2 + CH3CHC(CH3)2 CH3CHCH(CH3)2 CH3CH2C(CH3)2 Cl (40%) (60%) Cl Rearrangement of Carbocation 1,2-hydride shift a more stable carbocation Rearrangement of Carbocation 1,2-methyl shift a more stable carbocation Carbocation Rearrangement Ring Expansion a more stable carbocation Carbocation does not always rearrange … Addition of Halogens to Alkene QuickTime™ and a Video decompressor are needed to see this picture. Addition of Water to Alkene (alcohols) QuickTime™ and a QuickTime™ and a Graphics decompressor Graphics decompressor QuickTime™ and a to see this picture. are are needed to needed see this picture. Graphics decompressor are needed to see this picture. Acid-Catalyzed Addition of Alcohol (ethers) Addition of Halogens in the Presence of Water (halohydrins) Oxymercuration and Mercuration of Alkene (alcohols w/o carbocation rearrangement) Addition of Borane Hydroboration–Oxidation Anti-Markovnikov’s rule in product formation (less substituted alcohols) Vs. Markovnikov’s rule in product formation (more substituted alcohols) Formation of Alkyl Boranes Anti-Markovnikov Addition Boron adds to least hindered carbon Anti-Markovnikov Addition Markovnikov Addition Boron adds to least hindered carbon and is replaced w/ -OH by oxidation Formation of the most stable carbocation (A type of pericyclic reaction; important reaction and mechanism in directing reactions both regio- and stereoselectively.) Examples of Anti-Markovnikov Addition of an OH Group Carbene: another reactive intermediate Reaction with an Alkene Synthesis of Bromobutane Isomers Generation of Free Radicals Using 1/2 arrows for the movement of one electron Addition of Radicals to Alkenes Initiation Propagation Termination Relative Stabilities of Alkyl Radicals Addition of Hydrogen to Alkenes Catalytic Hydrogenation of an Alkene