* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Mon Feb 15 lecture
Kinetic resolution wikipedia , lookup
George S. Hammond wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Petasis reaction wikipedia , lookup
Ene reaction wikipedia , lookup
1,3-Dipolar cycloaddition wikipedia , lookup
Aza-Cope rearrangement wikipedia , lookup
Asymmetric induction wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Hydroformylation wikipedia , lookup
Aromaticity wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Polythiophene wikipedia , lookup
Stille reaction wikipedia , lookup
2-Norbornyl cation wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Aromatization wikipedia , lookup
Homoaromaticity wikipedia , lookup
Vinylcyclopropane rearrangement wikipedia , lookup
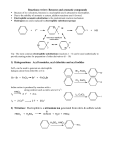
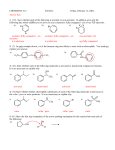
Lecture outline 4. Friedel-Crafts reactions — a. Alkylation + R AlCl3 R–Cl (+ HCl) (catalyst) (R = methyl, 1°, 2°, 3°; R ≠ vinyl, aryl) After initial complexation with the Lewis acid catalyst,... (add the charges!) R—Cl + AlCl3 R—Cl—AlCl3 — 2° and 3° alkyl halides react via an SN1-type mechanism (i.e. via 2° or 3° carbocation): (add the lone pairs, charges, and curved arrows!!!) H R—Cl—AlCl3 – AlCl4 – H R R 1. loss of leaving group 2. addition of nucleophile etc Cl—AlCl3 R (+ HCl + AlCl3 ) — methyl and 1° alkyl halides react via an SN2-type mechanism (i.e. direct displacement): H + R—Cl—AlCl3 – AlCl4 – H R etc keep in mind that carbocations sometimes rearrange... not Cl + AlCl3 write the mechanism: ...and rearrangements are sometimes observed even with 1° halides — in these cases, a hydride or alkyl shift is concerted with loss of the l.g., — this pathway avoids the intermediacy of a high-E 1° cation. (This is frequently observed in carbocation chemistry; we've already seen a similar concerted rearrangement and leaving group (water) departure in the reaction of some 1° alcohols with strong acid.) not Cl + AlCl3 Add the curved arrows to show the concerted H shift and loss of the leaving group. H – AlCl4 – Cl AlCl3 CH3 usual EAS mech H CH2 How are the 2-step (via the 1° cation) and 1-step routes analogous to SN1 and SN2 mechanisms, respectively? What functions as the "nucleophile" in the concerted pathway? Note that the reactions shown on p 692 of the text likely involve a competition between SN2 mechanism to attach the 1° C directly to the aromatic ring and a concerted rearrangement similar to the one shown above to produce a 2° (or 3°) cation directly. There is no compelling experimental evidence that localized 1° cations are ever formed under these conditions. The mechanism in the study guide for problem 17.14 (benzene + 1-chloropropane + AlCl3 —> propylbenzene + isopropyl benzene) is almost certainly wrong. Let's write a more reasonable mechanism for each pathway — 2° and 3° cations can also be generated in other ways — what reagents might we use to convert isobutylene and t-butyl alcohol to t-Bu cation? ... EAS OH b. Acylation + R O AlCl3 C (catalyst) Cl (an acyl chloride) O C R (+ HCl) Complete the mechanism below by adding charges, lone pairs, and curvy arrows. Loss of the leaving group generates an acylium ion, which is resonance stabilized, and thus has no tendency to rearrange. Show both resonance structures, then show this cation attaching to benzene via the usual EAS mechanism. O R C + Cl R O R O AlCl3 C C – AlCl4 – Cl AlCl3 AlCl3 Cl dead end Friedel-Crafts acylation provides a way to avoid rearrangements that often accompany FC alkylation. For example, benzene cannot be converted to isobutyl benzene directly by rxn with iBu–Cl with AlCl3 (direct route below; another product is formed instead — what is it and how is it formed? Hint: you just saw this 2 pages back!) Cl AlCl3 AlCl3 Cl O O Zn(Hg), aq HCl or H2NNH2, HO –, H2O, ! This can be avoided by (see the lower 2-step route) F–C acylation followed by either (a) Clemmensen reduction (zinc-amalgam and aqueous acid) or (b) Wolff-Kishner reduction (hydrazine, base, protic solvent, and lots of heat) avoids the rearrangement problem. (We’ll learn a bit more about these when we get to carbonyl chemistry.) — F-C acylation is quite a general procedure; a variety of different acyl groups can be introduced by this method. Here an a couple of other examples — draw the products formed from the following reactions: benzene + AlCl3 PhCOCl O AlCl3 Cl 5. H+ (D+) as the electrophile — D D + D2O (excess) C6H6 D D D D D C6D6 (an NMR solvent) (desulfonation is another example of this — in that case, an H+ adds and an SO3H+ is later lost)