* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download a PDF of the paper on this project published in the OCEANS 2005
Ellipsometry wikipedia , lookup
Diffraction topography wikipedia , lookup
Super-resolution microscopy wikipedia , lookup
Ultrafast laser spectroscopy wikipedia , lookup
Johan Sebastiaan Ploem wikipedia , lookup
Confocal microscopy wikipedia , lookup
Harold Hopkins (physicist) wikipedia , lookup
Ultraviolet–visible spectroscopy wikipedia , lookup
Optical tweezers wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Optical coherence tomography wikipedia , lookup
Chemical imaging wikipedia , lookup
3D optical data storage wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Vibrational analysis with scanning probe microscopy wikipedia , lookup
Fiber-optic communication wikipedia , lookup
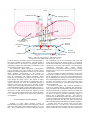
Development of a Free-Drifting Submersible Digital Holographic Imaging System D.W. Pfitsch Johns Hopkins University ([email protected]) E. Malkiel Johns Hopkins University([email protected]) Y. Ronzhes Johns Hopkins University S.R. King Johns Hopkins University J. Sheng Johns Hopkins University J. Katz Johns Hopkins University([email protected]) I. INTRODUCTION Abstract – Submersible film-based holographic systems have demonstrated the unique ability of holography to provide high resolution, three-dimensional, in-situ images of marine organisms. Inherently, use of film limits the frame rate and total number of holograms. This paper describes a submersible, free-drifting, digital holographic cinematography system that has a real-time fiber optic communication link. This system collects dual-view digital holograms at a rate of 15 frames per second, enabling the user to observe the behavior of marine plankton and distinguish motile organisms from abiotic particles. In order to follow the particles in time, the sample should have as little motion relative to the cameras as possible. To achieve this goal, the submersible is neutrally buoyant, and has high drag generating elements at the height of the sample volume. In addition, the components surrounding the sample are streamlined and designed to minimize the local flow disturbance. The data from the two digital cameras and other sensors are transmitted at 120 MB/s through a 1 km long, 250 µm diameter, fiber optic cable to an acquisition system located on a research vessel. The optical fiber is spooled out from the submersible by a powered mechanism, as the submersible drifts away from the vessel. Releasing the fiber out at a rate greater than that of the drifting speed minimizes the transmission of forces through the cable, effectively decoupling the submersible from the cable and vessel dynamics. A variable buoyancy system provides vertical position control while still allowing the system to drift vertically with the surrounding fluid, i.e. follow internal waves. The dual-view holo-camera records two in-line holograms from orthogonal directions, each with a volume of 40.5 cm3. Without lenses, the resolution in the 3.4 cm3 volume where the beams cross each other, is about 7.4 µm in all three directions. Outside of the overlapping region, the resolution in the beam axial direction is lower, but the lateral resolution remains 7.4 µm. An optional 2X lens doubles the resolution, but reduces the sample volume. The first field deployment for this system took place in June 2005, in the Ria de Pontevedra, Spain. It was used for examining thin layers of harmful algal blooms. Harmful algal booms (HAB) are becoming an increasingly significant concern for fisheries and for human health. The specific motivation for the present study is the toxic dinoflagellate, dynophysis, a 30 µm diameter motile organism that causes shell fish poisoning [12]. While traditional water sampling techniques combined with microscope examination provide considerable insight into the behavioral characteristics of micro-organisms [16], it does not provide information on their behavior in their natural environment, such as interaction with other organisms/particles and with the surrounding flow. Observations on plankton behavior in-situ are essential for understanding the reasons for the algae bloom formations and life cycles. Determination of what combination of fluid dynamics, e.g. horizontal shear, and migratory behavior would result in formation of thin layers [11] of harmful algal blooms has received little attention because of the difficulty of performing such observations. Two-dimensional digital and video imaging [4, 6, 15], while informative, are limited in the extent of behavioral information that can be gained due to the short time frame in which the subject remains in focus. Submersible film holography has been used to acquire 3-D still images of a sample volume containing plankton [2, 8, 10, 17], and can even be used to measure the instantaneous velocity by recording double exposure holograms, as has been done in laboratory settings [1, 14, 18]. This approach has been successful in measuring 3D velocity fields and determining particle distribution, but lacks a suitable time frame that could be used to observe plankton behavior. Furthermore, the data base is inherently limited, in part due to the need to submerge film rolls or plates, and in part due to the cumbersome process of film development followed by optical reconstruction. However, the film resolution is much higher than that of any digital recording medium, enabling use of off-axis holography that can resolve an order of magnitude more particles [18]. Three main criteria should be met in order to capture organism behavior in-situ. First, the system must be able to record a series of images at a framerate that enables observations and analysis of behavior. Second, the imaging technique must 1 1230 GPS/RF Unit Flashing Beacon Sample Volume 15 fps Digital Cameras Drag Generating Elements Streamlined Hull Laser Beams Pressure Housing Electronics, Battery and Laser Acoustic Transponder SeaBird CTD Ballast Tank Figure 1: The hull, drag generators, and sample volume arrangement of the submersible holography system. the (undisturbed part of the) illuminating beam with light be able to measure 3D motions, and have extended depth to scattered from objects in the sample volume, i.e. it contains all track objects regardless of their direction. Third, the motion of the information needed for reconstructing the location and shape the system relative to the subjects of investigation should be of particles over the entire sample volume. The holograms are minimized in order to allow observations on the behavior of the reconstructed numerically using Fresnel Huygens same organisms for as long as possible. transformation, as described in detail in [8] and [9], providing The submersible digital holographic cinematography detailed, in-focus images of any desired plane located within the stystem described in this paper satisfies all of these criteria. sample volume.. Holography maintains high lateral resolution over an extended The 3D coordinates of particles measured by a single in-line depth, enabling measurements of 3D motion of hologram are accuarate to about pixel resolution in directions micro-organisms, which would rapidly move out of the focal that are perpendicular to optical axis of the illuminating beam, plane of conventional, 2D imaging techniques. Digital but are substantially less accurate in the beam direction due to holographic cinematography can follow the same subject as it the so-called depth of focus effect [8, 18]. In order to obtain 3D passes through the sample volume. The number of frames data at the same resolution, we use a dual-view system, which containing the same object depends on magnification, relative records two in-line holograms of the same sample volume from motion, and frame rate. Thus, the submersible platform is orthogonal directions. designed to drift with the local current, and have minimal As sketched in Figures 1 and 3, coherent illumination is velocity relative to the fluid and particles in the sample volume. achieved by spatially filtering, expanding and collimating a The submersible holography system is described in Figures 1-3. beam generated by a Q-switched, diode pumped ND-YLF laser, Figure 1 shows the submersible platform highlighting elements which is manufactured by Crystalaser. The red wavelength, 660 that enable it to drift with the local flow. Figure 2 provides nm, is chosen specifically because few organisms in the ocean details on the control and data transmission systems, and Figure can detect red light [3], therefore minimizing changes in 3 is a schematic description of the optical setup. Details are behavior due to phototrophic responses. The dual-view provided below. holograms are recorded using two 2K x 2K pixels, 15 frames per second, 8 bit digital cameras manufactured by Pulnix. Electronic shuttering synchronized with the laser pulses minimizes the II. IN LINE DIGITAL HOLOGRAPHY effect of ambient sunlight, which allows for daytime operation. Without lenses, the total sample volme of each in-line Capturing an in-line digital hologram consists of hologram is 40.5 cm3. Over the 3.4 cm3 volume where the two back-illuminating a sample volume with a collimated, coherent light and recording the resulting diffraction pattern on a CCD sample volumes intersect, the resolution is about 7.4 µm (pixels array. This recorded image is a result of interference between pitch) in all three directions. Installing a 2X lens in front of each 2 Vessel: Data Acquisition and Tracking Sytem Fiber Optic MUX/ DEMUX 1510 nm 1530 nm 1570 nm 1550 nm CTD Camera Camera GPS/RF Digital to Optical Converter Digital to Optical Converter Four Terabyte Data Acquisition System Optical to Digital Converter Digital Compass Tracking Computer Acoustic Transponder 1510 nm 1530 nm Fiber Optic MUX/ DEMUX 1570 nm 1550 nm 250µm Single Mode Fiber optic Cable (1000m) Ballast System Controller Optical to Digital Converter GPS Acoustic USBL Transciever Digital Holographic Submersible Beacon Control Computer Drop Weight Figure 2: Components and communication lines of the system area is selected to maintain a relative velocity of less than 10 mm/s based on a simplified analysis involving the system inertia and drag forces on different components. Recognizing that typical coastal flows are non-uniform, it is essential to concentrate the drag force at the same elevation as the sample volume. Thus, the main pressure housing of the submersible is surrounded by a fiberglass, streamlined hull (Figure 1). Communication as well as data transfer to and from the submersible are performed using a 1 km long (typically), 250 µm diameter, single mode fiber optic cable. The cable spool is mounted on the bottom of the submersible, and is spooled off using motorized drive at a rate that is slightly faster than the drifting speed. This approach decouples the submersible from the drag forces on the cable, and from the dynamics of the researh vessel where the operator is sitting. The submersible is neutrally buoyant, and is equipped with a ballast system that enables control of its vertical position. The ballast is varied using a positive displacement pump, which pumps water into and out of a 1.6 L rubber tube located within a pressure housing under the main hull. This depth control method allows the controller to bring the submersible to a desired depth, without creating major disturbances. such as those caused by thrusters or compressed air-ballast systems. Once camera about doubles the resolution, but decreases the sample volume. Use of microscope objectives [13] can increase the resolution to sub-micron levels. III. THE FREE DRIFTING PLATFORM In accordance with the third criteria mentioned in the introduction, the submersible platform is designed to drift with minimal velocity relative to the fluid in sample volume, and to minimize the disturbance to the local flow. Consequently, it can view the behavior of the same organisms for as long as possible. To minimize the flow disturbances, the sample volume is located between two streamlined towers/fins, 367 mm away from the main body. The fins are flat on inner (sample volume) side, and convex on the outer side in order to minimize the fluid acceleration as it passes between the towers. As illustrated in Figures 1 and 3, the collimated laser beams are directed into each tower, and then transmitted through windows and sample volumes into the opposite tower, where the corresponding digital cameras are located. Large drag-generating structures are attached to the outer sides of each tower, at the same height as the sample volume. While the system drifts, these drag generators are aligned perpendicularly to the relative flow. Their surface 3 Overlapping sample region Windows Prism 30 mm expanded beam Digital Cameras Laser 50-50 Beamsplitter Figure 4: Deployment of the submersible holography system. lifting brackets attached to the towers. Once in the water and floating, the submersible is realeased, leaving the fiber optic cable as the only link to the research vessel. To track the submersible during deployment, it is equipped with an acoustic transponder. A TrackLink USBL transciever, manufactured by LinkQuest is intalled off the side of the vessel to query the transponder postion. Used in conjuction with a digital compass and GPS unit, this acoustic tracking system monitors the absolute position of the submersible as it drifts away from the vessel. In a typical deployment, after being released the ballast is adjusted to make the submersible dive quickly to below the target depth, and then rise slowly into the desired sampling elevation. This procedure minimizes the effect of vertical motion on the flow within the sample volume. Limited by battery power, the submergence period is about 1 hour. When the desired data has been acquired, the submersible rises to the surface. Once outside of the water, a Garmin GPS unit with RF transmitter located at the top of one of the towers can be polled to provide the location of the Spatial Filter Figure 3: Optical setup of the dual beam digital holography system. Only the centerline of the beam is shown for clarity. The blue lines depict the in-water sample volume adjusted, the neutrally buoyant platform can also drift vertically with the surrounding fluid, aided by vertical drag forces on the large surface area of the main hull. Prior to deployment, the system is adjusted to be nuetrally bouyant when the ballast tube is half full, by mounting syntactic foam on the drag elements (Figure 4), which also helps in maintaining the system stability. Additional elements are installed within the streamlined hull. IV. SYSTEM CONTROL & DEPLOYMENT The system is internally powered by a Lithum-Ion battery pack that enables deployment for about one hour, and lasts for about two hours. As noted before and sketched in Figure 2, we communicate and transmit all the data through a single fiber optic cable. Two semi-custom ARVOO Optilink units convert the digital images and RS-232 signals of the CTD and communication lines into four optical signals, each at a different laser frequency (wavelengths of 1510, 1530, 1550 and 1570 nm). A MUX/DEMUX unit from AFOP then combines all four optical signals into a single optical fiber. An identical MUX/DEMUX unit separates the four optical signals at the other end of the cable on the research vessel, and each is converted back to a digital signal using ARVOO Optilink units. In the present configuration, the image acquisition rate is about 120 MB/s. A computer integrated by Boulder Imaging, containing two 1 TB RAID drives and 2 TB of back-up hard-disk space, acquires the digital images and CTD data. These data are viewed in real time, enabling the operator to select whether and which holograms to save. A separate laptop computer runs the communication software that controls and monitors the submersible “vital functions,” e.g. bouyancy, battery voltage, fiber optic cable release mechanism and leak detectors. To deploy the submersible digital holography system, it is lowered over the side of the vessel by two time (minutes) 0 0 10 20 30 40 depth (m) 5 10 15 20 25 Figure 5: Sample depth record of the platform as measured by the on-board CTD during a deployment in the Ria de Pontevedra. 4 1mm Figure 6: A Tiny medusa traversing downward at 45º at a rate of 6.8 mm/s during contraction and virtually not moving during the resting phase of its swimming cycle. platform. A flashing beacon located on the other tower facilitates recovery of the submersible at night. The GPS system and beacon have independent power sources, and can operate for extended periods. A drop-weight supported by a solenoid-controlled pin is located at the very bottom of the submersible. It is designed to be released, which quickly brings the system to the surface in case of an emergency. The drop-weight is released automatically if communication or power are lost, but can also be released by a command over the fiber optic cable. When recoverd, the lithium-ion battery can be replaced for a quick turn around or can be recharged, which can takes up to 3 hours. The 1000 m optical fiber is clipped and a new spool is installed after each deployment. Since we use an unprotected fiber, its cost is minimal, and there is no compelling reason or provision to re-spool it. If we only use part of the fiber, the remaining spool can be reconnectorized. Since the GPS system utilizes an independent power source, it can still be used for finding the submersible platform even if all other means of communication fail. project called HABIT [5, 12], which studies thin layers of the HAB, Dinophysis acuminata. The European collaborators included biological and physical oceanographers from Spain, France, UK, and Ireland. Various sampling methods were used to detect the dinoflagellates, and provide approximate depths and locations, where the submersible should be deployed to observe their behavior. As is common on the first deployment of complicated instruments, we encountered many technical problems. We resolved most, but due to time constraints could not solve problems with the optical fiber release mechanism. Thus, the 250 µm fiber was spooled out off the deck of the research vessel. Furthermore, to ensure that the instrument would not be lost during these preliminary runs, we attached a bouyant safety tether to the submersible. The other end of this tether was attached to a small zodiak, which followed the platform, as it drifted with the current. By continuously maintaining slack in the tether, we did our best to minimize its effect on the trajectory and velocity of the submersible platform. Despite these setbacks, we completed 10 successful deployments and recorded over 2 TB of digital holograms. After reconstruction of multiple planes, these data will amount to over 250 TB of images. The longest deployment took 50 minutes, during which the the submersible performed two consecutive dives to a depth of 25 m, and the system drifted close to 150 m away from from the research vessel. A sample depth record for one of these V. RIA DE PONTEVEDRA The first series of deployment tests of the free-drifting digital holography system took place during June 2005, in the Ria de Pontevedra, on the coast of northwest Spain. The Johns Hopkins University group was working in collaboration with a European research team working on 5 in studying ocean particle dynamics,” Opt. Eng. Vol 18, pp. 524-525, 1979. [3] R. B. Forward, “Diel vertical migration: Zooplankton photobiology and behaviour,” Oceanography and Marine Biology—An annual Review, vol. 26, pp. 361-393, 1988. [4] M. Gallager, C. S. Davis, A. W. Epstein, A. Solow, and R. C. Beardsley, “High resolution observations of plankton spatial distributions correlated with hydrography in the Great South Channel, Georges Bank,” Deep-Sea Res., vol. 2, no. 43, pp. 1627-1663, 1996. [5] P. Gentien, M. Lunven, M. Lehaitre, and J. L. Duvent, “In-situ depth profiling of particle sizes,” Deep-Sea Res., vol. 42, pp. 1297-1312, 1995. [6] J. S. Jaffe, and P. J. S. Franks, “Simultaneous imaging of phytoplankton and zooplankton distributions,” Oceanography, vol 11, no. 1, pp. 24-29, 1998. [7] E. Malkiel, O. Alquaddoomi, and J. Katz, “Measurements of plankton distribution in the ocean using submersible holography,” Meas. Sci. Technol.,, vol. 10, pp. 529-551, April 1999. [8] E. Malkiel, J. Sheng, J. Katz, and J. R. Strickler, “The three-dimensional flow field generated by a feeding calanoid copepod measuered using digital holography,” J. of Exp. Bio., vol. 206, pp. 3657-3666, 2003. [9] J. H. Milgram and W. C. Li, “Computational reconstruction of images from holograms,” Appl. Optics, vol 41, pp 853-864, 2002. [10] T. J. O’Hern, L. D’Agostino, and A. J. Acosta, “Comparison of Holographic and Coulter counter measurements of cavitation nuclei in the ocean,” J. Fluids Eng. Vol. 110, pp. 200-207, 1988. [11] T. Osborn, “Finestructure, microstructure, and thin layers,” Oceanography, vol. 11, no. 1, pp. 36-43, 1998. [12] B. Reguera, I. Bravo, C. Marcaillou-Le Baut, P. Masselin, M. L. Fernandez, A. Miguez, and A. Martinez, “Monitoring of Dinophysis spp and vertical distribution of okadaic acid on mussel rafts from Ria de Pontevedra (NW Spain),” In: T. J. Smayda and Y. Shimizu (Eds.), Toxic Phytoplankton Blooms in the Sea. Elsevier, Amsterdam, pp. 553-558, 1993. [13] J. Sheng, E. Malkiel, and J. Katz, “A digital holographic microscope for measuring three dimensional particle distributions and motions” submitted to Applied Optics, 2005. [14] B. Tao, J. Katz, and C. Meneveau, “Statistical geometry of subgrid-scale stresses determined from holographic particle image velocimetry measuremtents,” J. Fluid Mech., vol 457, pp. 35-78, 2002. [15] P. Tiselius, “An in-situ video camera for plankton studies: design and preliminary observations,” Mar. Ecol. Prog. Series, vol. 64 pp. 293-299, 1998. [16] C. R. Tomas (Ed.), Identifying Marine Phytoplankton, San Diego, CA, Academic Press, 1997. [17] J. Watson, S. Alexander, G. Craig, D. C. Hendry, P. R. Hobson, R. S. Lampitt, J. M. Marteau, H. Nareid, M. A. Player, K. Saw et al. “Simultaneous in-line and off-axis subsea holographic recording of plankton and other marine particles,” Meas. Sci. Technol., vol 12, pp. L9-L15, 2001. [18] J. Zhang, B. Tao, and J. Katz, “Turbulent flow measurement in a square duct with hybrid holographic PIV,” Exp. Fluids, vol. 23, pp. 373-381, 1997. deployments, as measured by the CTD, is presented in Figure 5. A very small fraction of the holograms has been reconstructed to date. However, we have already reconstructed selected sequences of holograms containing images of organism behavior. In some cases, we are able to observe the very same organism in up to 20 frames. Figure 6 is a sample time series of reconstructed holograms, showing images of a 2 mm wide medusa jellyfish. Unlike organisms of this size, whose signatures can be readily identified in the original holograms, we have to reconstruct multiple planes to detect the 20-30 µm Dinophysis acuminata. We are only at the beginning of an extensive data analysis phase, which will most likely require development of several new processing tools. Some of these tools will be adopted from procedures developed during earlier analysis of film-based holograms [8]. VI. CONCLUSION Several unique features of our free-drifting, submersible digital holography system make it suitable for in-situ observation on behavior of marine organisms. Holography enables us to maintain the same lateral resolution over an extended depth. Use of dual perpendicular views provides the same resolution in all directions. Cinematography and deployment as a free-drifting platform enable us to follow and examine the behavior of the same micro-organisms over many frames. Preliminary analysis of data obtained during its first deployment, demonstrates an ability to study the behavior of organisms at unprecedented levels of detail. At this point, we have just begun the data analysis phase. The full potential of this instrument, and subsequent insights that the data will provide will soon be realized. As noted before, we still have to resolve some problems encountered during the first deployment. As we gain more experience in tracking and recovery operations, we will feel more confident in removing the safety tether, which may affect the motion of the system relative to the fluid in the sample volume. Acknowledgments The development of the digital holographic submersible was sponsered by the National Science Foundation under grant no. 0402792. Some of the equipment has been purchased using funds provided by NSFMRI grant no. 0079674. The authors would also like to thank Beatriz Reguera of IEO, our collaborator and host in Vigo, Spain, Walt Krug & Mike Franckowiak, superb machinists at JHU, Dan Ursu from JHU for his help in Vigo, Tom Osborn of JHU for his input regarding physical oceanography and plankton dynamics, Louis Whitcomb of JHU for the useful suggestion of using optical fiber, and finally the captain and crew of the Jose Maria Navaz. REFERENCES [1] D. H. Barnhart, R. J. Adrian, and G. C. Papen, “Phase-conjugate holographic system for high-resolution particle image velocimetry,” Appl. Opt. vol. 30, pp. 7159-7170, 1994. [2] K. L. Carder, “Holographic microvelocimeter for use 6