* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download On the specificity of antibiotics targeting the large ribosomal subunit
Discovery and development of tubulin inhibitors wikipedia , lookup
Discovery and development of direct thrombin inhibitors wikipedia , lookup
5-HT3 antagonist wikipedia , lookup
DNA-encoded chemical library wikipedia , lookup
Discovery and development of neuraminidase inhibitors wikipedia , lookup
Discovery and development of non-nucleoside reverse-transcriptase inhibitors wikipedia , lookup
Discovery and development of ACE inhibitors wikipedia , lookup
Metalloprotein wikipedia , lookup
Discovery and development of direct Xa inhibitors wikipedia , lookup
Discovery and development of antiandrogens wikipedia , lookup
NK1 receptor antagonist wikipedia , lookup
Drug design wikipedia , lookup
Antibiotics wikipedia , lookup
Nicotinic agonist wikipedia , lookup
Discovery and development of integrase inhibitors wikipedia , lookup
Discovery and development of cephalosporins wikipedia , lookup
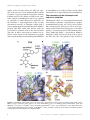
Ann. N.Y. Acad. Sci. ISSN 0077-8923 A N N A L S O F T H E N E W Y O R K A C A D E M Y O F SC I E N C E S Issue: Antimicrobial Therapeutics Reviews On the specificity of antibiotics targeting the large ribosomal subunit Daniel N. Wilson1,2 1 Center for integrated Protein Science Munich (CiPSM), Germany. 2 Gene Center and Department of Biochemistry, Ludwig-Maximilians-Universität München, Feodor-Lynenstr 25. München, Germany Address for correspondence: Daniel N. Wilson, Gene Center and Department of Biochemistry, Ludwig-Maximilians-Universität München, Feodor-Lynenstr. 25, 81377 München, Germany. [email protected] The peptidyltransferase center of the large ribosomal subunit is responsible for catalyzing peptide bonds. This active site is the target of a variety of diverse antibiotics, many of which are used clinically. The past decade has seen a plethora of structures of antibiotics in complex with the large ribosomal subunit, providing unprecedented insight into the mechanism of action of these inhibitors. Ten distinct antibiotics (chloramphenicol, clindamycin, linezolid, tiamulin, sparsomycin, and five macrolides) have been crystallized in complex with four distinct ribosomal species, three bacterial, and one archaeal. This review aims to compare these structures in order to provide insight into the conserved and species-specific modes of interaction for particular members of each class of antibiotics. Coupled with the wealth of biochemical data, a picture is emerging defining the specific functional states of the ribosome that antibiotics preferentially target. Such mechanistic insight into antibiotic inhibition will be important for the development of the next generation of antimicrobial agents. Keywords: antibiotics; protein synthesis; ribosome; RNA; species specificity; translation; X-ray crystallography Introduction The synthesis of proteins in the cell occurs on large macromolecular complexes called ribosomes (reviewed by Schmeing and Ramakrishan1 ). Ribosomes provide the platform upon which the codons of the mRNA are decoded by the anticodons of the tRNAs. In this way, tRNAs can deliver the appropriate amino acid to the ribosome so that it can be incorporated into the growing nascent polypeptide chain. The ribosome has three tRNA binding sites, the A, P, and E sites. The A site is where incoming aminoacyl-tRNA (aa-tRNA) enters the ribosome. Prior to peptide bond formation, the P site contains the peptidyl-tRNA, that is, the tRNA bearing the polypeptide chain. The E site binds exclusively deacylated or uncharged tRNAs, that is, those tRNAs that have incorporated their amino acid into the polypeptide chain and are ready to exit from the ribosome. Thus, during translation the tRNAs move from A→P→E. Peptide-bond formation occurs at the peptidyltransferase center (PTC) of the large ribosomal subunit (Fig. 1A) and requires the accurate positioning of the 3 terminal CCA-ends of the aa-tRNA at the A site and peptidyl-tRNA in the P site. The ribosome represents a major target in the cell for antibiotics, with many clinically used antibiotics that interfere with the process of peptide-bond formation.2 The wealth of decades of biochemical studies on antibiotic action on ribosomes can now be interpreted in the light of the crystal structures of the ribosome in different functional states and in complex with many different classes of antibiotics (reviewed by Wilson3 ). Indeed, multiple crystal structures of the same antibiotic in complex with ribosomal particles from a variety of different species have been determined (Table 1). Comparisons of such structures have already provided some surprising differences in the manner by which the same member of an antibiotic class interacts with distinct ribosomal particles.4 Here the aim is to provide an update on the diverse modes of antibiotic interaction with the large subunit, by comparing the available X-ray structures doi: 10.1111/j.1749-6632.2011.06192.x c 2011 New York Academy of Sciences. Ann. N.Y. Acad. Sci. 1241 (2011) 1–16 1 Antibiotics targeting the large ribosomal subunit Wilson Figure 1. Chloramphenicol (CAM) binds at the A site of the PTC. (A) Overview of the large subunit, showing relative positions of P-tRNA (green), A site (pink), polypeptide chain (tan), and ribosomal proteins L1 (blue) and L11 (purple). (B) Chemical structure of CAM. (C) Binding position of CAM-16 (gold) and CAM-29 (cyan) relative to A- (blue) and P-tRNA (green).74 (D) Comparison of CAM1 from D50S,8 E70S,6 and T70S5 structures. (E) Binding position of CAM1-E70S6 (gold), CAM1-T70S5 (orange), CAM-29 (cyan), and ERY-E70S6 (tan) relative to A-tRNA74 (blue) and TnaC peptidyl-tRNA27 (green). of antibiotics that have been crystallized in complex with the large ribosomal subunit from different species, namely the chloramphenicols, macrolides, oxazolidinones, pleuromutilins, and sparsomycins bound to the large 50S subunit of Deinococcus radiodurans (D50S) and Haloarcula marismortui (H50S) as well as the 70S ribosome of Escherichia coli (E70S) or Thermus thermophilus (T70S) (Table 1). Understanding how variations in the drug composition, as well as the drug target, can influence the binding and inhibitory activity of antibiotics will not only provide insight into the mechanism of drug action, but will also be important for future development of new improved antimicrobial agents. The orientation of chloramphenicol at the A site Chloramphenicol (CAM), originally isolated from Streptomyces venezuelae, consists of a para- 2 nitrophenyl ring attached to a dichloroacetamido tail (Fig. 1B). CAM displays broad-spectrum activity, inhibiting a wide range of Gram-positive and -negative bacteria, but not translation on eukaryotic cytosolic ribosomes (reviewed by Wilson3 ). CAM has been crystallized in complex with three bacterial ribosomal particles (Table 1), revealing a primary CAM-1 binding site at the PTC (Fig. 1C). In the T70S5 and E70S structures,6 the phenyl ring binds analogously and planar to a phenyl-moiety of an A-tRNA (Fig. 1C), consistent with the observation that CAM interferes with the puromycin reaction as well as the binding of small tRNA fragments to the A site of the PTC.7 An earlier CAM-D50S structure at lower resolution8 reported CAM-1 with a different orientation, rotated by ∼180◦ , with respect to the CAM-T70S/E70S structures (Fig. 1D). Furthermore, the phenyl ring was orthogonal to the plane of the phenyl-moiety of the A-tRNA. In contrast, no c 2011 New York Academy of Sciences. Ann. N.Y. Acad. Sci. 1241 (2011) 1–16 Wilson Antibiotics targeting the large ribosomal subunit Table 1. Structures of similar antibiotic–ribosome complexes available from different species Antibiotic Chloramphenicols Chloramphenicol Chloramphenicol Chloramphenicol Chloramphenicol Lincosamides Clindamycin Clindamycin Clindamycin Macrolide/ketolides Azithromycin Azithromycin Azithromycin Azithromycin Carbomycin A Carbomycin (Josamycin) Erythromycin Erythromycin Erythromycin Erythromycin Erythromycylamine Lankamycin Methymycin Rapamycin RU-69874 Solithromycin (CEM-101) Telithromycin Telithromycin Telithromycin Telithromycin Triacyloleandomycin Troleandromycin Tylosin Oxazolidinones Linezolid Linezolid Pleuromutilins Tiamulin Tiamulin Sparsomycin Sparsomycin Sparsomycin Complex Species Res (Å) PDB ID Reference 50S subunit 50S subunit 70S ribosome 70S ribosome D.r H.m E.c T.t 3.5 3.0 3.2 3.0 1K01 1NJI 3OFA-D 3OGE/Y, 3OH5/7 1 2 3 4 50S subunit 70S ribosome 50S subunit–G2099A D.r E.c H.m 3.1 3.2 3.0 1JZX 3OFX-Z, 3OG0 1YJN 1 3 5 50S subunit 50S subunit 50S subunit–G2099A 70S ribosome 50S subunit 50S subunit 50S subunit 50S subunit–G2099A 70S ribosome 70S ribosome 50S subunit 50S subunit 50S subunit 50S subunit 50S subunit 70S ribosome 50S subunit 50S subunit–G2099A 70S ribosome 70S ribosome 50S subunit 50S subunit 50S subunit D.r H.m H.m T.t H.m D.r D.r H.m E.c T.t D.r D.r D.r D.r D.r E.c D.r H.m E.c T.t H.m D.r H.m 3.2 3.2 2.4 3.0 3.0 3.3 3.5 2.7 3.1 3.0 3.6 3.3 3.7 3.8 3.6 3.3 3.4 2.6 3.3 3.1 2.9 3.4 3.0 1NWY 1M1K 1YHQ 3OHY/Z/0/1 1K8A 2O44 1JZY 1YI2 3OFO-R 3OHC/D/J/K 2O43 3PIO 3FWO 1Z58 2O45 1VT2, 3OR9/A/B 1P9X 1YIJ 3OAQ-T 3OI2-5 3I56 1OND 1K9M 6 7 5 4 7 8 1 5 3 4 8 9 10 11 8 12 13 5 3 4 14 15 7 50S subunit 50S subunit D.r H.m 3.5 2.7 3DLL 3CPW 16 17 50S subunit 50S subunit D.r H.m 3.5 3.2 1XBP 3G4S 6 18 50S subunit 50S subunit D.r H.m 3.5 3.2 1NJM/N 1M90, 1VQ8/9 19 2, 20 T.t, Thermus thermophilus; D.r, Deinococcus radiodurans; E.c, Escherichia coli ; H.m, Haloarcula marismortui. c 2011 New York Academy of Sciences. Ann. N.Y. Acad. Sci. 1241 (2011) 1–16 3 Antibiotics targeting the large ribosomal subunit Wilson CAM-1 binding site was observed in H50S structures solved with 20 mM CAM; however, a second binding site (CAM-2) was detected deeper within the tunnel, overlapping the binding position of the macrolide erythromycin (ERY) (Fig. 1E).9 Equilibrium dialysis studies reported two binding sites for CAM on bacterial ribosomes, one with high affinity (K d 2 M) and the other of low affinity (K d 200 M),10 which could reflect the CAM-1 and CAM-2 sites, respectively. Although no evidence for CAM-2 was seen in the E70S structure determined using 0.5 mM CAM,6 cross-linking of CAM to E. coli and archaeal H. halobium ribosomes identified modifications within the ERY binding site consistent with the CAM-2 position.11 Nevertheless, it seems unlikely that the CAM-2 site is critical for the inhibitory mechanism of the drug because of its low affinity and that most of the CAM resistance mutations and modifications cluster around CAM-1 within the A site of the PTC.3,12 CAM is well known as an elongation inhibitor since addition of the drug to growing bacterial cells stabilizes polysomes. The inhibitory action of CAM is dependent on the nature of the substrates: translation of synthetic mRNAs encoding bulky aromatic side chains, such as poly(U) for Phe, or poly(UA) for Tyr/Ile, is less effectively inhibited by CAM than translation of mRNAs encoding smaller or charged amino acids, such as poly(A) for Lys, poly(C) for Pro, or poly(UC) for Ser/Leu (reviewed by Pestka13 ). Similarly, higher concentrations of CAM are required to inhibit the ribosome binding of the tRNA fragments, such as CCA-Phe, compared to CCALys, CCA-Leu, or CCA-Ser.10 Collectively, these data suggest that some aa-tRNAs compete better with CAM due to their higher affinity for the A site of the ribosome. The nature of the P site substrate also influences CAM activity: CAM inhibits the puromycin reaction with AcPhe-tRNA in the P site, but not with Ac(Phe)2 -tRNA and CAM can even induce dissociation of Ac(Phe)2–4 -tRNAs from the ribosome,14 suggesting that the polypeptide chain attached to the P site can antagonistically influence activity of CAM bound in the A site. In contrast, CAM can also act synergistically with the P site substrate and has been shown to stimulate the binding of CACCA-NAcLeu fragments to the P site of the ribosome.15 Thiamphenicol (an analog of CAM where the para-nitro moiety is replaced by a methylsulfonyl moiety) is also reported to stabilize the 4 binding of fMet- and AcPhe-tRNAs to the P site of the ribosome.16 These data are consistent with the CAM-1 position from the E70S (or T70S) structures5,6 where the para-nitro/methyl-sulfonyl moiety of CAM/thiamphenicol is in close proximity to the aminoacyl moiety of the P-tRNA. Linezolid and interaction with the P-tRNA Linezolid (LIN), a synthetic compound belonging to the oxazolidinone class of antibiotics, is used clinically to treat a variety of Gram-positive infections (reviewed by Leach17 ). LIN comprises three aromatic rings with an acetamidomethyl tail attached to the pharmacokinetic oxazolidinone ring A (Fig. 2A). A wealth of biochemical and structural evidence (reviewed by Wilson3 ) indicates that LIN binds at the PTC of the large subunit, in a position overlapping with the aminoacyl-moiety of an A site bound tRNA (Fig. 2B). There is an excellent overall agreement in the LIN binding position derived from cross-linking data18 and subsequently visualized using X-ray crystallography (Fig. 2C).19,20 In each case, the acetamidomethyl tail of LIN extends down the tunnel toward A2503 (E. coli numbering is used throughout) of the 23S rRNA, whereas the morpholino ring C approaches U2585 (Fig. 2D). Differences are nevertheless observed when comparing the structures of the bacterial LIN-D50S20 and archaeal LIN-H50S19 structures. First, the fluorophenol ring B of LIN is rotated by ∼70◦ relative to the oxazolidinone ring A between the two structures (Fig. 2C). The outcome being that in the LIN-D50S structure the fluorophenyl ring is sandwiched between A2451 and U2506, whereas in the LIN-H50S the same ring stacks on C2452. This may result partially from the shifted position of U2506 in D50S, which would clash with LIN-H50S binding position (Fig. 2D). But the difference could also be due to the presence of the CCA-Phe bound in the P site of the H50S-LIN structure since it would encroach on the fluorine atom of LIN in the D50S structure (Fig. 2D). Second, differences are observed in the location and flexibility of U2504 between bacterial and archaeal ribosome structures: in the former, U2504 is observed stacking upon the oxazolidinone ring A of LIN. In contrast, an alternate conformation is observed for U2504 in archaeal (H50S) structures, except when LIN is bound. This indicates that binding of LIN to H50S reorients U2504 to allow stacking with the oxazolidinone ring of LIN as seen in c 2011 New York Academy of Sciences. Ann. N.Y. Acad. Sci. 1241 (2011) 1–16 Wilson Antibiotics targeting the large ribosomal subunit Figure 2. Linezolid (LIN) binds at the A site of the PTC. (A) Chemical structure of LIN. (B) Binding position of LIN20 (gold), relative to A- (blue) and P-tRNA (green).74 (C) Comparison of LIN from D50S,20 H50S,19 and E50S18 structures. (D) Binding position of LIN at the PTC of D50S,20 (gold) and H50S (blue) with P-tRNA74 (green). (E) Comparison of 23S rRNA nucleotides neighboring U2504: bacteria have A2572 (yellow-orange), whereas archaea have U2572 (blue). Arrows indicates different position of U2504 in native H50S75 (light blue) versus LIN-H50S19 (dark blue). the LIN-D50S (arrowed in Fig. 2E). In bacteria, the presence of a bulkier adenine at position 2572 (instead of uridine, as in H50S) would prevent U2504 from rotating back (Fig. 2E).19 High concentrations (5 mM) of LIN were used in the LIN-H50S structure, which may have been necessary to mobilize U2504,19 whereas 1,000-fold lower concentrations (5 M) were used for the bacterial structure.20 Surprisingly, however, the archaea Halobacterium halobium is reported as being sensitive to LIN, with a minimal inhibitory concentration of 3 M,21 making it unclear why such high LIN concentrations were used for H50S. Despite the detailed structural characterization of LIN on the ribosome, the exact mechanism of action of oxazolidinones still remains unclear. Based on the binding position, LIN should prevent correct placement of the aminoacyl-moiety of the A-tRNA and in doing so inhibit peptide-bond formation. Surprisingly, there are conflicting reports as to the ability of different oxazolidinone members to inhibit the puromycin reaction.3 Moreover, when inhibition is reported, nonphysiologically high concentrations of a drug (∼1 mM) are required. Similarly, while oxazolidinones are observed to compete effectively for ribosome binding with CAM, lincosamides, and puromycins, the IC50 s are in the ∼1 mM range.22,23 In contrast, the IC50 for LIN determined using in vitro translation systems is significantly lower (∼1– 10 M),23–25 suggesting that LIN targets a particular functional state of the ribosome. In this respect it is interesting that Ippolito et al.19 report that the c 2011 New York Academy of Sciences. Ann. N.Y. Acad. Sci. 1241 (2011) 1–16 5 Antibiotics targeting the large ribosomal subunit Wilson quality of the electron density for LIN was superior in the presence of an aminoacyl-tRNA mimic CCA-Phe, suggesting that the interaction with the P-tRNA increases the affinity of LIN for the ribosome. Indeed, oxazolidinones have been reported to crosslink to small tRNA-sized molecules on the ribosome.26 Superimposition of the recently determined structure of ribosome stalled with a nascent polypeptide chain attached to the P-tRNA,27 predicts that LIN would also interact with the C-terminal 2–4 amino acids of the nascent chain (Fig. 2B). It will be interesting to examine the influence of the nature of the aminoacyl or peptidy moiety of the P-tRNA on the binding and inhibition of oxazolidinones in order to define exactly which functional state is targeted by this class of antibiotics. The orientation of the clindamycin tail and A site inhibition Clindamycin (CLN) is a semisynthetic lincosamide that contains a galactose sugar linked to a propylpyrrolidinyl moiety (Fig. 3A). CLN is active against most Gram-positive bacteria as well as some protozoa, such as Plasmodium falciparum (reviewed by Spizek28 ). Crystal structures of CLN bound to bacterial (D50S and E70S)6,8 and archaeal (H50S)29 ribosomes (Table 1) locate the drug to the A site of the PTC (Fig. 3B). The galactose sugar of CLN is Figure 3. Clindamycin (CLN) binds at the A site of the PTC. (A) Chemical structure of CLN. (B) CLN bound to D50S8 (pink), H50S29 (orange), and E70S6 (gold), relative to A- (blue) and P-tRNA (green).74 (C) Binding position of CLN-E70S6 (gold), and ERY-E70S6 (tan) relative to A-tRNA74 (blue) and TnaC peptidyl-tRNA27 (green). (D) Conformation of 23S rRNA nucleotides comprising the CLN binding pocket of D50S8 (pink), H50S29 (orange), and E70S6 (gold). The arrow indicates the different position of the propyl-tail of CLN in D50S versus E70S and H50S. 6 c 2011 New York Academy of Sciences. Ann. N.Y. Acad. Sci. 1241 (2011) 1–16 Wilson Antibiotics targeting the large ribosomal subunit Figure 4. Sparsomycin (SPAR) stabilizes the P-tRNA at the PTC. (A) Chemical structure of SPAR. (B) SPAR (orange) bound to H50S9 (yellow), relative to A- (blue) and P-tRNA (green).74 Difference of A2602 conformation in SPAR-H50S (yellow) and PRE-state H50S (cyan) is arrowed. (C) as in (B), but including SPAR (blue) and A2602 (light blue) from the SPAR-D50S structure.36 located in a position approaching the binding position of the desosamine sugar of the macrolide ERY, whereas the pyrrolidinyl moiety overlaps the binding site of CAM as well as the aminoacyl moiety of an A-tRNA (Fig. 3C). Consistently, lincosamides have been shown to directly inhibit the transfer of fMet or AcPhe to puromycin30,31 as well as compete with both CAM and ERY for ribosome binding.32 The galactose sugar of CLN is located similarly in all three structures,6,8,29 where it interacts with A2503, A2058, and A2059 (Fig. 3D), providing an explanation as to how modification or mutation of these nucleotides can confer resistance to lincosamides.12,33 In contrast, the propyl-pyrrolidinyl moiety of CLN in the D50S structure8 is rotated by ∼90◦ when compared with the H50S29 and E70S6 structures (Fig. 3B and D). As stated previously,4 the lack of density for the propyl extension of CLN in the D50S structure led to a placement based on the available small molecule structure, and it was also noted that the conformation of pyrrolidinyl moiety of CLN as reported for the H50S structure would fit the density equally well. Support for the H50S/E70S conformation comes, first, from the orientation of U2504 that is invariant in the available bacterial structures and, second, from observation that the CLN-D50S position8 requires some adjustment to U2504 and C2452 to accommodate the propyl tail (Fig. 3D). Nevertheless, the poor density for the propyl tail in the D50S structure, suggests that it is highly flexible and therefore may contribute less to the binding of CLN to the ribosome. From a drug–design perspective, this region of the molecule may provide avenues for modification to develop successive generations of lincosamide antibiotics with improved ribosome interactions and/or pharmacological properties. Sparsomycin interaction with A2602 promotes translocation Sparsomycin (SPAR), a nucleoside analog of uracil (Fig. 4A) produced by Streptomyces sparsogenes, is a potent inhibitor of peptidyltransferase activity in bacteria, archaea, and eukaryotes (reviewed by Lazaro et al.34 ). SPAR has been crystallized together with tRNA mimics in complex with both archaeal (H50S)9,35 and bacterial (D50S)36 ribosomes (Table 1). In both cases, although the uracil moiety of SPAR was seen to stack upon A2602, consistent with previous crosslinking data,37 dramatic differences in the overall SPAR binding positions were observed (Fig. 4B and C). Bound to H50S, SPAR stacks between A2602 and the P site CCA-Phe analog (Fig. 4B), consistent with the observation that SPAR requires a P site substrate for ribosome binding.38 Binding of the CCA-Phe analogs normally distributes evenly between the A and P sites in the H50S crystals, but in the presence of SPAR, CCAPhe occupies only the P site.9,35 This is understandable since the conjugated tail of SPAR overlaps with the aminoacyl-moiety of an A-tRNA (Fig. 4B) and thus would prevent binding not only of A-tRNA, but also CAM and lincomycin in agreement with previous reports.34,39 The binding site of SPAR in the D50S structures in contrast spans across the P site and does not encroach on the A site (Fig. 4C).36 Although SPAR was also crystallized together with a tRNA substrate in the D50S structure, no interaction was observed between SPAR and the tRNA c 2011 New York Academy of Sciences. Ann. N.Y. Acad. Sci. 1241 (2011) 1–16 7 Antibiotics targeting the large ribosomal subunit Wilson substrate. In fact, an identical SPAR binding position was observed in the absence of the tRNA substrate.36 The SPAR-D50S binding position is hard to reconcile with the ability of SPAR to stabilize the P site substrate, since the reported binding position would clash with P-tRNA (Fig. 4C). Unlike the H50S crystals, binding of the tRNA substrates in the D50S crystals appears to favor the A site,36 which may have precluded formation of the stable functional state observed in the H50S structure. Pretranslocation (PRE) state ribosomes with tRNAs in both A and P sites are not protected from the action of SPAR, since SPAR can induce translocation of the tRNAs with the outcome that the peptidyl-tRNA is stabilized at the P site and the A site is blocked by the drug.40 A2602 is at the center of the rotational symmetry of the PTC where it has been proposed to play a role in guiding the CCA-end of the A-tRNA to P site during translocation.41 SPAR appears to induce a rotation of A2602 compared to the PRE state conformation (Fig. 4B), which may reflect the two-step reaction mechanism of SPAR—a slow ini- tial step that isomerizes slowly to adopt a more stable conformation.42 In this regard, the position of SPAR observed in the D50S structure (Fig. 3C) may reflect an initial binding event, postpeptide bond formation, where the deacylated tRNA is driven by SPAR from the P to the E site. Subsequently, the peptidyl-tRNA can then move into the P site where it is stabilized by SPAR, as observed in the H50S structures9,35 (Fig. 2B). Pleuromutilins overlap both the A and P sites at the PTC Pleuromutilin was discovered in the 1950s as a natural product of two basidiomycete species, Pleurotus mutilis and Pleurotus passeckerianus, and displays activity against Staphlococcus aureus strains (reviewed by Novak43 ). Modification of the pleuromutilin C14 tail led to the development of semi-synthetic derivatives, such as tiamulin (TIA, Fig. 5A),44 which was approved for veterinary usage in the late 1970s. TIA has been crystallized in complex with bacterial (D50S)45 and archaeal (H50S)46 Figure 5. Tiamulin (TIA) binds across the A and P sites at the PTC. (A) Chemical structure of TIA. (B) TIA bound to D50S45 (gold) and H50S46 (blue), relative to A- (blue) and P-tRNA (green).74 (C) TIA bound to D50S45 (gold), relative to A-tRNA74 (blue) and TnaC peptidyl-tRNA27 (green). (D) Conformation of 23S rRNA nucleotides comprising the TIA binding pocket of D50S45 (gold) and H50S46 (blue). The arrow indicates the different position of U2504 in D50S versus H50S. (E) as (D), but including additional pleuromutilin structures.49 8 c 2011 New York Academy of Sciences. Ann. N.Y. Acad. Sci. 1241 (2011) 1–16 Wilson large subunits (Table 1). These structures reveal that the tricyclic mutilin core of TIA (Fig. 5A) overlaps the binding site of aminoacyl-moiety of an A-tRNA (Fig. 5B and C), consistent with the finding that TIA competes with CAM and puromycin for ribosome binding and prevents binding of aa-tRNAs to the A site.47,48 In both structures, the C14-tail of TIA extends toward the P site, although there is some difference in the extent of overlap with the P-tRNA (Fig. 5B and C). This distinction results partially from the shifted position of the mutilin core, which is located deeper in the A site of the H50S, but mainly from the more extended conformation of the C14-tail of TIA in the D50S structure (Fig. 5C). In TIA-D50S, U2504 make an important contribution to the binding pocket of the mutilin core of TIA, whereas in the H50S structure the equivalent base is shifted away. This probably explains why 1 mM TIA was used for TIA-H50S,46 compared to 10 M (100× less) in TIA-D50S.45 Nevertheless, the shifted position of U2504 is unlikely to account for the shift in location of the mutilin core between TIA-H50S and TIA-D50S, since subsequent structures of other pleuromutilin derivatives bound to D50S49 reveal identical mutilin core placements as in the TIA-H50S, yet the U2504 maintains a similar confirmation to that in TIA-D50S (Fig. 5D). Biochemical analyses indicate that TIA can destabilize binding of fMet-tRNA to the ribosome.47,50 The structures indicate that the C14-tail could perturb the placement of the aminoacyl-moiety of the P-tRNA, accounting for tRNA drop-off. However, the destabilizing effect is modest, indicating that simultaneous cohabitation of the initiator tRNA and TIA may occur. Nevertheless, any initiation complexes that do form are nonproductive, since TIA is a potent peptidyltransferase inhibitor by preventing A-tRNA binding.47 Pleuromutilins cannot, however, inhibit translating ribosomes,50 suggesting that binding of TIA to ribosomes containing peptidyl-tRNAs is prevented. This is easy to envisage since (i) TIA binding, in particular the accommodation of the C14-tail, would be sterically blocked by the presence of a polypeptide chain (Fig. 5C), and furthermore (ii) the polypeptide chain in the tunnel stabilizes the P-tRNA, preventing peptidyltRNA drop-off. Thus, the mechanism of action of pleuromutilins is likely to be, on one hand, to prevent initiation complex formation by perturbing the stable binding of the fMet-tRNA to the P site—an Antibiotics targeting the large ribosomal subunit ability that is likely to be influenced by the length and nature of the C14-tail of the pleuromutilin, and on the other hand, if initiation complex formation does occur, then the A site location of the mutilin core would prevent delivery of the aa-tRNA to the A site and thus formation of the first peptide bond. Such a model is in agreement with the observation that addition of pleuromutilins to intact cells causes a loss of polysomes and a concomitant stabilization of 70S monosomes.50 The influence of species specificity on macrolide-ribosome interaction Macrolides represent a large class of polyketide compounds synthesized by actinomycetes, which inhibit protein synthesis on eubacterial, but not archaeal or eukaryotic ribosomes.51,52 Macrolide antibiotics bind adjacent to PTC, within the tunnel through which the polypeptide chain traverses during translation (Fig. 6A). The binding site of macrolides is vacant on free or initiating ribosomes, but unavailable in elongating ribosomes.53,54 Generally, the presence of macrolide antibiotics within the ribosomal tunnel restricts protein synthesis to short oligopeptides, which eventually dissociate from the ribosome in the form of oligopeptidyl-tRNAs.55–57 However, recent evidence has indicated that the inhibitory effect of macrolides appears to be also dependent on the sequence of the nascent polypeptide chain, such that some sequences can even escape the inhibitory effect of the drug.58 Clinically used macrolides have 14-, 15-, or 16-membered lactone rings to which amino sugars are attached at varying positions. For example, the macrolide ERY has a 14-membered ring with cladinose and desosamine sugars attached at the C3 and C5 positions, respectively (Fig. 6B). To date, there are X-ray structures of ERY bound to four different ribosomal particles; three bacterial (E70S, T70S, and D50S) and one archaeal (H50S) (Table 1). Comparison of the structures reveals that the binding position of ERY is identical in the T70S,5 E70S6 and H50S29 complexes (Fig. 6C). Within the limits of the resolution, the conformation and placement of the lactone ring and amino sugars as well as the interactions established with the ribosome appear to be conserved. This contrasts with the ERY-D50S8 and chemical similar erythromycylamine-D50S (ERC-D50S)59 structures, where the lactone ring conformations are markedly divergent (Fig. 6C). c 2011 New York Academy of Sciences. Ann. N.Y. Acad. Sci. 1241 (2011) 1–16 9 Antibiotics targeting the large ribosomal subunit Wilson Figure 6. The macrolides ERY and TAO bind in the ribosomal tunnel. (A) Transverse section of the large subunit showing the ribosomal tunnel and the relative positions of P-tRNA (green), macrolide (red), and path of the polypeptide chain (tan). (B–C) Chemical structures of ERY and TAO. (C–D) Comparison of the binding positions of ERY bound to D50S (salmon), E70S6 (gold), T70S5 (tan), and H50S29 (cyan) with erythromycylamine59 (ERC, pink), lankamycin60 (LNK, green), and RU6987459 (RUM, blue) bound to the D50S. (E) Comparison of the binding positions of ERY bound to E70S6 (gold) with TAO bound to D50S62 (pink) and H50S61 (cyan). The arrow indicates the difference between L22 in the native D50S (ribbon)76 and TAO-D50S62 (ball-trace). This is particularly surprising since subsequent D50S structures of the 14-membered macrolides RU69874 (RUM)59 and lankamycin (LNK)60 are nearly identical to the ERY-E70S structure (Fig. 6D). This observation is consistent with the re-examination of the ERY-D50S at higher resolution, which indicated that the conformation of lactone ring of ERY-D50S is in fact similar to that reported in the ERY-H50S structure.4 What was not addressed is the conformation of 14-membered macrolide troleandomycin (TAO). TAO is chemically similar to ERY (Fig. 6B) and, as expected, binds to H50S61 in a very similar fashion to all other 14-membered macrolides, e.g., ERY (Fig. 6E). In contrast, it is hard to rationalize why a completely different TAO binding site is reported in D50S structure.62 In D50S, TAO is reported to be located 10 deeper in the tunnel, with the C11-acetylation overlapping the Arg111 sidechain of ribosomal protein L22.62 This steric clash is suggested to be sufficient to completely destabilize the tip of the -hairpin of L22, causing it to flip into the lumen of the tunnel (Fig. 6E).62 The flipped L22 position was proposed to play a role in translational stalling;62 however, no such conformational change in L22 is evident in the recent structures of translationally stalled ribosomes.27,63,64 Nevertheless, detachment of the tip of L22 has been observed when three amino acids are deleted in L17 (L22 homolog) of H50S.29 The emergence of bacterial resistance to macrolide antibiotics has led to the development of ketolides, such as telithromycin (TEL), which are semisynthetic derivatives of macrolides where the C3-sugar is replaced with a keto group c 2011 New York Academy of Sciences. Ann. N.Y. Acad. Sci. 1241 (2011) 1–16 Wilson Antibiotics targeting the large ribosomal subunit Figure 7. Binding of TEL and AZI within the ribosomal tunnel. (A) Chemical structure of TEL. (B–C) Comparison of the binding positions of ERY-E70S6 (gold) with (B) TEL bound to E70S6 (blue) and H50S29 (cyan), and (C) TEL bound to T70S5 (green) and D50S65 (salmon). (D) Chemical structure of AZI. (E–F) Comparison of the binding positions of AZI-T70S5 (orange) with (E) ERY-E70S6 (gold) and AZI-H50S29 (cyan) and (F) AZI-D50S69 (salmon). (G) Binding positions of AZI-1 and AZI-2 in D50S69 (red), relative to L22 (orange). (Fig. 7A). The broader spectrum of activity of the ketolides is also related to the presence of additional modifications and side chains, such as the alkyl–aryl sidechain of TEL (Fig. 7A). TEL has been crystallized in complex with the D50S,65 H50S,29 T70S,5 and E70S6 (Table 1). Comparison of TEL in the H50S, E70S, and T70S structures indicates that the lactone ring and desosamine sugar are positioned in an identical fashion as ERY bound to E70S (Fig. 7B and C). Although the lactone ring of the TEL in the D50S structure appears to deviate significantly,65 reexamination of the TEL-D50S structure at higher resolution indicated that the conformation of lactone ring is in fact similar to that reported in the TEL-H50S structure.4 In contrast to the lactone ring, the placement of the alkyl–aryl side chain of TEL differs dramatically between different species: bound to the archaeal H50S,4 the TEL-side chain folds back across the lactone ring and stacks upon C2609 whereas in the bacterial E70S6 the side chain of TEL is rotated by 120◦ and stacks upon the A752-U2609 base-pair. This Watson–Crick base-pair cannot form in H50S because the equivalent bases to A752 and U2609 are both pyrimidines, providing an explanation for the alternate position in H50S. A similar stacking arrangement of the alkyl–aryl side chain is also seen for the fluoroketolide solithromycin (CEM-101) bound to the E70S66 as well as for TEL bound to T70S (Fig. 7C).5 Like H50S, D50S also has two pyrimidines at the equivalent positions to 752 and 2609; however, unlike H50S, the side chain of TEL does c 2011 New York Academy of Sciences. Ann. N.Y. Acad. Sci. 1241 (2011) 1–16 11 Antibiotics targeting the large ribosomal subunit Wilson not stack upon U2609, but rather adopts a unique conformation contacting C790 (Fig. 7C).65 In H50S, the equivalent base to C790 is rotated away and unavailable for interaction (arrowed in Fig. 7C). While C790 is available for stacking in E70S and T70S, the interaction with A752-U2609 appears to be energetically more favorable. Similar interactions with the A752-U2609 are predicted for the Kosan ketolide K1325, where the alkyl–aryl side chain is attached to the lactone ring at the C13 position.67 Thus, in contrast to the lactone ring, the attached heterocyclic side chain of TEL interacts with fewer conserved regions of the ribosome, which allows distinct conformations to be adopted across different organisms, and even between distinct bacterial species. Azalide antibiotics, such as azithromycin (AZI), are semisynthetic derivatives of ERY composed of a 15-membered lactone ring. AZI differs from ERY by the absence of a keto-oxygen (C9) and the addition of a methyl-nitrogen at the C10 position (Fig. 7D). The insertion of the methyl-substituted nitrogen in the lactone ring increases the acid stability and bioavailability compared to ERY. AZI has been crystallized in complex with the H50S,29,68 T70S,5 and D50S69 (Table 1). Given the chemical similarity to ERY, it is not surprising that the binding mode of AZI on the H50S and T70S is near identical to ERY-E50S (Fig. 7E). A similar binding site is observed on D50S; however, a slight shift is reported in the position of the desosamine sugar as well as a deviation in the location of the distal region of the lactone ring (Fig. 7F).69 It is possible that the latter deviation in the lactone results, in part, from the unexpected finding that a second molecule of AZI (AZI-2) interacts with this region of AZI-1 in D50S (Fig. 7G).69 In addition, AZI-2 interacts predominantly with the tip of L22 (Fig. 7G), suggesting that differences in the sequence of this region of L22 are responsible for influencing the binding of AZI-2 to ribosomes of different species. Kinetic and binding data also support the cooperative interaction of two AZI molecules in D50S and a single-binding site on E70S ribosomes.70 The 16-membered macrolides, such as tylosin (TYL) and carbomycin (CAR), generally contain mycaminose-mycarose disaccharides attached to the C5 position (Fig. 8A and B). The crystal structure of TYL bound to the H50S68 reveals that despite the larger size, the placement of the lactone ring and C5-sugar is very similar to that observed for ERY 12 (Fig. 8C). Interestingly, TYL contains a C6-ethyl aldehyde (Fig. 8A and B) that forms a covalent interaction with the N6 of A2602 (Fig. 8C and D). Biochemical studies have indicated that modifications that abolish the potential of TYL to form a covalent bond with A2062 dramatically reduce the binding and inhibitory properties of the drug.58 CAR, and the structurally related josamycin (JOS), bind to the ribosome analogously as TYL and also form a carbinolamine bond with A2062 (Fig. 8D).59,68 One difference of CAR and JOS from TYL is the presence of an isovalerate extension on the C5-disaccharide (compare Fig. 8A and B). In the context of the ribosome, the C5-sugars extend from the macrolide binding site in the tunnel back up toward the PTC, such that the isovalerate extension of CAR/JOS overlaps the binding position of the A-tRNA (Fig. 8E). This is consistent with the correlation between the length of the C5-extension and the ability to inhibit the peptidyltransferase activity, i.e., CAR completely inhibits, TYL has a moderate effect (60%), and ERY does not inhibit the reaction at all.71 The corollary is that macrolides with C5-disaccharides, such as TYL, generally permit synthesis of 2–4 amino acids before peptidyl-tRNA drop-off occurs, whereas macrolides with C5-monosaccharides like ERY allow synthesis of oligopeptides of 6–8 amino acids in length.57 Curiously, the crystal structure of a small 12-membered monosugar macrolide mythymycin (MYT) bound to the D50S reveals that the drug does not bind in the tunnel analogously to the other macrolides, but rather at the PTC in the position overlapping the A-tRNA72 (Fig. 8F). Based on its binding position, MYT would be expected to inhibit peptide-bond formation and prevent synthesis of oligopeptides. In contrast, although the large polyketide compound rapamycin (RAP) was shown to bind within the tunnel of the D50S,73 the binding site is located adjacent to canonical macrolide binding site (Fig. 8F). In this position, the lumen of the tunnel remains unobstructed, explaining the lack of effect that RAP has on translation in bacteria.73 Conclusion The vast plethora of crystal structures of antibiotic– ribosome complexes has strengthened our understanding of the conserved features that antibiotics use to interact with the ribosome. At the same time, these structures also highlight some differences that arise due to species-specific differences as well as the c 2011 New York Academy of Sciences. Ann. N.Y. Acad. Sci. 1241 (2011) 1–16 Wilson Antibiotics targeting the large ribosomal subunit Figure 8. Binding of TYL, CAR, and JOS within the ribosomal tunnel. (A–B) Chemical structure of (A) TEL and (B) CAR. (C) Comparison of the binding positions of ERY-E70S6 (gold) and TYL-H50S68 (cyan). (D) Comparison of the binding positions of JOS-D50S59 (salmon) and CAR-H50S68 (cyan). (E) Comparison of ERY-E50S6 (gold), TYL-H50S68 (cyan), and JOS-D50S (salmon), relative to A- (blue) and P-tRNA (green).74 (F) Comparison of ERY-E50S6 (gold), relative to MYT-D50S72 (green), RAP-D50S73 (cyan), ribosomal protein L22, and A-tRNA (blue).74 functional state of the ribosome—aspects that are likely to be critical for the binding and inhibitory activity of the antibiotics. This review provided a structural basis for the species-specific differences and indicated, where possible, how the nature of the tRNA substrates and nascent chain can have a dramatic influence on the effectiveness of antibiotic action. A challenge for the future is to better define the physiological functional states that are targeted by different classes of antibiotics, both biochemically and structurally. Such studies will not only provide insight into the mechanism of action of the antibiotics but could also provide a platform upon which to design new improved antimicrobial agents. Conflicts of interest The authors declare no conflicts of interest. References 1. Schmeing, T.M. & V. Ramakrishnan. 2009. What recent ribosome structures have revealed about the mechanism of translation. Nature 461: 1234–1242. 2. Sohmen, D., J.M. Harms, F. Schlunzen & D.N. Wilson. 2009. Enhanced SnapShot: antibiotic inhibition of protein synthesis II. Cell 139: 212–212 e211. 3. Wilson, D.N. 2009. The A–Z of bacterial translation inhibitors. Crit. Rev. Biochem. Mol. Biol. 44: 393–433. 4. Wilson, D.N., J.M. Harms, K.H. Nierhaus, et al. 2005. Species-specific antibiotic–ribosome interactions: implications for drug development. Biol. Chem. 386: 1239– 1252. c 2011 New York Academy of Sciences. Ann. N.Y. Acad. Sci. 1241 (2011) 1–16 13 Antibiotics targeting the large ribosomal subunit Wilson 5. Bulkley, D., C.A. Innis, G. Blaha & T.A. Steitz. 2010. Revisiting the structures of several antibiotics bound to the bacterial ribosome. Proc. Natl. Acad. Sci. USA 107: 17158–17163. 6. Dunkle, J.A., L. Xiong, A.S. Mankin & J.H. Cate. 2010. Structures of the Escherichia coli ribosome with antibiotics bound near the peptidyl transferase center explain spectra of drug action. Proc. Natl. Acad. Sci. USA 107: 17152–17157. 7. Celma, M.L., R.E. Monro & D. Vazquez. 1971. Substrate and antibiotic binding sites at the peptidyl transferase centre of E. coli ribosomes: binding of UACCA-leu to 50S subunits. FEBS Lett. 13: 247–251. 8. Schlünzen, F. et al. 2001. Structural basis for the interaction of antibiotics with the peptidyl transferase centre in eubacteria. Nature 413: 814–821. 9. Hansen, J.L., P.B. Moore & T.A. Steitz. 2003. Structures of five antibiotics bound at the peptidyl transferase center of the large ribosomal subunit. J. Mol. Biol. 330: 1061–1075. 10. Lessard, J.L. & S. Pestka. 1972. Studies on the formation of transfer ribonucleic acid-ribosome complexes: 23. Chloramphenicol, aminoacyl-oligonucleotides, and Escherichia coli ribosomes. J. Biol. Chem. 247: 6909–6912. 11. Long, K.S. & B.T. Porse. 2003. A conserved chloramphenicol binding site at the entrance to the ribosomal peptide exit tunnel. Nucleic Acids Res. 31: 7208–7215. 12. Kehrenberg, C., S. Schwarz, L. Jacobsen, et al. 2005. A new mechanism for chloramphenicol, florfenicol and clindamycin resistance: methylation of 23S ribosomal RNA at A2503. Mol. Microbiol. 57: 1064–1073. 13. Pestka, S. 1977 In Molecular Mechanisims of Protein Biosynthesis. H. Weissbach & S. Pestka, Eds.: 467–553. Academic Press Inc. New York. 14. Rheinberger, H.J. & K.H. Nierhaus. 1990. Partial release of AcPhe-Phe-transfer RNA from ribosomes during poly(U)dependent poly(Phe) synthesis and the effects of chloramphenicol. Eur. J. Biochem. 193: 643–650. 15. Ulbrich, B., G. Mertens & K.H. Nierhaus 1978. Cooperative binding of 3’ fragments of tRNA to the peptidyltransferase centre of E. coli ribosomes. Arch. Biochem. Biophys. 190: 149–154. 16. Contreras, A., M. Barbacid & D. Vazquez. 1974. Binding to ribosomes and mode of action of chloramphenicol analogues. Biochem. Biophys. Acta 349: 376–388. 17. Leach, K.L., S.J. Brickner, M.C. Noe & P.F. Miller. 2011. Linezolid, the first oxazolidinone antibacterial agent. Ann. N.Y. Acad. Sci. 1222: 49–54. 18. Leach, K.L. et al. 2007. The site of action of oxazolidinone antibiotics in living bacteria and in human mitochondria. Mol. Cell 26: 393–402. 19. Ippolito, J.A. et al. 2008. Crystal structure of the oxazolidinone antibiotic linezolid bound to the 50S ribosomal subunit. J. Med. Chem. 51: 3353–3356. 20. Wilson, D.N. et al. 2008. The oxazolidinone antibiotics perturb the ribosomal peptidyl-transferase center and effect tRNA positioning. Proc. Natl. Acad. Sci. USA 105: 13339– 13344. 21. Kloss, P., L. Xiong, D.L. Shinabarger & A.S. Mankin. 1999. Resistance mutations in 23 S rRNA identify the site of action of the protein synthesis inhibitor linezolid in the ribosomal peptidyl transferase center. J. Mol. Biol. 294: 93–101. 14 22. Lin, A.H., R.W. Murray, T.J. Vidmar & K.R. Marotti. 1997. The oxazolidinone eperezolid binds to the 50S ribosomal subunit and competes with binding of chloramphenicol and lincomycin. Antimicrob. Agents Chemother. 41: 2127–2131. 23. Skripkin, E. et al. 2008. R chi-01, a new family of oxazolidinones that overcome ribosome-based linezolid resistance. Antimicrob. Agents Chemother. 52: 3550–3557. 24. Eustice, D.C., P.A. Feldman & A.M. Slee. 1988. The mechanism of action of DuP 721, a new antibacterial agent: effects on macromolecular synthesis. Biochem. Biophys. Res. Commun. 150: 965–971. 25. Shinabarger, D.L. et al. 1997. Mechanism of action of oxazolidinones: effects of linezolid and eperezolid on translation reactions. Antimicrob. Agents Chemother. 41: 2132–2136. 26. Colca, J.R. et al. 2003. Crosslinking in the living cell locates the site of action of oxazolidinone antibiotics. J. Biol. Chem. 278: 21972–21979. 27. Seidelt, B. et al. 2009. Structural insight into nascent polypeptide chain-mediated translational stalling. Science 326: 1412–1415. 28. Spizek, J., J. Novotna & T. Rezanka. 2004. Lincosamides: chemical structure, biosynthesis, mechanism of action, resistance, and applications. Adv. Appl. Microbiol. 56: 121–154. 29. Tu, D., G. Blaha, P. Moore & T. Steitz. 2005. Structures of MLSBK antibiotics bound to mutated large ribosomal subunits provide a structural explanation for resistance. Cell 121: 257–270. 30. Kallia-Raftopoulos, S. & D.L. Kalpaxis. 1999. Slow sequential conformational changes in Escherichia coli ribosomes induced by lincomycin: kinetic evidence. Mol. Pharmacol. 56: 1042–1046. 31. Kouvela, E.C., A.D. Petropoulos & D.L. Kalpaxis. 2006. Unraveling new features of clindamycin interaction with functional ribosomes and dependence of the drug potency on polyamines. J. Biol. Chem. 281: 23103–23110. 32. Fernandez-Munoz, R., R.E. Monro, R. Torres-Pinedo & D. Vazquez. 1971. Substrate- and antibiotic-binding sites at the peptidyl transferase centre of E. coli ribosomes. Studies on the chloramphenicol, lincomycin and erythromycin sites. Eur. J. Biochem. 23: 185–193. 33. Poehlsgaard, J., P. Pfister, E.C. Bottger & S. Douthwaite. 2005. Molecular mechanisms by which rRNA mutations confer resistance to clindamycin. Antimicrob. Agents Chemother. 49: 1553–1555. 34. Lazaro, E., A.S. Felix, L.A.G.M. Vandenbroek, et al. 1991. Interaction of the antibiotic sparsomycin with the ribosome. Antimicrob. Agents Chemother. 35: 10–13. 35. Schmeing, T.M., K.S. Huang, D.E. Kitchen, et al. 2005. Structural insights into the roles of water and the 2’ hydroxyl of the P site tRNA in the peptidyl transferase reaction. Mol. Cell 20: 437–448. 36. Bashan, A. et al. 2003. Structural basis of the ribosomal machinery for peptide bond formation, translocation, and nascent chain progression. Mol. Cell 11: 91–102. 37. Porse, B.T., S.V. Kirillov, M.J. Awayez, et al. 1999. Direct crosslinking of the antitumor antibiotic sparsomycin, and its derivatives, to A2602 in the peptidyl transferase center of 23S-like rRNA within ribosome-tRNA complexes. Proc. Natl. Acad. Sci. USA 96: 9003–9008. c 2011 New York Academy of Sciences. Ann. N.Y. Acad. Sci. 1241 (2011) 1–16 Wilson 38. Monro, R.E., M.L. Celma & D. Vazquez. 1969. Action of sparsomycin on ribosome-catalysed peptidyl transfer. Nature 222: 356–358. 39. Pestka, S. 1969. Studies on the formation of transfer ribonucleic acid–ribosome complexes: XI. Antibiotic effects on phenylalanyl–oligonucleotide binding to ribosomes. Proc. Natl. Acad. Sci. USA 64: 709–714. 40. Fredrick, K. & H.F. Noller. 2003. Catalysis of ribosomal translocation by sparsomycin. Science 300: 1159–1162. 41. Agmon, I. et al. 2003. On peptide bond formation, translocation, nascent protein progression and the regulatory properties of ribosomes. Eur. J. Biochem. 270: 2543–2556. 42. Ioannou, M., C. Coutsogeorgopoulos & D. Drainas. 1997. Determination of eukaryotic peptidyltransferase activity by pseudo-first-order kinetic analysis. Anal. Biochem. 247: 115– 122. 43. Novak, R. & D.M. Shlaes. 2010. The pleuromutilin antibiotics: a new class for human use. Curr. Opin. Investig. Drugs 11: 182–191. 44. Egger, H. & H. Reinshagen. 1976. New pleuromutilin derivatives with enhanced antimicrobial activity: II. Structureactivity correlations. J. Antibiot. (Tokyo) 29: 923–927. 45. Schlunzen, F., E. Pyetan, P. Fucini, et al. 2004. Inhibition of peptide bond formation by pleuromutilins: the structure of the 50S ribosomal subunit from Deinococcus radiodurans in complex with tiamulin. Mol. Microbiol. 54: 1287–1294. 46. Gurel, G., G. Blaha, P.B. Moore & T.A. Steitz. 2009. U2504 determines the species specificity of the A-site cleft antibiotics: the structures of tiamulin, homoharringtonine, and bruceantin bound to the ribosome. J. Mol. Biol. 389: 146– 156. 47. Hodgin, L.A. & G. Hogenauer. 1974. The mode of action of pleuromutilin derivatives. Effect on cell-free polypeptide synthesis. Eur. J. Biochem. 47: 527–533. 48. Hogenauer, G. 1975. The mode of action of pleuromutilin derivatives. Location and properties of the pleuromutilin binding site on Escherichia coli ribosomes. Eur. J. Biochem. 52: 93–98. 49. Davidovich, C. et al. 2007. Induced-fit tightens pleuromutilins binding to ribosomes and remote interactions enable their selectivity. Proc. Natl. Acad. Sci. USA 104: 4291–4296. 50. Dornhelm, P. & G. Hogenauer. 1978. The effects of tiamulin, a semisynthetic pleuromutilin derivative, on bacterial polypeptide chain initiation. Eur. J. Biochem. 91: 465–473. 51. Poehlsgaard, J. & S. Douthwaite. 2003. Macrolide antibiotic interaction and resistance on the bacterial ribosome. Curr. Opin. Investig. Drugs 4: 140–148. 52. Mankin, A.S. 2008. Macrolide myths. Curr. Opin. Microbiol. 11: 414–421. 53. Tai, P.-C., B.J. Wallace & B.D. Davis. 1974. Selective action of erythromycin on initiating ribosomes. Biochemistry 13: 4653–4659. 54. Contreras, A. & D. Vazquez. 1977. Cooperative and antagonistic interactions of peptidyl-tRNA and antibiotics with bacterial ribosomes. Eur. J. Biochem. 74: 539–547. 55. Otaka, T. & A. Kaji. 1975. Release of (oligo)peptidyl-tRNA from ribosomes by erythromycin A. Proc. Natl. Acad. Sci. USA 72: 2649–2652. Antibiotics targeting the large ribosomal subunit 56. Menninger, J. & D. Otto. 1982. Erythromycin, carbomycin and spiramycin inhibit protein synthesis by stimulating the dissociation of peptidyl-tRNA from ribosomes. Antimicrob. Agents Chemother. 21: 811–818. 57. Tenson, T., M. Lovmar & M. Ehrenberg. 2003. The mechanism of action of macrolides, lincosamides and streptogramin B reveals the nascent peptide exit path in the ribosome. J. Mol. Biol. 330: 1005–1014. 58. Starosta, A. et al. 2010. Interplay between the ribosomal tunnel, nascent chain, and macrolides influences drug inhibition. Chem. Biol. 17: 1–10. 59. Pyetan, E., D. Baram, T. Auerbach-Nevo & A. Yonath. 2007. Chemical parameters influencing fine-tuning in the binding of macrolide antibiotics to the ribosomal tunnel. Pure Appl. Chem. 79: 955–968. 60. Belousoff, M.J. et al. 2011. Crystal structure of the synergistic antibiotic pair, lankamycin and lankacidin, in complex with the large ribosomal subunit. Proc. Natl. Acad. Sci. USA 108: 2717–2122. 61. Gurel, G., G. Blaha, T.A. Steitz & P.B. Moore. 2009. Structures of triacetyloleandomycin and mycalamide A bind to the large ribosomal subunit of Haloarcula marismortui. Antimicrob. Agents Chemother. 53: 5010–5014. 62. Berisio, R. et al. 2003. Structural insight into the role of the ribosomal tunnel in cellular regulation. Nat. Struct. Biol. 10: 366–370. 63. Bhushan, S. et al. 2010. Structural basis for translational stalling by human cytomegalovirus (hCMV) and fungal arginine attenuator peptide (AAP). Mol. Cell 40: 138–146. 64. Bhushan, S. et al. 2011. SecM-stalled ribosomes adopt an altered geometry at the peptidyltransferase center. PLoS Biol. 19: e1000581. 65. Berisio, R. et al. 2003. Structural insight into the antibiotic action of telithromycin on resistant mutants. J. Bact. 185: 4276–4279. 66. Llano-Sotelo, B. et al. 2010. Binding and action of CEM-101, a new fluoroketolide antibiotic that inhibits protein synthesis. Antimicrob. Agents Chemother. 54: 4961–4970. 67. Kouvela, E.C., D.L. Kalpaxis, D.N. Wilson & G.P. Dinos. 2009. Distinct mode of interaction of a novel ketolide antibiotic that displays enhanced antimicrobial activity. Antimicrob. Agents Chemother. 53: 1411–1419. 68. Hansen, J.L. et al. 2002. The structures of four macrolide antibiotics bound to the large ribosomal subunit. Mol. Cell 10: 117–128. 69. Schlunzen, F. et al. 2003. Structural basis for the antibiotic activity of ketolides and azalides. Structure 11: 329–338. 70. Petropoulos, A.D. et al. 2009. Time-resolved binding of azithromycin to Escherichia coli ribosomes. J. Mol. Biol. 385: 1179–1192. 71. Poulsen, S.M., C. Kofoed & B. Vester. 2000. Inhibition of the ribosomal peptidyl transferase reaction by the mycarose moiety of the antibiotics carbomycin, spiramycin and tylosin. J. Mol. Biol. 304: 471–481. 72. Auerbach, T. et al. 2009. Structural basis for the antibacterial activity of the 12-membered-ring mono-sugar macrolide methymycin. Biotechnologia 1: 24–35. c 2011 New York Academy of Sciences. Ann. N.Y. Acad. Sci. 1241 (2011) 1–16 15 Antibiotics targeting the large ribosomal subunit Wilson 73. Amit, M. et al. 2005. A crevice adjoining the ribosome tunnel: hints for cotranslational folding. FEBS Lett. 579: 3207– 3213. 74. Hansen, J.L., T.M. Schmeing, P.B. Moore & T.A. Steitz. 2002. Structural insights into peptide bond formation. Proc. Natl. Acad. Sci. USA 99: 11670–11675. 16 75. Ban, N., P. Nissen, J. Hansen, et al. 2000. The complete atomic structure of the large ribosomal subunit at 2.4 Å resolution. Science 289: 905–920. 76. Harms, J.M. et al. 2008. Translational regulation via L11: molecular switches on the ribosome turned on and off by thiostrepton and micrococcin. Mol. Cell 30: 26–38. c 2011 New York Academy of Sciences. Ann. N.Y. Acad. Sci. 1241 (2011) 1–16