* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Answer Key to Assignment #7
Kinetic resolution wikipedia , lookup
Discodermolide wikipedia , lookup
Bottromycin wikipedia , lookup
Woodward–Hoffmann rules wikipedia , lookup
Elias James Corey wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Ene reaction wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Stille reaction wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Vinylcyclopropane rearrangement wikipedia , lookup
Hydroformylation wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Petasis reaction wikipedia , lookup
George S. Hammond wikipedia , lookup
Diels–Alder reaction wikipedia , lookup
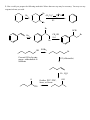
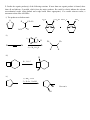
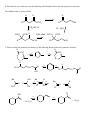
Chem 2600 Assignment 7 Answer Key 1. a) Predict the major product for each of the following reactions: Br + HBr + HBr Br ROOR (Peroxide) b) Rationalize the observed regiochemistry, where appropriate, for each of the preceding reactions. If your answer involves a key intermediate, give its structure. The top reaction proceeds through a cation intermediate. The most stable available carbocation gives rise to the observed Markovnikov product. The bottom reaction proceeds through a free radical mechanism, so a different intermediate leads to the observed product. Br Br 2. How would you prepare the following molecules? More than one step may be necessary. You may use any reagents/solvents you wish. Br Br2 (H3C)3C O K E2 Br Br OCH3 CH3 OH SN1 Br2 Br Br OH SOBr2 Convert OH to leaving group - either halide or sulfonate Br E2 (t-Butoxide) H+, H2O O Oxidize, PCC, PDC, Jones, or Swern CH3 OH 3. Predict the organic product(s) of the following reactions. If more than one organic product is formed, show them all and indicate, if possible, which is/are the major products. Be careful to clearly indicate the relevant stereochemical results (using dashed and wedge bonds where appropriate). If a racemic mixture results, a statement to that effect will suffice. i) The products are both racemic. O H O2N CO 2 Et CO 2 Et Et O 2 C O O2N O H ii) 1) O B H O Ph Ph Ph Ph 2 ) C H 3 CO 2 H iii) H H H H 2 , Pd / C H iv) 1) BH 3 , T HF 2) H2 O 2 , N a O H H OH NO2 Ra c e m ic 4. Develop syntheses of the following compounds using 1-methylcyclopentene. O3, H 2O2 HO O O C C O3, S(CH3)2 H 5. 1) NaCN CH3 O O C C Target 2) H+ CH3 OH, H+ Target CH 3 i) Both hydrogens of acetylene can be removed by reaction with n-butyllithium to give dilithium acetylide – an ambident nucleophile. With this in mind, show how you would make the following molecule from acetylene and benzaldehyde. H H n-BuLi Li Li 2 PhCHO HO OH Ph Ph H2 , Pd/C OH HO Ph Ph ii) Comment on the stereochemical outcome of this reaction. The product has two new chiral centers. You will get three stereoisomeric products: a meso compound (RS) and a racemic mixture of RR and SS products. 6. Describe how you would carry out the following transformations. More than one step may be necessary. The aldehyde must be protected first, O O O H H OCH3 CH3 OH, H+ OCH 3 O H3CO H+, H2O LAH, ether H OH OCH3 H3CO OCH3 H OH 7. What would be the products of ozonolysis of the following alkenes followed by reductive workup? O O OH O ox HO r ed O HO O O ox H O OH O r ed O O H Bu OH O Bu ox r ed O HO H Ph H Ph Bu H O O H Ph H OH ox O + HCO2 H r ed O + CH2 O 8. How might you distinguish between the following two isomeric alkenes using a chemical test? Ozonolysis of the first will give a single product, propanal (via the reductive workup). The second will give a mixture of two products, butanal and acetaldehyde. 9. How would you prepare the following compound using a Diels-Alder reaction followed by reductive ozonolysis? Note the stereochemistry. O O H H H 3 CO 2 C H 3 CO 2 C H 3 CO 2 C Diels-Alder reaction of cyclopentadiene and methyl acrylate gives the endo adduct shown. Reductive ozonolysis gives the final product. Note that the stereochemistry of the Diels-Alder reaction has allowed you to fix the relative stereochemistry (not absolute stereochemistry) of three chiral centers. 10. Suggest syntheses of the following alcohols, all of which can be derived from methylcyclopentene. The number of arrows indicate the number of reactions that are needed. 1 ) KMn O4 or O s O4 CH 3 OH HO H CH 3 OH 2 ) Pe r a cid t o for m t h e ep oxid e , CH 3 fo llo w ed b y e p o xi d e o p e n in g w ith H Na O H o r a q . H 2 SO4 2 3 1 H OH 4 H OH 5 CH 3 H CH 3 OH HH HO H 3 ) Hy d r o b or a t io n follo w ed b y r ea c t ion wit h ba sic H2 O 2 4 ) Co n ve r s ion of t h e h y d r oxy l gr ou p in t o a s u lfo n a t e es t e r l ea v in g gr ou p . (CH3 SO 2 Cl, p y rid in e ) Th en SN 2 r e a ct io n w it h h y d r oxid e . Migh t give a lo t o f E2 , t h o u gh . 5 ) Aq . H 2 SO4