* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download drug design and thalidomide - School of Medical Sciences
Survey
Document related concepts
Orphan drug wikipedia , lookup
Compounding wikipedia , lookup
Polysubstance dependence wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Psychopharmacology wikipedia , lookup
Neuropharmacology wikipedia , lookup
Theralizumab wikipedia , lookup
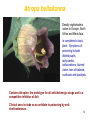
Pharmacogenomics wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Drug interaction wikipedia , lookup
Drug design wikipedia , lookup
Prescription drug prices in the United States wikipedia , lookup
Pharmacognosy wikipedia , lookup
Prescription costs wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Drug discovery wikipedia , lookup
Transcript
DRUG DESIGN AND THALIDOMIDE PHARMACOLOGY Dr Trudie Binder Department of Pharmacology 1 History of Pharmacology 2 The age of Herbs, potions and Magic • Ancient civilizations around the world have used plant extracts as medicines. • Many of these are merely placeboes but some ancient remedies are truly effective. – Willow bark; opium In many cultures the administration of remedies was accompanied by magic or religious rituals. An appreciation that natural products themselves had the power to cure began to emerge. Many poisonous mixtures were made. 3 The birth of modern Science and Pharmacology. • The 16th and 17th centuries were notable for the simultaneous development of physiology, chemistry and physics. • Physiologists documented the way in which the body functioned and chemists began to develop the technologies which permitted extractions, purification, and the synthesis of chemical substances from plants. • These trends began to merge as the discipline of Pharmacology: the study of the actions of drugs or chemical substances on physiological processes. The term Pharmacology was first used in 1692 and is derived from the Greek ‘pharmakon’ meaning medicine First Professor in Pharmacology was appointed in 1847 at the Imperial Russian University in Dorp; Estonia. 4 What is a drug? 5 What is a drug? An agent that interacts with specific target molecules in the body and produces a physiological effect 6 Drugs have side effects • “Medicine sometimes grants health, sometimes destroys it…..” Ovid, Tristia • “If you want to explain any poison properly, what then isn’t a poison? All things are poison; nothing is without poison; the dose alone causes a thing NOT to be a poison.” (Parcelsus; 1493 – 1541 AD) 7 Side effects • Because: receptors for a drug occur in several tissue, not just the target tissue. • Because: of non specificity/selectivity of drugs. 8 Salix sp: Willow Bark Willow bark has been used throughout the centuries in China and Europe to reduce fever and inflammation. In 1829 Scientists discovered that salicin was the active principal constituent. This led to the synthesis of salicylic acid and acetyl salicylic acid [Asprin]. New uses for Aspirin continue to be found: Cardioprotective: anticoagulant. 9 Papaver Somniferum • The Opium poppy is an annual herb native to Southeastern Europe and Western Asia. • Around the 8th Century Arab traders brought opium to India and China. • In 1806, Friedrich Serturner isolated a pure constituent of opium hat he named morphine. • The main clinical use for opioids such as morphine is in the treatment of pain. 10 Digitalis Purpurea • The purple foxglove is native to most of Europe. • From the leaves of this plant the cardiac glycosides digitoxin and digoxin were isolated. These drugs are still used to treat heart failure. • The leaves flowers and seeds of Digitalis are all poisonous and can be fatal if eaten. • At the right dosage the digitalis toxin can cause the heart to beat more strongly preventing heart failure. Symptoms of Digitalis poisoning include a low pulse rate, nausea, vomiting, cardiac arrest and finally death. 11 Atropa belladonna Deadly nightshade is native to Europe, North Africa and West Asia. Is considered a toxic plant. Symptoms of poisoning include: dilated pupils, tachycardia, hallucinations, blurred vision, loss of balance, confusion and paralysis. Contains Atropine, the prototype for all anticholinergic drugs and is a competitive inhibitor of Ach. Clinical uses include as an antidote to poisoning by anticholinesterases. 12 The ‘Dark Side’ • The advances of the 19th Century did not come without a price. • Active constituents were more potent then the parent compound they were derived from, but they also demonstrated more serious side effects. • No clinical trials were conducted and no tests on toxicity, drug addiction and tolerance. Scientists often tested new drugs on themselves. – Sigmund Freud tested the effects of Cocaine on himself – Friedrich Serturner tested morphine on himself • At the end of the 19th Century, drugs could be bought without prescription; – Heroin could legally be purchased in grocery shops. 13 Regulations • Until the 1930’s drugs did not need to be tested for safety or effectiveness. • ‘Elixir of Sulfonamide Tragedy’ of 1937 led to the requirement that drugs be tested for safety before they reached the market. – 107 children died. Establishment of the Food, Drug and Cosmetic Act of 1938 in the USA; requiring details of a drug’s uses and proof of its safety. Clinical trials were not required. 14 Thalidomide • Thalidomide was introduced onto the European market as a safe sedative/hypnotic in the late 1950’s. • Many pregnant women took it for morning sickness. • Tested on male rats only; no teratogen testing. • Thalidomide was found to be a teratogen which causes birth defects. All drugs capable of crossing the placenta are capable of affecting the foetus. ‘Life’ article on thalidomide “In Britian an armless baby’s play” 15 Critical exposure periods for thalidomide during human development. 16 Kim J H , and Scialli A R Toxicol. Sci. 2011;122:1-6 Linking Birth Defects to Thalidomide • 1961, the Australian Dr William McBride and the German Dr Widukind Lenz separately linked the use of thalidomide in early pregnancy to birth defects. ‘In recent months I have observed that the incidence of multiple severe abnormalities in babies delivered of women who were given the drug thalidomide (‘Distival’) during pregnancy, as an anti-emetic or as a sedative, to be almost 20%.’ WG McBride, The Lancet, Dec 1961 17 Withdrawal of thalidomide • Thalidomide was withdrawn in Australia, Germany and the UK by the end of 1961. • Eight months after the withdrawal of thalidomide, babies stopped being born with the characteristic limb defects. • About 40% of babies damaged by the effects of thalidomide died in their first year. • There are adults alive today who are living with disabilities caused by thalidomide. 18 Mechanism of action of Thalidomide • Mechanism of action not fully understood • Thalidomide possesses immunomodulatory, anti-inflammatory and anti-angiogenic properties. • May be related to suppression of excessive TNF-α production and down-modulation of selected cell surface adhesion molecules involved in leukocyte migration. 19 Mechanism of teratogenic effects?? • More than 30 hypotheses have been proposed to explain how thalidomide causes limb defects • Including disruption of molecular pathways including DNA intercalation, acetylation of macromolecules, interference of glutamate metabolism and folic acid antagonism. 20 New Uses for Thalidomide • Used for the treatment of leprosy and multiple myeloma • Thalidomide administration to these patients is useful in suppressing these conditions. 21 1. THALOMID thalidomide 200mg hard capsule blister pack Product Specific Indications MULTIPLE MYELOMA - Thalomid in combination with melphalan and prednisone is indicated for the treatment of patients with untreated multiple myeloma aged 65 years and over or ineligible for high dose chemotherapy. Thalomide, as monotherapy, is indicated for the treatment of multiple myeloma after failure of standard therapies. ERYTHEMA NODOSUM LEPROSUM (ENL)Thalomid is indicated for the acute treatment of the cutaneous manifestations of moderate to severe erythema nodosum leprosum (ENL). Thalomid is also indicated as maintenance therapy for prevention and suppression of the cutaneous manifestations of ENL recurrence. Thalomid is prescribed and dispensed through the Thalidomide Risk Management Programme 22 Therapeutic goods administration Consumer Medicine Information warning for thalidomide Severe life-threatening birth defects: Thalidomide has caused severe birth defects when taken during pregnancy. Thalidomide should never be used by women who are pregnant or who could become pregnant whilst taking the drug or could become pregnant within 4 weeks after stopping the drug. Even a single dose can cause severe birth defects. Product Information warning for thalidomide Teratogenic effects: Thalidomide has caused severe birth defects when taken during pregnancy. Thalidomide should never be used by women who are pregnant or who could become pregnant whilst taking the drug or could become pregnant within 4 weeks after stopping the drug. Even a single dose can cause birth defects. TGA: Fifty years of independent expert advice on prescription medicines 23 Continued use of Thalidomide 1. Prescribers must educate patients on the potential benefits and side-effects of thalidomide 2. Patients must receive contraceptive counselling and regular pregnancy testing 3. Patients must provide informed consent 24 Further Regulations • New Regulations required proof of quality, safety and efficacy or effectiveness. • The Australian government established: – The Australian Drug Evaluation Committee [ADEC]: 1962 – Registry of adverse drug reactions: 1962 – Adverse Drug reactions Advisory Committee [ADRC]:1971 • It now takes many years of research before a new drug can be registered. – Adverse drug reactions still occur e.g. Rofecoxib (viox) 25 The Drug Design & Development Process Target Selection Target Validation Lead Identification Lead Optimisation Preclinical Development Clinical Development Drug Design & Development Jargon Target: what the drug works on (e.g. receptor, enzyme, DNA, channel) Lead: a molecule that has potential to be a drug after further modification Pre-clinical: testing done in the test tube or in animals to determine safety/ toxicity Clinical: testing in humans to determine (i) safety and (ii) if the drug works 26 26 Rang 2006 “Drug Discovery and Development” The Drug Design & Development Process Changes Over Time <1980 Target Selection Serendipity Insights from pathophysiology Lead Identification Lead Optimisation 1990 Defined molecular targets Genomics & other “omics” Screening of 100-1000 compounds at a time Virtual screening of vast libraries of compounds Testing 1-10 compounds at a time Compounds synthesized one at a time Trial and error 2000 Combinatorial chemistry Synthesis of 100-1000 compounds at a time Computational prediction27 27 Is the twenty-first century the era of personalised medicine? Up to 80% of potential drugs fail clinical testing. They might actually succeed if tested only on subpopulations of patients known (from genetic testing) to be susceptible to the benefits and not susceptible to the side effects of the drug. 28 28 29 29 http://www.nigms.nih.gov/About/Budget/Statements/February25_1999.htm