* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Rickettsia, Chlamydia, Mycoplasma, Legionella, and Gardnerella
Neglected tropical diseases wikipedia , lookup
West Nile fever wikipedia , lookup
Orthohantavirus wikipedia , lookup
Gastroenteritis wikipedia , lookup
Hepatitis C wikipedia , lookup
Anaerobic infection wikipedia , lookup
Herpes simplex virus wikipedia , lookup
Dirofilaria immitis wikipedia , lookup
Yellow fever wikipedia , lookup
Middle East respiratory syndrome wikipedia , lookup
Chagas disease wikipedia , lookup
Brucellosis wikipedia , lookup
Human cytomegalovirus wikipedia , lookup
Hepatitis B wikipedia , lookup
Trichinosis wikipedia , lookup
1793 Philadelphia yellow fever epidemic wikipedia , lookup
Typhoid fever wikipedia , lookup
Neisseria meningitidis wikipedia , lookup
Onchocerciasis wikipedia , lookup
Visceral leishmaniasis wikipedia , lookup
Sarcocystis wikipedia , lookup
Neonatal infection wikipedia , lookup
Marburg virus disease wikipedia , lookup
African trypanosomiasis wikipedia , lookup
Yellow fever in Buenos Aires wikipedia , lookup
Oesophagostomum wikipedia , lookup
Sexually transmitted infection wikipedia , lookup
Schistosomiasis wikipedia , lookup
Fasciolosis wikipedia , lookup
Hospital-acquired infection wikipedia , lookup
Leptospirosis wikipedia , lookup
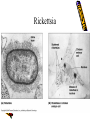
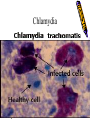
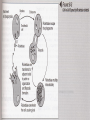
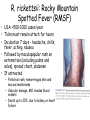
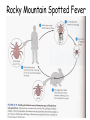
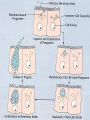
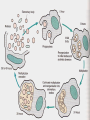
Obligate Intracellular Pathogen Rickettsia Chlamydia D. Rickettsia - Virus-like bacteria Infect cells lining the capillaries (intracellular). Transmitted by insects (arthropods). Defective bacteria - leaky plasma membranes, very small 0.3-1.0 micrometers. Ex. Rocky Mountain Spotted Fever, typhus, Q fever. E. Chlamydia - “virus-like” (intracellular) Spread from one human to the next. Very small: 0.2-0.7 micrometers. Defective bacteria - can’t make their own ATP. Complicated reproductive cycle. Diseases: blindness, urethritis, and pneumonia. Rickettsia Chlamydia F. Mycoplasma Sometimes form long strands that resemble fungi in microscopic appearance. • No cell walls - instead contains sterols. • Ameboid locomotion - disease: “walking” pneumonia • The smallest bacteria - 0.2 micrometers Mycoplasma “fungus-form” Chlamydia & Rickettsia General Characteristics • Obligate intracellular organisms • Can not be cultured on agar plates • Dependant on a host for survival Rickettsia • Intracellular, gram negative organism • Requires host to replicate and survive • Cause febrile illness through the bite of an arthropod • Patient often presents with a rash • Posses a cell wall Rickettsia and Related Organisms Disease Agent Arthropod Vector Rocky Mountain spotted fever Rickettsia rickettsii Wood tick Rickettsial pox Rickettsia akari House mouse mites Murine typhus Rickettsia typhii Rat flea Epidemic typhus Rickettsia prowazekii Human body louse Scrub typhus Orientia tsutsugamushi Chigger mites Ehrlichiosis Ehrlichia chaffeenis Lone star tick Q fever Coxiella burnetti None- spead by inhalation Family Rickettsiaceae: Genera • Zoonotic infection – Human microbial pathogens ~61% zoonotic – Rickettsia are arthropod-borne infections • Spotted Fever Group – Rickettsia rickettsii – Rocky Mountain spotted fever; rodent, tick • Typhus Group – Rickettsia typhi – Endemic typhus; rodent, flea – Rickettsia prowazekii – Epidemic typhus; mammal, louse Rickettsia: Gram Stain and Culture • Gram (-) small, pleomorphic coccobacilli • Gram stain poorly, observed by Giemsa stain of infected cell • Grow in phagocytic, nonphagocytic cells • Lab culture in embryonated eggs or cell tissue culture (similar for virus) • Cultivation costly and hazardous; aerosol transmission occurs easily Chlamydia, Rickettsia, Virus Rickettsia: Lab ID • Giemsa, or Immunofluorescence assay (IFA) - direct detection MO in tissue • Weil-Felix reaction – Nonspecific test – Rickettsial antibody agglutinate Proteus vulgaris – Presumptive evidence of typhus group infection – Not very sensitive or specific, many false positives • Agglutination or Complement Fixation (CF) assay - use specific Rickettsial antigen, test for infection and antibody Rickettsia: Virulence Factors • Induced phagocytosis, intracelluular growth – protected from host immune clearance • Replicates in endothelial cells – cell damage, vasculitis • Recruitment of actin - intracellular spread Rickettsia: Infection and Disease • Disease worldwide, USA • Arthropod reservoir/vector (tick, mite, louse, flea) • Diseases characterized by fever, headache, myalgias, usually rash R. rickettsii: Rocky Mountain Spotted Fever (RMSF) • USA ~500-1000 cases/year • Ticks must remain attach for hours • Incubation 7 days - headache, chills, fever, aching, nausea • Followed by maculopapular rash on extremities (including palms and soles), spread chest, abdomen • If untreated – Petechial rash, hemorrhages skin and mucous membranes – Vascular damage, MO invades blood vessels – Death up to 20%, due to kidney or heart failure Rocky Mountain Spotted Fever Rickettsia: Typhus Group • Incubation 5-18 days • Symptoms - severe headache, chills, fever, maculopapular rash (subcutaneous hemorrhaging as MOs invade blood vessel) • Rash begins on upper trunk; spread to whole body except face, palms of hands, soles of feet • Lasts ~2 weeks • Patient may have prolonged convalescence R. typhi : Endemic Typhus Fever • “typhus” “fever” • Disease worldwide in warm, humid areas (Gulf states, So Cal.; S. America, Africa, Asia, Australia, Europe) • Murine typhus - rat primary reservoir, transmitted to human by rat flea • Disease occurs sporadically • Clinically same, but less severe than epidemic typhus • Restricted to chest, abdomen; generally uncomplicated, lasts <3 weeks • Low fatality R. prowazekii : Epidemic Typhus Fever • • • • Disease C & S Americas, Africa; less common USA Human, squirrel primary reservoir Transmitted by louse; bites, defecates in wound At risk - people living in crowded, unsanitary conditions; often war, famine, natural disaster • Complications - myocarditis, CNS dysfunction • Mortality high untreated cases, up to 20% • Brill-Zinsser disease - individual may harbor MO, latent infection with occasional relapses Rickettsia: Treatment and Prevention • RMSF – Doxycycline drug of choice – Avoid ticks, wear protective clothing, use insect repellents, insecticides – In infested areas, check and remove ticks immediately • Typhus Fever – Doxycycline effective – Improve personal hygiene and living conditions, reduce lice by insecticides, control rodent population – Inactivated vaccine for epidemic typhus Laboratory Diagnosis of Rickettsial Disease • Immunohistochemical detection • Serological tests • PCR Family Chlamydiaceae: Genera Chlamydia trachomatis – STD, eye infection Chlamydophila pneumoniae – pneumonia Chlamydophilia psittaci – pneunomia (psittacosis); birds, humans Obligate intracellular parasite Cell wall similar G(-) bacilli, lack peptidoglycan Energy parasites, use ATP of host cell Chlamydia Characteristics • Unique growth cycle because they are deficient in independent energy metabolism • Replication involves elementary body (EB) and reticulate body (RB) – EB’s are infectious and non-metabolically acitve – RB’s are noninfectious and metabolically active Chlamydia: Life Cycle – Elementary Body (EB) • Circular, infectious form; 300-400 nm • Metabolically inactive • Resistant to harsh environments • 0 hour - EB binds to host cell, induced phagocytosis • Outer membrane of EB prevents lysosome fusion, survives in phagosome • 8 hours - EB reorganizes into Reticulate Body (RB) Chlamydia: Life Cycle – Reticulate Body (RB) • Noninfectious form, larger, less dense, 800-1000 nm • Metabolically active • 8-30 hours – – – – Synthesize new materials Multiply by binary division Form inclusion body Reorganize, condense into EB • 35-40 hours - cell lyses, releases EB, begins cycle again Chlamydia: Lab ID • Stain tissue – Giemsa stain – Direct fluorescent antibody (DFA) – ELISA – Less sensitive • Cell culture – More sensitive method – Grow MO in tissue culture, stain infected cells • DNA amplification test – Recently developed – Specific, sensitive – Now routine test of choice Chlamydia: Virulence Factors • Intracellular replication – protected from host immune defense • Prevent fusion of phagolysome – evades phagocytic killing • Repeated infections by C. trachoma result in cell pathology • Serotypes A-K and L1, L2, L3 - serotype identifies strain’s clinical manifestation Chlamydia pneumoniae • Important respiratory pathogen (acute respiratory disease, pneumonia, and pharyngitis) • Common (50% of adults have antibodies) • College age students most susceptible • Implicated in asthma • Risk factor for Guillain-Barre’ syndrome • Reinfection common • Biphasic clinical picture – Prolonged sore throat and hoarseness, followed by flu-like lower respiratory symptoms – Pneumonia and bronchitis Chlamydia trachomatis • Most commonly sexually transmitted bacterial pathogen in U.S. – Only HPV is a more commonly sexually transmitted disease – Major cause of sterility in U.S. – May be transmitted to newborns during delivery • Results in conjunctivitis • Other sites of infection – Trachoma – infection of the conjunctiva, resulting in scarring and blindness (Mostly in India and Egypt) – Lymphogranuloma venereum • Infects lymph nodes Chlamydia psittaci • Causes psittacosis (parrot fever) • Identification based on history of close contact with birds and serologic evaluation “parrot” “parrot fever” Naturally infects avian species Mild to severe respiratory infections Human infection by contact infected bird Infection - subclinical to fatal pneumonia Commonly causes atypical pneumonia with fever, chills, dry cough, headache, sore throat, nausea, and vomiting Chlamydia trachomatis: Trachoma • • • • • • • • • “rough” “trachoma” granulations on conjunctiva Serotypes A-C Single, greatest cause blindness developing countries Infections mainly children (reservoir), infected first three months life Transmission eye-to-eye, direct contact (droplet, hand, clothing, fly) Chronic infection, reinfection common Conjunctival scarring, corneal vascularization Scars contract, upper lid turn in so eyelashes cause corneal abrasions Leads to secondary bacterial infections, blindness C. trachomatis: Lymphogranuloma Venereum • Venereal disease, occurs developing, tropical areas • Primary stage - painless lesion (vesicle or an ulcer) occurs site of entry in few days, heals with no scarring; but widespread dissemination • Secondary stage - occurs 2-6 weeks later, symptoms of regional suppurative lymphadenopathy (buboes), may drain for long time, accompanied by fever and chills. Arthritis, conjunctival, CNS symptoms • Tertiary stage - urethrogenital perineal syndrome; structural changes, such as non-destructive elephantiasis of the genitals, rectal stenosis C. trachomatis: • Urogenital tract infection - serotypes D-K • Major cause of nongonococcal urethritis; frequently found concomitantly with N. gonorrhoeae • In males - urethritis, dysuria, sometimes progresses to epididymitis • In females - mucopurulent cervical inflammation, can progress to salpingitis and PID C. trachomatis: Inclusion Conjunctivitis • Newborns and adults • Genital tract infection source of eye infection (serotypes D-K) • Benign, self-limited conjunctivitis, heals with no scarring • Newborns infected during birth process: – 1-2 weeks, mucopurulent discharge – Lasts 2 weeks, subsides – Some develop afebrile, chronic pneumonia • In adults – causes an acute follicular conjunctivitis with little discharge Chlamydia: Treatment and Prevention • Genital tract infection and conjunctivitis: – Adult - azithromycin or doxycycline, prompt treatment of patients and partners – Newborn – erythromycin – Public Health education • Trachoma: – Need prompt treatment, prevent reinfection – Systemic tetracycline, erythromycin; long term therapy necessary – Improve living, sanitary conditions – Difficult to prevent endemic disease in developing countries due to lack of resources, medical care Laboratory Diagnosis • If cultured, must be in cells • Direct microscopic examination to find EB’s – visualized with fluorescein-conjugated antibodies • Enzyme immunoassay • Nucleic acid probes with and without amplification (PCR) • Serologic tests are method of choice for detection (Four-fold rise in titer) Case Study 9 - Questions • 1. Why is penicillin ineffective against Chlamydia? What antibiotic can be used to treat this patient? • 2. Describe the growth cycle of Chlamydia. What structural features make the EBs and RBs well suited for their environment? • 3. Describe the differences among the three species in the family Chlamydiaceae that cause human disease. Normal Flora: Commensals are regularly found in certain human microbiotopes. The normal human microflora is thus the totality of these commensals. Table 1.7 lists the most important microorganisms of the normal flora with their localizations. Bacteria are the predominant component of the normal flora. They proliferate in varied profusion on the mucosa and most particularly in the gastrointestinal tract, where over 400 different species have been counted to date. The count of bacteria per gram of intestinal content is 101–105 in the duodenum, 103–107 in the small intestine, and 1010–1012 in the colon. Over 99% of the normal mucosal flora are obligate anaerobes, dominated by the Gram-neg. anaerobes. Although life is possible without normal flora(e.g., pathogen-free experimental animals), commensals certainly benefit their hosts. One way they do so is when organisms of the normal flora manage to penetrate into the host through microtraumas, resulting in a continuous stimulation of the immune system. Commensals also compete for living space with overtly pathogenic species, a function known as colonization resistance .On the other hand, a potentially harmful effect of the normal flora is that they immunocompromised individual. can also cause infections in