* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Glenn Fennelly - Pediatric Multi-Drug Resistance Bacterial Infections
Survey
Document related concepts
Transmission (medicine) wikipedia , lookup
History of virology wikipedia , lookup
Bacterial cell structure wikipedia , lookup
Human microbiota wikipedia , lookup
Infection control wikipedia , lookup
Traveler's diarrhea wikipedia , lookup
Methicillin-resistant Staphylococcus aureus wikipedia , lookup
Gastroenteritis wikipedia , lookup
Bacterial morphological plasticity wikipedia , lookup
Staphylococcus aureus wikipedia , lookup
Clostridium difficile infection wikipedia , lookup
Carbapenem-resistant enterobacteriaceae wikipedia , lookup
Urinary tract infection wikipedia , lookup
Neonatal infection wikipedia , lookup
Anaerobic infection wikipedia , lookup
Transcript
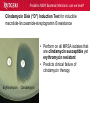
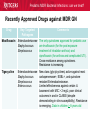
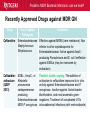
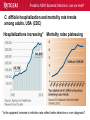
Pediatric Multi-Drug Resistant Bacterial Infections: Can We Treat Effectively? Glenn Fennelly, MD, MPH Professor and Chair, Department of Pediatrics Professor, Department of Microbiology and Molecular Genetics Rutgers-New Jersey Medical School Pediatric MDR Bacterial Infections: can we treat? Pediatric MDR Bacterial Infections: can we treat? • Learning objectives: At the conclusion of the presentation, the participants should be able to – Cite emerging challenges in the management of hospital-acquired (HAI) MDR gram-negative and MRSA infections in children – Select appropriate antimicrobials for treatment of HAI or MRSA in children – Identify opportunities to prevent HAI and MRSA in children Pediatric MDR Bacterial Infections: can we treat? Pediatric MDR Bacterial Infections: can we treat? • Emerging challenges in MDR infections – Gram positive infections – Gram negative infections – Clostridium difficile • Opportunities and novel approaches to prevent HAI in children – Infection Prevention – Prudent diagnosis and antibiotic use – Vaccination Pediatric MDR Bacterial Infections: can we treat? NO DRUGS FOR BAD BUGS ?? Rise in % Resistant Isolates Number of New Antibacterial Approvals Spellberg, et. al., CID 2004 US, CDC Pediatric MDR Bacterial Infections: can we treat? Timeline of Key Antibiotic Resistance Events CDC Pediatric MDR Bacterial Infections: can we treat? An estimated 721,800 HAI in U.S. in 2011. • • • • • 22% pneumonia, 22% surgical-site, and 17% GI Clostridium difficile the most common pathogen (12%) 14% in children < 18 years of age 26% device-associated (CLABSI, Foley-UTI, and VAP) HAI kill at least 23,000 people each year CDC and Magill S et al. 2014 Pediatric MDR Bacterial Infections: can we treat? Emerging challenges in MDR gram positive infections • MDR S. pneumoniae – Do not treat viral URIs with antibiotics! – Antibiotic overuse (amoxicillin largely sufficient) • Methicillin-resistant S. aureus (MRSA) – Community-acquired (CA) versus HA Pediatric MDR Bacterial Infections: can we treat? State-to-state variation in antibiotic prescriptions per 1000 persons of all ages: opportunities to prevent unnecessary treatment Pediatric MDR Bacterial Infections: can we treat? Judicious Use of Antibiotics for URIs in Children • Doctors prescribe antibiotics 62% of the time if they perceive parents expect them but only 7% of the time if they don’t. Mangione-Smith R et al.1999 • Risks: diarrhea, dermatitis, C difficile colitis, antibiotic resistance • 1st-line therapy • AOM: Watchful waiting if > 2 years old, Amoxicillin + clavulanate • Sinusitis: Amoxicillin + clavulanate • Confirmed GAS pharyngitis: Amoxicillin (once a day) or penicillin • Avoid azithromycin and oral 3rd-generation cephalosporins Hersh AL et al. 2013 * * * * Formerly, increasing incidence of MDR serotype 19A Prevnar 13 is effective against serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19 A, 19F, and 23F Kyaw et al 2006 Pediatric MDR Bacterial Infections: can we treat? Effectiveness of Various Antibiotics Against Penicillin Resistant Pneumococcus Antibiotic Amoxicillin MIC 90 ( g/ml) S I R 0.03 0.1-1 2-4 Peak Concentrations Serum MEF 3.5-7 1-6* Cefuroxime Ceftriaxone Cefprozil 0.12 0.06 0.25 1-4 1 4-8 4-16 1-4 32 2-7 171 6-26 1-2 35 2 Loracrabef Cefixime 2 0.5 64 16 128 64 13-19 3-4 2-4 1-2 *Doubling the dose would increase MEF peak concentrations to exceed the MIC 90 of resistant strains Dowell PIDJ 1999 Pediatric MDR Bacterial Infections: can we treat? Which the optimal empiric antibiotic for hospitalized child with community-acquired (CAP) lobar pneumonia? A. B. C. D. High dose ampicillin Extended-spectrum cephalosporin Macrolide Vancomycin • Narrow- as effective as broad-spectrum empiric antibiotics (duration of O2 and fever, and LOS). Queen MA et al. 2014 • If PCN MIC < 2 g/ml, consider high-dose PCN • If 3rd-gen ceph MIC > 8, consider vancomycin or clindamycin Pediatric MDR Bacterial Infections: can we treat? S. pneumoniae ceftriaxone in vitro “resistance” (up to 40%): What is the clinical relevance? • Ceftriaxone remains clinically effective against “resistant” blood/lung infections due to high achievable serum levels • CNS infections may fail treatment due to lower achievable levels in CSF: – Susceptible* – Resistant* MIC <0.5 (meningitis) MIC >2.0 (meningitis) <1.0 (non-meningitis)^ >4.0 (non-meningitis)^^ – ^Use of interpretive data for non-meningitis requires doses appropriate for severe pneumococcal infection – ^^Use empiric vancomycin + 3rd generation cephalosporin to treat meningitis due to resistant strains *revised NCCLS MIC break-points (microgms/mI) Pediatric MDR Bacterial Infections: can we treat? Guidance on Empiric Treatment of Severe Pneumococcal Infections (USA) • Pneumococcal conjugate vaccines have reduced pneumococcal disease incidence • There is an increasing likelihood that a case of invasive pneumococcal infection will be MDR • Pneumonia – Avoid macrolides (high resistance rates) – “High dose” ampicillin effective against intermediate/high PCN resistance • Meningitis (beyond the neonatal period) – Empiric ceftriaxone plus vancomycin – Add rifampin if high-level ceftriaxone resistant Pediatric MDR Bacterial Infections: can we treat? Emerging challenges in MDR gram positive infections: Methicillin-resistant S. aureus (MRSA) The most effective oral antibiotic for empiric treatment of community-acquired cellulitis in a 5- year old child in Newark in 2014 is A. Clindamycin B. TMP/SMX C. Cephalexin D. Doxycycline E. TMP-SMX F. A or C Pediatric MDR Bacterial Infections: can we treat? Considerations in Empiric Antibiotic Treatment of Skin/Soft Tissue Infections Purulent abscess •Usually S. aureus •I&D without antibiotics may be sufficient •Perform wound cultures routinely Isolated non-purulent skin/soft tissue cellulitis •Usually S. aureus or GA HS •Consider therapy against GA HS, MSSA, and MRSA streptococcus (GA HS is resistant to TMP/SMX!) Pediatric MDR Bacterial Infections: can we treat? Optimal empiric antibiotic therapy of skin/soft tissue infections in an era of increasing MRSA rates • MRSA rates among all S. aureus infections generally > 50% – 50% MRSA among all S. aureus isolates at Jacobi Pediatrics by 2009 – > 10-fold increase (from 0.8% to 9.2) in CA-MRSA colonization in children in Nashville between 2001-2004 Creech B et al. 2007 • Consider disease severity when selecting therapy . Pediatric MDR Bacterial Infections: can we treat? CA-MRSA differs genotypically and is distinct from HA-MRSA (not a hospital strain that “escaped”) • SCCmecIV (contains mecA that makes PBPs inaccessible) inserted in to virulent MSSA strains • USA300, a predominant strain implicated in unrelated outbreaks of CA-MRSA across the US Kazakova SV et al 2005, Moran GJ et al. 2006 – Contain Panton-Valentine leukocidin gene (a secreted tissue toxin) – Linked to more severe SSTI, pneumonia, and skeletal infections – Contains the msr(A) erythromycin resistance gene and SCCmec type IV. • Often retain susceptibility to clindamycin, TMP-SMX, doxycycline, fluoroquinolones or rifampin Pediatric MDR Bacterial Infections: can we treat? The optimal IV antibiotic choice for empiric treatment of hematogenous osteomyelitis in a previously healthy 12 year old is A. Vancomycin B. Clindamycin C. Linezolid D. Daptomycin E. TMP/SMX Pediatric MDR Bacterial Infections: can we treat? Emerging challenges in MRSA infections: optimal empiric antibiotic therapy for skeletal infections • Consider clindamycin, vancomycin OR linezolid • S. aureus is isolated in half of all culture positive casesadditional bacterial pathogens include GA HS, GB HS, E. coli, S. pneumoniae, K. kingae* *Often resistant to vancomycin and clindamycin yet susceptible to PCN, cephalothin, cefotaxime, gent, chloramphenicol, tetracycline, TMP/SMX, and CIPRO Pediatric MDR Bacterial Infections: can we treat? Is CA-MRSA associated with worse clinical outcomes? • Skeletal infections: increased rate of bone abscess (Saavedra-Lozano J, et al. 2008) and prolonged fever (Carrillo-Marquez MA et al. 2009) relative to MSSA. • USA300 MRSA infection independently predicts early complications for hospitalized adults with pneumonia (OR, 2.6; P = .02) but not CLABSI. (Lessa FC et al. 2012) Pediatric MDR Bacterial Infections: can we treat? A two-year old male with a small BSA (2%) third-degree burn develops high fever, tachycardia, hypotension, anemia and a rash (Figure). Appropriate initial management should include A. Empiric Linezolid B. Debridement plus empiric Clindamycin C. Debridement plus empiric Vancomycin D. Debridement plus empiric Vancomycin plus Nafcillin + Clindamycin Pediatric MDR Bacterial Infections: can we treat? Clindamycin Disk (“D”) Induction Test for inducible macrolide-lincosamide-streptogramin B resistance • Perform on all MRSA isolates that are clindamycin susceptible yet erythromycin resistant • Predicts clinical failure of clindamycin therapy Erythromycin Clindamycin Pediatric MDR Bacterial Infections: can we treat? Increasing Clindamycin Resistance Among MRSA in 57 Northeast US Military Treatment Facilities. (Braun L et al. 2005) Pediatric MDR Bacterial Infections: can we treat? Caveats: empiric use of clindamycin against skin, soft tissue and skeletal infections • Group B Streptococcus (GBS) infections in early infancy period (birth-3 months), clindamycin resistance in 38.4% of perinatal GBS rectovaginal cultures obtained in 2010-2011 Back EE et al. 2012 • Strains that are phenotypically clindamycin-susceptible yet harbor inducible MLSB gene may become resistant • Kingella kingae skeletal infections-high rates of resistance to clindamycin and inherent resistance to vancomycin Yagupsky P et al. 2012 Pediatric MDR Bacterial Infections: can we treat? Standard dosing of vancomycin for children with invasive MRSA may not be adequate • Active surveillance of MRSA MIC trends is important • If MRSA/MSSA with MIC of > 1.0 g/mL is common – Standard empiric vancomycin dose (40 mg/kg/d) may not achieve the optimal pharmacodynamic target of AUC24/MIC 400 – Use 60 mg/kg/d – If MIC > 2.0 g/mL, consider alternatives to vancomycin • Further pharmacokinetic and pharmacodynamic studies are needed in children to optimize vancomycin dosing Fymoyer A et al. 2009 Pediatric MDR Bacterial Infections: can we treat? Vancomycin-resistant Staphylococcus aureus • MRSA strains with vancomycin MIC>1-1.5 g/mL are becoming increasingly common • Vancomycin susceptible: ≤1 g/mL – Maintain trough concentration ≥ 10 mg/L to avoid resistance (≥ 15 mg/L if the MIC is 1 g/mL) • Vancomycin intermediate (VISA): 4 to 8 g/mL • Vancomycin resistant (VRSA): ≥16 g/mL /mL Pediatric MDR Bacterial Infections: can we treat? Alternatives to vancomycin to treat MRSA in children: Limited armamentarium? • Linezolid is a suitable alternative to vancomycin for infections caused by MRSA, especially those also resistant to clindamycin • Only two new classes of antibiotics approved since the 1970s – oxazolidinones (linezolid 2000) and – cyclic lipopeptides (daptomycin - 2003) • All other antibiotics are derivatives of existing classes Pediatric MDR Bacterial Infections: can we treat? Recently Approved Drugs against MRSA (CDC) Drug (year approved) Key Targeted Pathogens Comments Linezolid (2000) Staphylococcus Enterococcus (IV/PO) Resistance in targeted pathogens still uncommon Daptomycin (2003) Staphylococcus Streptococcus Enterococcus (IV) may be superior to either Linezolid or Vancomycin against CNS infections, bacteremia and R-sided endocarditis; not active in pneumonia Children 3 to 24 months of age require higher mg/kg doses (Bradley JS et al 2014) Emerging resistance in all of the targeted pathogens (albeit at low rates). Quinupristin/Dal foprisitin (1999) Staphylococcus Streptococcus Side effects common (not 1st-line) % resistance remains low in the US. Pediatric MDR Bacterial Infections: can we treat? Recently Approved Drugs against MRSA (CDC) Drug (year approved) Key Targeted Pathogens Comments Ceftaroline (2010) Staphylococcus A cephalosporin drug, effective against MRSA Streptococcus (resistance is rare). Telavancin (2008) Staphylococcus (IV) Approved for treatment of gram-positive Streptococcus SSTI. Should not be used in a woman of Enterococcus childbearing age without a pregnancy test. Marginal versus MRSA Gram-positive Phase III studies in ABSSSI and cSSTI show bacteria that tedizolid 200 mg/day X 6 days was noninferior to linezolid 600 mg twice daily X 10 days Tedizolid phosphate Pediatric MDR Bacterial Infections: can we treat? Recently Approved Drugs against MRSA (CDC) Drug Key Targeted Comments (year Pathogens approved) Dalbavancin Gram-positive Active against VISA, once weekly IV bacteria slowly cidal, not dialyzable if toxicity Oritavancin marginal versus VRSA Gram-positive active against both VISA and VRSA bacteria cidal Telavancin ods regimens Gram-positive Active against VISA, marginal versus VRSA Cidal bacteria ods regimens, Tigecycline Broadspectrum useful for mixed infections possibly including MRSA Pediatric MDR Bacterial Infections: can we treat? Emerging challenges in MDR gram negative infections • Community-acquired urinary tract infection • Hospital-acquired gram negative infections • Limited treatment options Antibiotic resistance is natural, ancient, and hard wired (Bhullar K et al. 2012) • Somewhere in nature there likely is present resistance to every potential natural antibiotic as will as the ability to evolve resistance to any synthetic agent • Use of any antibiotic exerts selective pressure to develop intrinsic, acquire from other bacteria, or mutate resistance • Certain organisms evolve resistance while on therapy, e.g. pseudomonas, MRSA (esp. in severe chronic illness) • Increasingly, spontaneous primary MDR infections • Carbapenems no longer a cure-all for GNR Pediatric MDR Bacterial Infections: can we treat? “Multi-drug resistant” Enterobacteriaceae: Definitions • “Resistance to antimicrobials usually kept in reserve” • Highly variable definitions in recent survey: 22 unique “MDR” definitions for Enterobacteriaceae Drees M et al. 2014 • Standard definitions* Magiorakos AP et al. 2012 • MDR: non-susceptible to ≥1 agent in ≥3 antimicrobial categories • XDR: non-susceptible to ≥1 agent in all but ≤2 categories. • PDR: non-susceptible to all available antimicrobial agent *For standard definitions see Clinical Microbiology and Infection, openly accessible at: http://onlinelibrary.wiley.com/doi/10.1111/j.14690691.2011.03570.x/pdf Pediatric MDR Bacterial Infections: can we treat? Multi-drug resistance among gram-negative bacilli • Most common in Enterobacteriaceae, Pseudomonas aeruginosa, Acinetobacter • Mobile genetic elements contain multiple resistance genes plus broad spectrum beta-lactamases. • Empiric broad-spectrum cephalosporin use associated with ESBL-bacteria in a NICU Le J. et al 2008 • Types of broad spectrum beta-lactamases – ESBL, AmpC (third generation cephalosporin resistance) – Carbapenemase (NDM, KPC, VIM, IMP, OXA-48) • May be difficult to impossible to treat! Pediatric MDR Bacterial Infections: can we treat? Clinical and Cost Impact of MDR GNB • Associated with longer LOS compared with antibioticsusceptible infection Folgori L et al. 2014 • ESBL-producing Enterobacteriaceae, MDR P. aeruginosa and carbapenem-resistant Acinetobacter HAI are associated with increased mortality rates (all others have no clear increased mortality risk) Giske CG et al. 2008 Pediatric MDR Bacterial Infections: can we treat? Increasing prevalence of antimicrobial resistant GNB in children developing countries • Bacteremia causes an estimated 6% of neonatal and 14% of under 5-year-old deaths worldwide • The lack of cheap antibiotics is critical constraints in lowand low-middle-income countries • Data from studies of 4710 neonates with gram-negative bacteremia in developing countries in Asia – median E. coli ampicillin resistance rate was 97% – median E. coli, Klebsiella and Enterobacter ceftriaxone resistance rates ranged from 74 to 80% Black RE et al. 2008 Pediatric MDR Bacterial Infections: can we treat? Common childhood uropathogens* Gram positive Gram negative • • • • • • E. coli* 54 - 67% Klebsiella 6 - 7% Proteus 5 - 12% Serratia Enterobacter Pseudomonas 2 - 6% • • • • Enterococcus 3 - 9% Group B strep (neonates) Staph aureus Staph saprophyticus – adolescent girls *Based upon multiple surveys in USA and Europe (2005-2008) ** E. coli resistance patterns: Ampicillin 38 – 65%, Amox/clav 7 – 43%, Bactrim 8 – 53%. Similar rates of resistance for Klebsiella and Proteus sp. Pediatric MDR Bacterial Infections: can we treat? Risk factors for UTI with drug resistant bacteria • Child care attendance • Family members colonized with resistant organisms • Age • Hospital exposure • Urinary tract malformations • Current or past antimicrobial exposure – Recent Rx for OM, pharyngitis, pneumonia, SSTI – Antimicrobial prophylaxis Pediatric MDR Bacterial Infections: can we treat? MDR CA-E. coli (UTI) at Jacobi Hospital: Few remaining options for oral therapy? • 17 of 335 E. coli urine isolates had in vitro resistance to amoxicillin/clav., cephalexin and TMP/SMX • All of 15 of these empirically treated with amoxicillin/clav. had clinical resolution and negative urine cultures 2-3 days later and no complications • Conclusions: – Clinical cure in cases of in vitro resistant CA amoxicillin/clav.-E. coli – Achievable urine levels of amoxicillin/clav. likely exceed standard in vitro MIC resistance cut off. Pediatric MDR Bacterial Infections: can we treat? Increasing Antibiotic-Resistant Pediatric UTIs • Contributing factors: Poor empiric prescribing, no urine testing, and nonselective use of prophylaxis 3rd-generation cephalosporin use for children with UTIs doubled in the US between 1998 to 2007 Copp H et al. 2011 • Practice changes to curb growing resistance rates: – Routine urinalysis and urine culture testing: treat only when indicated and tailor broad-spectrum therapy – Use antibiotic prophylaxis only in patients with high-grade VUR and high-grade hydronephrosis – Use local antibiograms, ideally pediatric-specific with inpatient versus outpatient data, to guide therapy Edlin RS et al. 2014 Pediatric MDR Bacterial Infections: can we treat? What’s on the Horizon….. • Increasing MDR gram-negative UTIs rates in children • Emerging severe MDR gram-negative threats: – Extended-spectrum β-lactamase–producing organisms (ESBL), – AmpC β-lactamase–producing organisms – Carbapenem-resistant Enterobacteriaceae (CRE), P. aeruginosa, and A. baumannii are associated with a 21% mortality rate in children < 15 years old Maltezou, HC et al. 2013 … and challenges: – – – – How to treat based on in vitro resistance breakpoints? How to define organ-specific or tissue-specific “breakpoints” How to predict and suppress emergence of resistance? How to treat nearly pan-drug resistant species? Pediatric MDR Bacterial Infections: can we treat? Effective therapy against MDR gram-negative infections in critically ill children requires… is hampered by… • Proper culture techniques • Limited antibiotic options • Timely initiation of therapy • Lack pharmacokinetic/ • Selection of active agents that pharmacodynamic and clinical penetrate to the site outcome data in children • Adequate dose and interval to • Dependence upon extrapolation optimize effectiveness from adult studies that lack • Prompt removal/drainage of effective comparators infected sources Must use in vitro data, observational data, and clinical experience to guide therapy Hsu AJ. 2014 Pediatric MDR Bacterial Infections: can we treat? Clinicians’ Knowledge, Attitudes, and Practices regarding Infections with MDR-GNB in ICUs True or False? Carbapenem agents are ineffective for GNB expressing extended-spectrum βlactamases Tigecycline is an option for hospitalassociated pneumonia caused by MDR Pseudomonas aeruginosa Carbapenem-resistant Klebsiella species are usually susceptible to quinolone agents Quinolone agents exhibit concentrationdependent killing After Zhou JJ et al. 2014 Maximizing pharmacodynamics-based dosing Aminoglycosides Daptomycin Fluoroquinolones Ketolides Aminoglycosides Daptomycin Fluoroquinolones Ketolides Azithromycin Clindamycin Oxazolidinones Vancomycin Carbapenems Cephalosporins Erythromycin Linezolid Penicillins Pharmacokinetic and pharmacodynamics parameters of antibiotics on a concentration vs time curve. Roberts JA, et al. 2009 Proposed treatment algorithm for carbapenem-resistant Gram-negative organisms. * Courter JD et al 2009 *Nonprotein bound meropenem serum concentrations that exceed the MIC for 40% of the time (T>MIC) for organisms with MICs up to 8 μg/mL are achieved with prolonged or continuous infusions in children Daikos GL 2012 Pediatric MDR Bacterial Infections: can we treat? Combination therapy with > 2 agents with differing kinetics and tissue penetration against MDR GNR 1. May achieve additive or synergistic killing (may antagonize!) – Bioreactor chamber testing models synergy in vivo (more reliable than checkerboard testing): synergy observed with the following combinations against AmpC, ESBL and CRE gram-negative bacilli • Doripenem plus colistin (against Klebsiella) • Meropenem plus levofoloxacin (against pseudomonas) • Colistin plus rifampin (against Pseudomonas or Acinetobacter) 2. May prevent the emergence of resistance! (as in TB therapy) – Tobramycin to prevent resistant to cefepime Drusano GL et al. 2003 – Avibactam plus ceftaroline prevents ESBL resistance Pediatric MDR Bacterial Infections: can we treat? Recently Approved Drugs against MDR GN Drug Key Targeted Pathogens Comments Moxifloxacin Enterobacteriaceae The only quinolones approved for pediatric use Staphylococcus are levofloxacin (for the post exposure Streptococcus treatment of inhalation anthrax) and ciprofloxacin (for anthrax and complicated UTI). Cross-resistance among quinolones. Resistance is increasing. Tigecycline Enterobacteriaceae Staphylococcus Streptococcus Enterococcus New class (glycylcyclines) active against most carbapenemase+, ESBL+, and quinolone resistant Enterobacteriaceae. Limited effectiveness against certain A. baumannii with MIC > 2 mg/L; poor clinical outcomes in and in CLABSI (despite demonstrating in vitro susceptibility). Resistance is emerging. Data in children > 8-years old Pediatric MDR Bacterial Infections: can we treat? Recently Approved Drugs against MDR GN Drug Key Targeted Pathogens Comments Gemifloxacin Enterobacteriaceae Streptococcus Treatment of mild to moderate communityassociated respiratory disease. Crossresistance with other fluoroquinolone drugs Doripenem Enterobacteriaceae P. aeruginosa Acinetobacter spp. Streptococcus spp. Data down to neonate May overcome low-level resistance of A. baumannii and P. aeruginosa strains. Inferior to imipenem for VAP. High-level resistance has emerged reducing its overall effectiveness Ertapenem Enterobacteriaceae Staphylococcus Streptococcus Approved for use in children > 3 months of age. Carbapenem-resistant Enterobacteriaceae (CRE) are spreading rapidly. Pediatric MDR Bacterial Infections: can we treat? Recently Approved Drugs against MDR GN Drug Key Targeted Pathogens Comments Ceftaroline Enterobacteriaceae Effective against MRSA (rare resistance). Non Staphylococcus inferior to other cephalosporins for Streptococcus Enterobacteriaceae. Active against AmpCproducing Pseudomona and E. coli. Ineffective against ESBLs (may be overcome by avibactam). Ceftazidimavibactam (QIDP 2013) ESBL-, AmpC-, or Klebsiella pneumoniae carbapenemaseproducing Enterobacteriaceae MDR P. aeruginosa Pediatric studies ongoing. The addition of avibactam to ceftazidime improves its in vitro activity against Enterobacteriaceae and P. aeruginosa. Inactive against Acinetobacter, Burkholderia, and most anaerobic gramnegatives. Treatment of complicated UTIs intra-abdominal infections (with metronidazole) Pediatric MDR Bacterial Infections: can we treat? Suggested Antimicrobials for the Treatment of MDR Gram-Negative Infections in Children ESBL–producing organisms First-Line Agents Meropenem Imipenem-cilastatin Second-Line Agents Ciprofloxacin Trimethoprim-sulfamethoxazole UTIs Ertapenem UTIs Piperacillin-tazobactam Nitrofurantoin (cystitis)* Oral fosfomycin (cystitis) Aminoglycosides (cystitis) AmpC β-lactamase– Meropenem producing organisms Imipenem-cilastatin Cefepime Trimethoprim-sulfamethoxazole Ciprofloxacin *The use of nitrofurantoin should be limited to cystitis as treatment failures have been observed when prescribed for pyelonephritis. Pediatric MDR Bacterial Infections: can we treat? Suggested Antimicrobials for Treatment of Severe MDR Gram-Negative Infections in Children First-Line Agents Carbapenem-resistant Prolonged infusion meropenem Enterobacteriaceae PLUS aminoglycoside OR fluoroquinolone OR colistin* 5 mg/kg/dose Carbapenem-resistant Prolonged infusion meropenem Pseudomonas PLUS aminoglycoside OR aeruginosa fluoroquinolone OR colistin Carbapenem-resistant Prolonged infusion meropenem Acinetobacter PLUS aminoglycoside OR baumannii fluoroquinolone OR colistin Second-Line Agents Tigecycline IV fosfomycin (limited availability) Oral fosfomycin (cystitis) Fosfomycin Ampicillin-sulbactam Tigecycline * Colistin (polymixin) is active against most gram-negative bacilli except for Pseudomonas mallei, Burkholderia cepacia, Proteus, Providencia , Serratia , Edwardsiella and Brucella , (12-17% of S. maltophilia are resistant) Pediatric MDR Bacterial Infections: can we treat? Polymixin (colistin) use and its toxicities in children are poorly understood • Cell-wall acting bactericidal and anti-endotoxin activity • Can be administered IV, IM or by nebulization • Susceptibility testing – Colistin disk: susceptible if the zone of inhibition is >11 mm – Broth micro dilution: susceptible MIC < 4 mg/L resistant MICs >8 mg/L • Nephro- and neurotoxicity are the most common toxicities – Dose-dependent, usually reversible if early discontinuation – Avoid use with other nephrotoxic agents. – Modify dose for renal failure M. Falagas M et al 2005 Pediatric MDR Bacterial Infections: can we treat? Polymixin synergy Landman et al. 2008. Pediatric MDR Bacterial Infections: can we treat? Use of IV Colistin Among Children in the US: Query of Pediatric ID specialists (2005-2011) Number of children prescribed IV colistin . Tamma PD et al. 2012 Pediatric MDR Bacterial Infections: can we treat? Use of IV Colistin Among Children in the US (2005-2011 ) • Prescribed by only 22% of 239 Pediatric ID specialists • Use to treat MDR Pseudomonas or Acinetobacter baumanii; CPE and ESBL-producing Enterobacteriaceae • Additional antimicrobials administered to 84% of cases • Resistance to colistin developed in 20.5% of isolates • Toxicities – Nephrotoxicity in 22% (more common in children ≥13 yrs) – Neurotoxicity (reversible) observed in 4.3% Tamma PD et al. 2012 Pediatric MDR Bacterial Infections: can we treat? Antibiotic Options for Multidrug-Resistant Gram-Negative Bacilli Nicasio AM et al. 2008 Pediatric MDR Bacterial Infections: can we treat? Novel strategies to treat MDR GN infections • Monte Carlo stimulation modeling to predict success based on (variable) population pK and MIC distributions of only 20 to 30 subjects • Use ANY FDA-approved ACTIVE drug even if not pediatric approved • Direct delivery to attempt to maximize concentration at infection site • Non-traditional susceptibility testing in tissue culture: macrolides inhibit pseudomonas influx pumps making it susceptible (in vivo correlate?) Buyck JM et al. 2012 • “High dose-Short course” to reduce bacterial load/avert resistance • Pipeline antibiotics – Ceftazidime + avibactam phase I pediatric study (7/14 completion) – All others only in adult pipeline, or not yet advanced to human trials Pediatric MDR Bacterial Infections: can we treat? C. difficile hospitalization and mortality rate trends among adults, USA (CDC) Hospitalizations increasing* Mortality rates plateauing *Is the apparent increase in infection rate reflect better detection or over diagnosis? Pediatric MDR Bacterial Infections: can we treat? Clostridium difficile (CDI) • Responsible for 12% of all HAI Magill S et al. 2014 • Toxin causes pseudomembranous colitis, toxic mega colon, colonic perforation, sepsis • Antibiotic exposure increases risk • Increasing rates and severity of CDI since 2003 due to – Increasing fluoroquinolone use in adults – Inadequate hand hygiene – Emergence of new C. difficile strain: Greater virulence, 20-fold higher toxin production, often fluoroquinolone resistant Pediatric MDR Bacterial Infections: can we treat? Age-specific incidence Clostridium difficile infection per 10,000 pediatric hospitalizations in US, 1997–2006 Health Care Utilization Project Kids' and Inpatient Database (CDC) Pediatric MDR Bacterial Infections: can we treat? Which is an appropriate testing strategy to confirm the diagnosis of Clostridium difficile infection? A. Stool culture B. C difficile toxin enzyme immunoassay on 3 consecutive stool specimens C. C difficile nucleic acid-based assay on a single stool specimen D. Stool ova/parasite trichrome stain Clostridium difficile Diarrhea in Children: Diagnosis, Management, and Prevention. Medscape. Jan 24, 2014. Pediatric MDR Bacterial Infections: can we treat? What is the recommended therapy for a child with moderate initial Clostridium difficile infection? A. Vancomycin given intravenously B. Metronidazole given IV and vancomycin given PO C. Metronidazole given orally D. Metronidazole given PO with an ant peristaltic medication Clostridium difficile Diarrhea in Children: Diagnosis, Management, and Prevention. Medscape. Jan 24, 2014. Pediatric MDR Bacterial Infections: can we treat? Guidelines for C. difficile treatment Bauer MP et al. 2009; Cohen SH et al. 2010 Pediatric MDR Bacterial Infections: can we treat? Guidelines for C. difficile treatment Bauer MP et al. 2009; Cohen SH et al. 2010 Pediatric MDR Bacterial Infections: can we treat? Guidelines for C. difficile treatment • Vancomycin use to treat the 1st episode of CDI in children admitted to children's hospitals in the US Increased significantly (P = 0.005) between 2006-2011. • There is no empirical evidence to suggest superiority of oral vancomycin over oral metronidazole. Schwenk HT et al. 2013 Pediatric MDR Bacterial Infections: can we treat? Prevention of Antibiotic-Associated Diarrhea • Appropriate antibiotic use: Reduced fluoroquinolone and cephalosporin use led to a 60% drop in CDI use in England • Probiotics? Controlled trials ongoing • C difficile spores persist: Use gowns and gloves! • Alcohol does not kill C difficile spores effectively: Hand washing with soap and water is optimal • Most disinfectants are not sporicidal: Use sodium hypochlorite to clean rooms • Hospitals following infection control recommendations lowered rates by 20% in less than 2 years Pediatric MDR Bacterial Infections: can we treat? Emerging challenges in MRSA infections: Summary • MRSA accounts for > 50% of CA S. aureus infections • Most CA isolates carry the PVL gene, associated with increased virulence and morbidity (USA 300) • Include anti- GA HS coverage in empiric Rx of SSTI • I&D alone may be sufficient for uncomplicated abscesses • Clindamycin-resistance frequency is increasing • Include vancomycin for severe suspected MRSA infections • Linezolid is a safe and effective alternative against VISA Pediatric MDR Bacterial Infections: can we treat? Emerging challenges in MDR GNB infections: Summary • Adversely affect cost and health outcomes • Carbapenems are no longer a cure-all for GNR • Broad-spectrum cephalosporin use contributes to emergence of ESBL-bacteria • Practice changes to curb growing UTI resistance rates: – Routine urinalysis and urine culture testing: – Selectively use antibiotic prophylaxis: – Use local antibiograms • Consider the possibility of MDR CRE, ESBL and/or quinolone resistant bacteria in choosing empiric Rx Pediatric MDR Bacterial Infections: can we treat? Emerging challenges in MDR GNB infections: Summary • Lack of pediatric pK/pD and clinical outcome data • Maximize pharmacodynamics-based dosing (e.g. continuous infusion for meropenem) • Use combination therapy for additive or synergistic effect, or to prevent emergence of resistance. • Newer compounds (e.g. avibactam plus ceftaroline) may improve options • Polymyxins: use as a last resort (limited pediatric pK/pD data, reversible nephro- and neurotoxicity) Pediatric MDR Bacterial Infections: can we treat? Emerging challenges in C. difficile infections: Summary • • • • • The most common HAI pathogen Overuse of flouroquinolones contributes to CDI Nucleic acid-based assay should be used to diagnose Overuse of oral vancomycin to treat in children Metronidazole PO is 1st-line agent for mild-moderate disease • Strict hand-hygiene and environmental decontamination are critical to control Pediatric MDR Bacterial Infections: can we treat? Opportunities and novel approaches to prevent HAI in children • Surveillance of MDR GNR in High-risk Neonates? Colonized infants are at risk for MDRGN infection important reservoir for nosocomial transmission Simon A et al. 2013 • Infection Prevention – Hand washing, etc – Fomites (stethoscopes, white coats, etc) • Diagnose and treat infections effectively • Vaccination- S. aureus, C. difficile, Pseudomonas? Always wash your hands! Pediatric MDR Bacterial Infections: can we treat? Fomites: White coats and Accessories • White coats are frequently contaminated with potentially harmful bacteria, such as Staphylococcus aureus, gramnegative bacteria, and others, including MDR pathogens. Banu A et al. 2012. • Bare Below the Elbows (BBE) bans the wearing of long sleeves watches, wristbands, bracelets, or rings to enable better hand and wrist hygiene. (No proven benefit). National Health Service UK 2007 . Pediatric MDR Bacterial Infections: can we treat? Fomites: Neckties, Identification Badges and Personal Items • Hand and stethoscope contamination correlate directly • Secure neckties and other personal apparel items by a white coat or other means to prevent contact with the patient • Any item that comes into direct contact with the patient or environment should be disinfected regularly, replaced, or eliminated Longtin Y, et al. 2014. Pediatric MDR Bacterial Infections: can we treat? Every time antibiotics are prescribed: 1. Order cultures before antibiotics are given and start drugs promptly. 2. Specify indication, dose, and expected duration in the patient record. 3. Reassess within 48 hours and adjust Rx if necessary or stop Rx if indicated. After CDC Vital Signs, 2014 Pediatric MDR Bacterial Infections: can we treat? Considerations for common prescribing situations • UTIs: Do culture results represent true infection and not colonization? – Assess for signs and symptoms of UTI. – Obtain urinalysis with every urine culture. • Pneumonia: Do symptoms truly represent pneumonia? • MRSA: Is MRSA growing in clinically relevant cultures? – Do not use vancomycin to treat infections caused by MSSA! • Always treat for recommended length of time After CDC Vital Signs, 2014 Pediatric MDR Bacterial Infections: can we treat? Common Clinical Syndromes: Durations of Antimicrobial Treatment • Community-Acquired Pneumonia: 10 days • Ventilator-Associated Pneumonia: 7 days, (15 days for nonfermenting Gram-negative organisms) • Urinary Tract Infections due to E. coli – Acute uncomplicated cystitis (treat for 3-10d): – Pyelonephritis: 7 days may be adequate • Uncomplicated SSTI: 5-10 days (5 generally adequate) • Complicated Intra-abdominal Infections: 4-7 days After Septimus EJ et al. 2012 Pediatric MDR Bacterial Infections: can we treat? Opportunities and novel approaches to prevent HAI in children: Vaccines • Nosocomial viruses – Rotavirus – Norovirus (pipeline) – Influenza • MDR pathogens – – – – Pneumococcus: conjugate (PCV13) and 23-valent polysaccharide (PPV23) S. aureus (pipeline) Pseudomonas (vaccine or mAb Rx) (pipeline) Klebsiella pneumoniae • C. difficile toxoid (pipeline) Use antibiotics wisely Do not! • treat viral infections with antibiotics • treat contamination or colonization • treat with mild doses of antibiotics over long periods • continue empiric treatment if no pathogen isolated • use vancomycin, quinolones or cephalosporins needlessly • use antimicrobial prophylaxis indiscriminately Do • use shortened antibiotic courses with proven efficacy (OM, pharyngitis) • use a combination of drugs to treat a bacterial infection • use local antibiogram data • promote adherence – (improve palatabity) • reduce use of antibiotics in domestic livestock • stop therapy when infection is cured or unlikely Pediatric MDR Bacterial Infections: can we treat? Opportunities and novel approaches to prevent HAI in children: parting thoughts • Changes in antibacterial regimens may reduce one problem yet opportunistic bacteria may fill the vacant niche. • Aim for a better balance between the accumulation of resistance and new antibacterial development. Livermore DM et al. 2003 Clinicians Hold the Solution (CDC)