* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Document
Survey
Document related concepts
Temperature wikipedia , lookup
Superconductivity wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Sessile drop technique wikipedia , lookup
Surface tension wikipedia , lookup
Gibbs paradox wikipedia , lookup
Thermodynamics wikipedia , lookup
Degenerate matter wikipedia , lookup
Ionic liquid wikipedia , lookup
Supercritical fluid wikipedia , lookup
Superfluid helium-4 wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Van der Waals equation wikipedia , lookup
Spinodal decomposition wikipedia , lookup
Equilibrium chemistry wikipedia , lookup
Glass transition wikipedia , lookup
Equation of state wikipedia , lookup
Liquid crystal wikipedia , lookup
Transcript
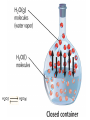
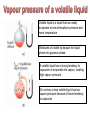
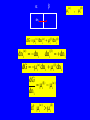
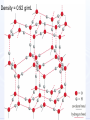
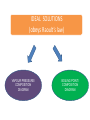
CHEM 21112 Phase Equilibria (5L) CHEM 21112 Basic Physical Chemistry II Course content: Quantum mechanics (9 h) Surface and colloid chemistry (6 h) Atomic and molecular spectroscopy (9h) Phase equilibria (6 h): Thermodynamical description of mixtures, partial molar quantities; partial molar volume and Gibbs free energy. Phases, components and degree of freedom, the phase rule, phase diagrams; interpretation, lever rule. Liquid-liquid phase diagrams; phase separation, critical solution temperatures. Temperature-composition diagrams; distillation of mixtures, zeotropes and azeotropes. Liquid solid phase diagrams; eutectics and three component systems. Textbooks "Physical Chemistry" P.W. Atkins Oxford University Press "Elements of Physical Chemistry" P.W. Atkins Oxford University Press Equilibrium two opposing processes occurring at the same rate a system at equilibrium is in balance H2O(l) H2O(g) Equilibrium is a state in which there are no observable changes as time goes by. Chemical equilibrium is achieved when: • the rates of the forward and reverse reactions are equal and they are not zero. • the concentrations of the reactants and products remain constant Chemical equilibrium N2O4 (g) 2NO2 (g) Physical equilibrium the changes that occur are physical processes H2O(l) H2O(g) Chemical equilibrium chemical equilibrium involves different substances as reactants and products N2O4 (g) 2NO2 (g) Phase Specific state of matter that is uniform through out in composition and physical state Phase State of matter A homogenous portion of a system that has uniform physical and chemical characteristics 1) The mixture of ice and water two phases, solid and liquid 2) The mixture of oxygen gas and nitrogen gas (the system is Homogeneous) 3) CaCO3(s) CaO(s) + CO2(g) one phase, gas phase = 3 phases (2 solid,1 gas) Phase Changes • • • • • Sublimation: solid → gas. Vaporization: liquid → gas. Melting or fusion: solid → liquid. Condensation: gas → liquid. Freezing: liquid → solid. Phase Changes Energy Changes Accompanying Phase Changes Enthalpy Enthalpy (H) ~ heat content (q) @ constant pressure ∆H = thermal (heat) energy change = q Physical process H2O (l) + energy H2O (g) ∆Hvap = 44 kJ/mole heat of vaporization What does the sign of the enthalpy tell you? Endothermic process or reaction ∆H > 0 or positive Exothermic process or reaction ∆H < 0 or negative Gas-Liquid Equilibration Heating Curves • Plot of temperature change versus heat added is a heating curve. • During a phase change, adding heat causes no temperature change. – These points are used to calculate ∆Hfus and ∆Hvap. Heating Curves Vapor Pressure Explaining Vapor Pressure on the Molecular Level • Some of the molecules on the surface of a liquid have enough energy to escape the attraction of the bulk liquid. • These molecules move into the gas phase. • As the number of molecules in the gas phase increases, some of the gas phase molecules strike the surface and return to the liquid. • After some time the pressure of the gas will be constant at the vapor pressure. Volatile liquid is a liquid that can easily evaporate at one atmospheric pressure and room temperature Molecules of volatile liq escape the liquid phase into gaseous phase. A volatile liquid has a strong tendency to vapourize or evaporate into vapour, creating high vapour pressure. On contrary a less volatile liquid has low vapour pressure because of lower tendency to vapourize Vapour pressure increases with increasing temperature due to its KE When a liquid evaporates in a closed vessel, its gaseous molecules formed above the liquid have high KE and exert a vapour pressure. sublimation Microscopic equilibrium between gas and liquid. Note that the rate of evaporation of the liquid is equal to the rate of condensation of the gas. Microscopic equilibrium between gas and solid. Note that the rate of evaporation of the solid is equal to the rate of condensation of the gas. Types of Molecules: the types of molecules that make up a solid or liquid determine its vapor pressure. If the intermolecular forces between molecules are: 1 ethyl ether (C4H10O) Pvapor (25oC) = 520 torr ethyl alcohol (C2H6O) Pvapor (25oC) = 75 torr •relatively strong, the vapor pressure will be relatively low. •relatively weak, the vapor pressure will be relatively high. Temperature: at a higher temperature, more molecules have enough energy to escape from the liquid or solid. At a lower temperature, fewer molecules have sufficient energy to escape from the liquid or solid. Low Temperature 2 High Temperature Vapor Pressure and Boiling Point • Liquids boil when the external pressure equals the vapor pressure. • Temperature of boiling point increases as pressure increases. • Two ways to get a liquid to boil: increase temperature or decrease pressure. – Pressure cookers operate at high pressure. At high pressure the boiling point of water is higher than at 1 atm. Therefore, there is a higher temperature at which the food is cooked, reducing the cooking time required. • Normal boiling point is the boiling point at 760 mmHg (1 atm). Phase Diagrams • Phase diagram: plot of pressure vs. Temperature summarizing all equilibria between phases. • Given a temperature and pressure, phase diagrams tell us which phase will exist. Features of a phase diagram: – Triple point: temperature and pressure at which all three phases are in equilibrium. – Vapor-pressure curve: generally as pressure increases, temperature increases. – Critical point: critical temperature and pressure for the gas. H2O Standard phase diagram for one component system B CO2 Critical point ??? O A ????? Phase Diagrams Gibbs Free Energy ? 2nd Law of Thermodynamics A reaction is spontaneous if ∆S for the universe is positive. ∆Suniverse = ∆Ssystem + ∆Ssurroundings ∆Suniverse > 0 for spontaneous process First calc. entropy created by matter dispersal (∆Ssystem) Next, calc. entropy created by energy dispersal (∆Ssurround) 29 Entropy, S One property common to spontaneous processes is that the final state is more DISORDERED or RANDOM than the original. Spontaneity is related to an increase in randomness. The thermodynamic property related to randomness is ENTROPY, S. Reaction of K with water 30 31 The entropy of liquid water is greater than the entropy of solid water (ice) at 0˚ C. 32 Entropy, S So (J/K•mol) H2O(liq) 69.95 H2O(gas) 188.8 S (gases) > S (liquids) > S (solids) Second law states: • Entropy of the Universe must increase in a spontaneous process ∆Suniv 〉 0 spontaneous ∆Suniv = 0 equilibrium ∆Suniv = ∆Ssys + ∆Ssurr Do we have to keep calculating ∆Suniv ? Not necessarily! A convenient way of using second law…… From last lecture…….. Phase Specific state of matter that is uniform through out in composition and physical state Phase Diagrams • • Phase diagram: plot of pressure vs. Temperature summarizing all equilibria between phases. Given a temperature and pressure, phase diagrams tell us which phase will exist. Gibbs Free Energy - New state function Gibbs Free Energy The Gibbs Free Energy is a new state function, defined as: G = H − TS At constant temperature and pressure, ∆G is ∆G = ∆H − T∆S We only have to think about the system, NOT the Universe…… Josiah Willard Gibbs (1839-1903) Gibbs Free Energy ‘G’ is extremely useful for chemistry and biochemistry, since so much takes place at constant temperature and pressure. The condition of constant T and P is very important when using G. Otherwise, the entropy change of the surroundings might be different leading to a different result. At constant T and P, consideration of ∆G will answer the question “Will a given reaction be spontaneous?” ∆G < 0 ∆G > 0 ∆G = 0 process is spontaneous reverse process is spontaneous Equilibrium The Gibbs Free Energy is a direct measure of spontaneity Phase Equilibria X(phase 1) X(phase 2) At equilibrium, ∆G = 0 H20 (g) So, µ(phase 1) = µ(phase 2) H20 (l) Chemical Potential (µ) Chemical potential of component A, µA, is defined as the partial molar Gibbs free energy: ∂G µ A = ∂n A T ,P ,n j ≠ n A This is the change in G with respect to a infinitesimal change in the amount of component A with all other parameters held constant. It is essentially the free energy increase (or decrease) associated with adding a little of A to the system. α β µ i( α ) dni dG = µ i( α ) dn i( α ) + µ i(β ) dn i(β ) dn (α) i = −dn i dn (β ) i = + dn i dG = −µ dn i + µ dn i (α) i (β) i dG (β) (α) = µi − µi dn i if µ i( α ) > µ i( β ) , µ i(β ) dG =0 dn i µ (α) i =µ (β ) i Phase Equilibria X(phase 1) X(phase 2) At equilibrium, ∆G = 0 H20 (g) So, µ(phase 1) = µ(phase 2) H20 (l) Equation of a phase boundary G Master Equation (α) m =G (β ) m dG = Vdp − SdT ((αα)) m m dG dG V dp dp −−SS dT dT == V ((αα)) m m ((αα)) m m and dG (β ) m = V dp − S dT (β ) m (β ) m dG (α) m = dG (β) m V dp − S dT = V dp − S dT (α) m (α) m (α) m dp(V (β) m (β ) m − V ) = dT (S (β ) m (α) m (α) m dp S = dT V (α) m −S ) (β ) m (β ) m (β ) m −S −V dp ∆Sm = dT ∆Vm Clapeyron equation What Clapeyron equation says….? dp ∆Sm = dT ∆Vm The Phase Diagrams of H2O and CO2 When water freezes, it expands. The density of solid water is less than the density of liquid water. Ice floats Why? Density = 0.92 g/mL Density = 1.00 g/mL Fig. 3-UN4 Ice: stable hydro- Liquid water: transient hydrogen gen bonds bonds Phase From last few lectures…….. Specific state of matter that is uniform through out in composition and physical state Phase Diagrams • • Phase diagram: plot of pressure vs. Temperature summarizing all equilibria between phases. Given a temperature and pressure, phase diagrams tell us which phase will exist. Gibbs Free Energy - New state function Chemical Potential (µ) At equilibrium, ∆G = 0 µ(phase 1) = µ(phase 2) What Clapeyron equation says….? dp ∆Sm = dT ∆Vm Standard phase diagram for water (H2O) Special case !!!!! TA curve = known as melting point or freezing point Represent the equilibrium between ice and liquid Has a negative slope Water as the liquid is denser than the solid (ice floats on water). That means that an increase of pressure favors the formation of liquid and that the melting point of water falls with increasing pressure. This unique properties of water is due to the network of hydrogen bonding in ice is more extensive than in liquid H2O Standard phase diagram for one component system B O A CO2 H2O CO2 dp ∆Sm = dT ∆Vm dp ∆Sm = dT ∆Vm ΔSpc qrev, pc q rev ,pc ∆Spc = Tpc ∆H pc = Tpc dp ∆H m = dT T∆Vm Clausius-Clapeyron Equation Rudolf Clausius 1822 – 1888 Emile Clapeyron 1799 - 1864 German Mathematical Physicist French Engineer Clausius-Clapeyron equation − ∆H m 1 ln p = + const. R T p2 − ∆H m ln = p1 R 1 1 − T2 T1 P1 and P2 are the vapor pressures at T1 and T2 respectively Common Applications • Calculate the vapor pressure of a liquid at any temperature (with known vapor pressure at a given temperature and known heat of vaporization) • Calculate the boiling point of a liquid at a nonstandard pressure p2 − ∆H m ln = p1 R 1 1 − T2 T1 ∆H can be determined from measurements of vapor pressure at multiple temperatures. ln P(atm) From Clausius-Clapeyron equation we see that –∆Hm/R is the slope lnP vs 1/T. − ∆H m 1 ln p = + const. R T liquid water solid ice ∆Hvap ∆Hsub water vapor 1/T Phase Diagrams Normal: Occurs at 1 atm. Critical Point: A combination of temperature and pressure beyond which a gas cannot be liquefied. • Critical Temperature: The temperature beyond which a • Critical Pressure: The pressure beyond which a liquid gas cannot be liquefied regardless of the pressure. cannot be vaporized regardless of the temperature. Supercritical Fluid: A state of matter beyond the critical point that is neither liquid nor gas. Triple Point: A point at which three phases coexist in equilibrium. www.abertay.ac.uk/careers Critical Temperature and Pressure • Gases liquefied by increasing pressure at some temperature. • Critical temperature: the minimum temperature for liquefaction of a gas using pressure. • Critical pressure: pressure required for liquefaction. Critical Temperature, Tc Transition to Supercritical CO2 Supercritical CO2 Used to Decaffeinate Coffee EXAMPLES OF GREEN CHEMISTRY • Safer dry cleaning – Initially gasoline and kerosene were used – Chlorinated solvents are now used, such as perchloroethylene – Supercritical/liquid carbon dioxide (CO2) The Phase Diagrams of H2O and CO2 • Water: – The melting point curve slopes to the left because ice is less dense than water. – Triple point occurs at 0.0098°C and 4.58 mmHg. – Normal melting (freezing) point is 0°C. – Normal boiling point is 100°C. – Critical point is 374°C and 218 atm. • Carbon Dioxide: – Triple point occurs at -56.4°C and 5.11 atm. – Normal sublimation point is -78.5°C. (At 1 atm CO2 sublimes it does not melt.) – Critical point occurs at 31°C and 73 atm. The Phase Diagrams of H2O and CO2 Phase From last few lectures…….. Specific state of matter that is uniform through out in composition and physical state Phase Diagrams • • Phase diagram: plot of pressure vs. Temperature summarizing all equilibria between phases. Given a temperature and pressure, phase diagrams tell us which phase will exist. Gibbs Free Energy - New state function Chemical Potential (µ) At equilibrium, ∆G = 0 µ(phase 1) = µ(phase 2) What Clapeyron equation says….? dp ∆Sm = dT ∆Vm p2 − ∆H m ln = p1 R 1 1 − T2 T1 Phase Rule Four phases of a single substance cannot coexist in mutual equilibrium. F=C–P+2 INTERNATIONAL FOOD POLICY RESEARCH INSTITUTE F = C – P +2 Degree of freedom or the number of independent variables The number of phase Number of component 2 variables (temperature and pressure) F=C–P+2 F = # degrees of freedom The number of intensive parameters that must be specified in order to completely determine the system P = # of phases Phases are mechanically separable constituents C = minimum # of components Chemical constituents that must be specified in order to define all phases 2 = 2 intensive parameters Usually temperature and pressure OR The number of chemical species that can explained the composition of all phase in a system The least number of different substances required to describe the composition of all phases in the system 1) water, CO2 = one component 2) Aqueous solution of potassium nitrate = 2 system component because have potassium nitrate salt and water. COMPONENT DEGREE OF FREEDOM (F) The number of variables that may be changed independently without causing the appearance of a new phase or disappearance of an existing phase UNIVARIANT TYPES BIVARIANT EXAMPLES CaCO3(s) CaO(s) F = C – P +2 =2–3+2 = 1 (univariant) + CO2(g) Calculate the degree of freedom (F) Means: only one variable, either temperature or pressure can be changed independently The number of components is not always easy to determine at first glance, and it may require careful examination of the pyhsical conditions of the system at equilibrium Standard phase diagram for water (H2O) ONE COMPONENT SYSTEM Standard phase diagram for carbon dioxide (CO2) Example 1 – Liquid region In a single phase region (two degrees of freedom), both temperature and pressure can be varied independently within the limits of the phase boundaries without INTERNATIONAL FOOD POLICY RESEARCH INSTITUTE changing the phase F=C–P+2 Example 2 – Freezing line When two phases are in equilibrium (one degree of freedom), either temperature or pressure can vary independently, but not both INTERNATIONAL FOOD POLICY RESEARCH INSTITUTE F=C–P+2 Example 3 – Triple point At the triple point (three phase equilibrium) there are no degrees of freedom. Therefore, in this situation, neither temperature or pressure may be varied without altering one or two of the phases INTERNATIONAL FOOD POLICY RESEARCH INSTITUTE Phase diagrams of mixtures Two component systems are better described by binary phase diagrams In these equilibrium diagrams we have a composition variable in addition to the usual temperature and pressure variables. We therefore need a three dimensional diagram to plot all three variables. To simplify binary phase diagrams are usually drawn at atmospheric pressure, showing variations of temperature and composition only. INTERNATIONAL FOOD POLICY RESEARCH INSTITUTE Raoult’s law – in explaining the effect of non-volatile solute on vapour pressure of solvent and its melting and boiling point Two completely miscible liquid – ideal, non-ideal, positive and negative deviation TWO COMPONENT SYSTEM Composition diagram vs boiling point composition for ideal, non-ideal, negative n positive deviation Eutectic system and cooling curves Fractional distillation and azeotropic system Single component systems Ex. H2O, CO2 Two component systems Liquid – liquid phase diagrams F=C–P+2 F=4–P F' = 3 – P Ideal solutions [obeys Raoult's law ] F=C–P+2 F=4–P F' = 3 – P 3 types : 1. Complete Miscible liquid 2. Half miscible liquid 3. Immiscible liquid Methanol and ethanol Liquid in liquid ether and water Oil and water Ideal solution- mostly involve the substance that have similar physicochemical properties. Ex: MeOH/EtOH, benzene/toluene Complete Miscible liquid 2 types of completely miscible liquids which are ideal and non-ideal solutions An ideal solution is a solution that obeys Raoult’s law and non-ideal solution disobey. A solution is a ideal solution when: A A = A B The intermolecular attractions between the mixture of same molecules are equal with the mixture of different molecules. The volume of the mixture is the total volume of both liquids. No heat changes (no endo-exothermic process) Obeys Raoult’s law Liquid – liquid phase diagrams • Consider a solution of two liquids, say benzene and toluene held at a constant temperature. • Benzene and toluene form very nearly ideal solutions Ideal solution obeys Raoult's law where pi is the partial pressure of component i above the liquid mixture (or the vapor pressure of i in the mixture), Xi is the mole fraction of component i in the liquid, and pi* is the vapor pressure of pure component i. IDEAL SOLUTIONS (obeys Raoult’s law) VAPOUR PRESSURE/ COMPOSITION DIAGRAM BOILING POINT/ COMPOSITION DIAGRAM Vapor Pressure Diagram Temperature versus composition We call this line a “tie line” It intersects the upper curve at the composition of the vapor in equilibrium with the liquid at the same temperature Mixtures of volatile liquids The Liquid–Vapor Temperature– Composition Phase Diagram of Benzene and Toluene at 1.00 atm. EXAMPLE !!!!! Which vapour sample rich at this point? composition Fractional distillation Boiling diagrams provide an explanation for fractional distillation. “Theoretical plates” Distillation – Fractional Vapor line Temperature °C 110 Liquid line 100 90 80 Mole % Toluene 0 Mole % Benzene 100 20 80 40 60 60 40 80 20 100 0 Composition (mole%) As the hot vapors leave the distilling flask, they condense on the first cold surface, completing one vaporization-condensation cycle. Vapors from the Distilling flask Suppose we distill the same 80:20 mixture of toluene to benzene we did in the simple distillation example Distillation – Fractional Vapor line Temperature °C 110 Liquid line 100 90 80 Mole % Toluene 0 Mole % Benzene 100 20 80 40 60 60 40 80 20 100 0 Composition (mole%) This surface begins to heat from the condensed vapors which are now 55:45 toluene-benzene Vapors from the Distilling flask This benzene enriched liquid now has a boiling point of ~94 °C (lower than the incoming vapors) and it begins to boil off this higher surface Distillation – Fractional Vapor line Temperature °C 110 Liquid line 100 90 80 Mole % Toluene 0 Mole % Benzene 100 20 80 40 60 60 40 80 20 100 0 Composition (mole%) These vapors are even further enriched in benzene (now 30:70, toluene:benzene) and condense on the next cold surface Vapors from the Distilling flask Distillation – Fractional Vapor line Temperature °C 110 Liquid line 100 90 80 Mole % Toluene 0 Mole % Benzene 100 20 80 40 60 60 40 80 20 100 0 Composition (mole%) This condensed liquid has an even lower boiling point (86 °C) and as this surface heats it begins to boil off this next higher surface Vapors from the Distilling flask Distillation – Fractional Vapor line Temperature °C 110 Liquid line 100 90 80 Mole % Toluene 0 Mole % Benzene 100 20 80 40 60 60 40 80 20 100 0 Composition (mole%) This vapor now condenses on the next cold surface (now 20:80, toluene:benzene) and the cycle continues Vapors from the Distilling flask Distillation – Fractional 1:99 toluene:benzene Vapor line Temperature °C 110 Liquid line 100 90 80 Mole % Toluene 0 Mole % Benzene 100 20 80 40 60 60 40 80 20 100 0 Composition (mole%) This cycle will continue until the top of the column is reached The liquid collected after seven cycles is now 99% benzene! Vapors from the Distilling flask 80:20 toluene-benzene Distillation – Fractional 1:99 toluene:benzene Vapor line Temperature °C 110 Liquid line 100 90 80 Mole % Toluene 0 Mole % Benzene 100 20 80 40 60 60 40 80 20 100 0 Composition (mole%) Each vapor-condensation (or mini-distillation) cycle is known as one theoretical plate Vapors from the Distilling flask 80:20 toluene-benzene The length of distillation column required to provide one theoretical plate of separation is known as the height equivalent theoretical plate (HETP) Single component systems Ex. H2O, CO2 Two component systems Liquid – liquid phase diagrams F=C–P+2 F=4–P F' = 3 – P Ideal solutions [obeys Raoult's law ] Non Ideal solutions [Azeotrope mixture] negative/positive deviation NON-IDEAL SOLUTIONS • Negative deviation • Positive deviation Intermolecular forces between molecules in the solution are stronger than those in pure liquid Therefore, vapour pressure of the solution is lower than vapour pressure of its components or pure liquid. Example : A A = B B WEAKER THAN A B the molecules in the solution have lower tendency to escape into vapour phase. Therefore the process is EXOTHERMIC Boiling diagrams for non-ideal solutions Azeotrope mixture Ex: Formed when the intermolecular forces between molecules in the mixture are weaker than those in pure liquids. A A = B B STRONGER THAN A Vapour pressure of the solution is higher than expected B The solution has a greater tendency to evaporate or escape into vapour The process is endothermic "low boiling azeotrope." AZEOTROPE It is known as a constant boiling mixture or an azeotropic mixture or an azeotrope. An azeotrope is a mixture of two (or more) miscible liquids that when boiled produce the same composition in the vapor phase as that is present in the original mixture. Single component systems Ex. H2O, CO2 Two component systems Liquid – liquid phase diagrams F=C–P+2 F=4–P F' = 3 – P Ideal solutions [obeys Raoult's law ] Non Ideal solutions [Azeotrope mixture] negative/positive deviation Liquid – Solid phase diagrams Eutectic systems Liquid –solid phase diagrams The knowledge of temperature-composition diagrams for solid mixtures guides the design of important industrial processes, such as the manufacture of liquid crystal displays and semiconductors. Liquid –solid phase diagrams Eutactic composition: the mixture with lowest melting point. A solid with a eutactic composition melts, without changing the composition, at the lowest temperature of any mixture.