* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chapter 4
Survey
Document related concepts
Transcript
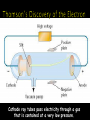
Chapter 4 Ted Ed: Just How Small is an Atom? Scale Atoms are the smallest particle of an element that still behaves like that element. Generally, atoms are arranged such that there is a dense center core (the nucleus) surrounded by a diffuse field. In (almost) every atom, there are the following three key subatomic particles: ◦ Proton ◦ Neutron ◦ Electron Below the level of protons, neutrons, and electrons are other subatomic particles. Without going into too much depth: ◦ Protons are made of three quarks, two “up” and one “down.” ◦ Neutrons are made of three quarks, two “down” and one “up.” ◦ Electrons are made of…electrons. Protons ◦ ◦ ◦ ◦ Location: Nucleus Charge: Positive Mass: 1 amu Symbol: p+ Neutrons ◦ ◦ ◦ ◦ Location: Nucleus Charge: None Mass: 1 amu Symbol: n0 Electrons ◦ Location: A cloud around the nucleus, moving at high speeds Actually, VERY FAR from the nucleus, too. ◦ Charge: Negative ◦ Mass: 0 amu (so small it’s rounded to virtually nothing) ◦ Symbol: e- Atomic Number Element Symbol • The number of protons in the nucleus of each atom of that element. • Identifies the element. • This is also equal to the number of electrons in a neutral atom. Element 6 # of protons 6 # of electrons 6 Phosphorus 15 15 15 Gold 79 79 79 Carbon Atomic # • The total number of protons and neutrons in the nucleus of an isotope. • Always round the mass number to the highest/lowest whole number. • Can be written as Na-23 or 23Naer Na-23 or 23Na. Mass # = p+ + n0 16 O 8 16 Arsenic Phosphorus 8 75 16 8 16 33 75 15 31 How many protons, electrons and neutrons are in the following atoms? 9Be 4 ◦ Protons = 4 Electrons = 4 Neutrons = 5 20Ne 10 ◦ Protons = 10 Electrons = 10 Neutrons = 10 35Cl 17 ◦ Protons = 17 Electrons = 17 Neutrons = 18 Build the following elements (don’t forget all the subatomic particles): ◦ Hydrogen, Oxygen, Lithium, Carbon Questions to ponder: ◦ What happens when you change the number of protons? ◦ What happens when you change the number of neutrons? ◦ What happens with you change the number of electrons? Changing the number of protons changes the element. In other words, the atomic number of an element never changes. ◦ It is always equal to the atomic number. Changing the number of neutrons in an atom changes the atomic mass, creating a new isotope. Isotopes are atoms of the same element that have different atomic masses. ◦ Example: Carbon always has 6 protons. Sometimes it has 6 neutrons, sometimes it has 8 neutrons. ◦ Thus, Carbon (mass 12) and Carbon (mass 14) are isotopes. Changing the number of electrons in an atom changes the charge, creating a ion. If an atom loses an electron it becomes positively charged and is called a cation. If an atom gains an electron it becomes negatively charged and is called a anion. Isotopes are atoms of the same element having different masses due to varying numbers of neutrons. Isotope Protons Electrons Neutrons Hydrogen–1 (protium) 1 1 0 Hydrogen-2 (deuterium) 1 1 1 Hydrogen-3 (tritium) 1 1 2 Nucleus Atomic mass is the weighted average of all the naturally occurring isotopes of that element. Isotope Symbol Composition of the nucleus % in nature Carbon-12.00 12C 6 protons 6 neutrons 98.89% Carbon-13.00 13C 6 protons 7 neutrons 1.11% Carbon-14.00 14C 6 protons 8 neutrons <0.01% Carbon = 12.011 The following table list the isotopes of copper with the relative abundances. Calculate the average atomic mass of copper. Isotope Symbol Composition of the nucleus % in nature Copper-63.00 63Cu 29 protons 34 neutrons 69.20% Copper-65.00 65Cu 29 protons 36 neutrons 30.80% Step 1 multiply the first isotope mass by the decimal of the % in nature. Repeat for any remaining isotopes. Step 2 Add to determine the atomic mass unit for that element. 63.00 * 0.6920 = 43.60 65.00 * 0.3080 = 20.02 43.60 + 20.02 Carbon = 63.62 amu Calculate the atomic mass of bromine. The two isotopes of bromine have atomic masses and relative abundances of 78.92 amu (50.69%) and 80.92 amu (49.31%). All elements are composed of tiny indivisible particles called atoms. An atom is the smallest particle of an element that retains the properties of that element Atoms of the same element are identical. The atoms of any one element are different from those of any other element. Atoms of different elements can physically mix together or can chemically combine in simple wholenumber ratios to form compounds. Chemical reactions occur when atoms are separated, joined or rearranged. Atoms of one element can never be changed into atoms of another element as a result of a chemical reaction. Cathode ray tubes pass electricity through a gas that is contained at a very low pressure. Cathode rays have identical properties regardless of the element used to produce them. All elements must contain identically charged electrons. Atoms are neutral, so there must be positive particles in the atom to balance the negative charges of the electron. Electrons have so little mass that atoms must contain other particles that account for most of the mass. Particles (helium nuclei) were fired at a thin sheet of gold foil. Particle hits on the detection screen are recorded. Most of the particles passed right through A few particles were deflected VERY FEW were greatly deflected CONCLUSIONS The nucleus is small The nucleus is dense The nucleus is positively charged Atomic masses were known, but mass of electron and proton didn’t add up to the total. There must be something else in the atom! Neutrons Contained in the nucleus No charge Mass equals that of a proton (1.67 x 10-24 g)