* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 5.9 M - Thierry Karsenti
Survey
Document related concepts
Transmission (medicine) wikipedia , lookup
Traveler's diarrhea wikipedia , lookup
Hospital-acquired infection wikipedia , lookup
Microorganism wikipedia , lookup
Phospholipid-derived fatty acids wikipedia , lookup
Disinfectant wikipedia , lookup
Triclocarban wikipedia , lookup
Human microbiota wikipedia , lookup
Bacterial cell structure wikipedia , lookup
Bacterial taxonomy wikipedia , lookup
Transcript
http://en.wikipedia.org/wiki/Amoeboid
Amoeboid
From Wikipedia, the free encyclopedia
Jump to: navigation, search
Amoeboid
Scientific classification
Classes and subclasses
Class Lobose pseudopods
Amoebozoa
Percolozoa
Class Filose pseudopods
Cercozoa
Vampyrellids
Nucleariids
Class Reticulose pseudopods
Foraminifera
Gymnophryids
Class Actinopods
Radiolaria
Heliozoa
Foraminiferan (Ammonia tepida)
Heliozoan (Actinophrys sol)
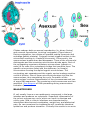
Amoeboids are unicellular lifeforms that mainly consist of contractile vacuoles, a
nucleus, and cytoplasm as their basic structure. They move and feed by means of
temporary cytoplasmic projections, called pseudopods (false feet). They have
appeared in a number of different groups. Some cells in multicellular animals may be
amoeboid, for instance human white blood cells, which consume pathogens. Many
protists also exist as individual amoeboid cells, or take such a form at some point in
their life-cycle. The most famous such organism is Amoeba proteus; the name amoeba
is variously used to describe its close relatives, other organisms similar to it, or the
amoeboids in general.
[edit] Morphological categories
Amoeboids may be divided into several morphological categories based on the form
and structure of the pseudopods. Those where the pseudopods are supported by
regular arrays of microtubules are called actinopods, and forms where they are not are
called rhizopods, further divided into lobose, filose, and reticulose amoebae. There is
also a strange group of giant marine amoeboids, the xenophyophores, that do not fall
into any of these categories.
•
Lobose pseudopods are blunt, and there may be one or several on a cell,
which is usually divided into a layer of clear ectoplasm surrounding more
granular endoplasm. Most, including Amoeba itself, move by the body mass
flowing into an anterior pseudopod. The vast majority form a monophyletic
group called the Amoebozoa, which also includes most slime moulds. A
second group, the Percolozoa, includes protists that can transform between
amoeboid and flagellate forms.
•
Filose pseudopods are narrow and tapering. The vast majority of filose
amoebae, including all those that produce shells, are placed within the
Cercozoa together with various flagellates that tend to have amoeboid forms.
The naked filose amoebae comprise two other groups, the vampyrellids and
nucleariids. The latter appear to be close relatives of animals and fungi.
•
Reticulose pseudopods are cytoplasmic strands that branch and merge to
form a net. They are found most notably among the Foraminifera, a large
group of marine protists that generally produce multi-chambered shells. There
are only a few sorts of naked reticulose amoeboids, notably the gymnophryids,
and their relationships are not certain.
•
Actinopods are divided into the radiolaria and heliozoa. The radiolaria are
mostly marine protists with complex internal skeletons, including central
capsules that divide the cells into granular endoplasm and frothy ectoplasm
that keeps them buoyant. The heliozoa include both freshwater and marine
forms that use their axopods to capture small prey, and only have simple
scales or spines for skeletal elements. Both groups appear to be polyphyletic.
. However, amoeboids have appeared separately in many other groups, including
various different lines of algae not listed above.
Δů==Subphylum Sarcodina== Sarcodina is a subphylum of the phylum
Sarcomastigophora, of unicellular life forms that move by cytoplasmic flow. Some
species use cytoplasmic extensions called pseudopodia for locomotion or feeding. The
subphylum includes such protozoa as the common amoeba and the Foraminifera and
Radiolaria. Most members of the subphylum reproduce asexually through fission,
although some reproduce sexually. Sarcodina is sometimes subdůivided into two
classes - Rhizopoda and Actinopoda.ÒΜκŁΔβΑhi mom.
[edit] External links
•
•
•
•
•
The Amoebae website brings together information from published sources.
Amoebas are more than just blobs
sun animacules and amoebas
Molecular Expressions Digital Video Gallery: Pond Life - Amoeba (Protozoa)
Some good, informative Amoeba videos.
Joseph Leidy's Amoeba Plates
Retrieved from "http://en.wikipedia.org/wiki/Amoeboid"
Categories: Protista | Cell biology | Amoeboids | Motile cells
Views
•
•
•
•
Article
Discussion
Edit this page
History
Personal tools
•
Log in / create account
Navigation
•
•
•
•
•
Main Page
Contents
Featured content
Current events
Random article
Interaction
•
•
•
•
•
•
About Wikipedia
Community portal
Recent changes
Contact Wikipedia
Donate to Wikipedia
Help
Search
Toolbox
•
•
•
•
•
•
•
What links here
Related changes
Upload file
Special pages
Printable version
Permanent link
Cite this page
Languages
•
•
•
•
•
•
•
•
•
•
•
Deutsch
Eesti
Español
Euskara
Français
Hrvatski
日本語
(Norsk (nynorsk)(
Polski
Svenska
Türkçe
•
This page was last modified on 28 March 2008, at 19:39.
•
•
•
•
All text is available under the terms of the GNU Free Documentation License.
(See Copyrights for details.)
Wikipedia® is a registered trademark of the Wikimedia Foundation, Inc., a
U.S. registered 501(c)(3) tax-deductible nonprofit charity.
Privacy policy
About Wikipedia
Disclaimers
http://en.wikipedia.org/wiki/Sporozoans
Apicomplexa
From Wikipedia, the free encyclopedia
(Redirected from Sporozoans)
Jump to: navigation, search
Apicomplexa
Scientific classification
Domain:
Eukaryota
Kingdom:
Chromalveolata
Superphylum: Alveolata
Phylum:
Apicomplexa
Classes & Subclasses
Aconoidasida
•
•
Haemosporasina
Piroplasmasina
Blastocystea
Conoidasida
•
•
Coccidiasina
Gregarinasina
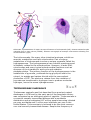
The Apicomplexa are a large group of protists, characterized by the presence of a
unique organelle called an apical complex (see also apicoplast). They are unicellular,
spore-forming, and exclusively parasites of animals. Motile structures such as flagella
or pseudopods are absent except in certain gamete stages. This is a diverse group
including organisms such as coccidia, gregarines, piroplasms, haemogregarines, and
malarias; some diseases caused by apicomplexan organisms include:
•
•
•
•
Babesiosis (Babesia)
Malaria (Plasmodium)
Cryptosporidiosis (Cryptosporidium)
Coccidian diseases including:
o Cryptosporidiosis (Cryptosporidium parvum)
o Cyclosporiasis (Cyclospora cayetanensis)
o Toxoplasmosis (Toxoplasma gondii)
Most members have a complex life-cycle, involving both asexual and sexual
reproduction. Typically, a host is infected by ingesting cysts, which divide to produce
sporozoites that enter its cells. Eventually, the cells burst, releasing merozoites which
infect new cells. This may occur several times, until gamonts are produced, forming
gametes that fuse to create new cysts. There are many variations on this basic pattern,
however, and many Apicomplexa have more than one host.
Generic life cycle of an apicomplexa: 1-zygote (cyst), 2-sporozoites, 3-merozoites, 4gametocytes.
Apicomplexan structure: 1-polar ring, 2-conoid, 3-micronemes, 4-rhoptries, 5nucleus, 6-nucleolus, 7-mitochondria, 8-posterior ring, 9-alveoli, 10-golgi apparatus,
11-micropore.
The apical complex includes vesicles called rhoptries and micronemes, which open at
the anterior of the cell. These secrete enzymes that allow the parasite to enter other
cells. The tip is surrounded by a band of microtubules, called the polar ring, and
among the Conoidasida there is also a funnel of rods called the conoid..[1] Over the
rest of the cell, except for a diminished mouth called the micropore, the membrane is
supported by vesicles called alveoli, forming a semi-rigid pellicle.
The presence of alveoli and other traits place the Apicomplexa among a group called
the alveolates. Several related flagellates, such as Perkinsus and Colpodella have
structures similar to the polar ring and were formerly included here, but most appear
to be closer relatives of the dinoflagellates. They are probably similar to the common
ancestor of the two groups.
Another similarity is that apicomplexan cells contain a single plastid, called the
apicoplast, surrounded by either 3 or four membranes. Its functions are thought to
include tasks such as lipid synthesis, it appears to be necessary for survival. They are
generally considered to share a common origin with the chloroplasts of
dinoflagellates, although some studies suggest they are ultimately derived from green
rather than red algae.
The Apicomplexa comprise the bulk of what used to be called the Sporozoa, a group
for parasitic protozoans without flagella, pseudopods, or cilia. Most of the
Apicomplexa are motile however. The other main lines were the Ascetosporea, the
Myxozoa (now known to be derived from animals), and the Microsporidia (now
known to be derived from fungi). Sometimes the name Sporozoa is taken as a
synonym for the Apicomplexa, or occasionally as a subset.
Contents
[hide]
•
•
•
•
1 Blood borne genera
2 Disease Genomics
3 References
4 External links
[edit] Blood borne genera
Within the Apicomplexa there are three groups of blood borne parasites. These
species lie within in three suborders.
•
•
suborder Adeleorina - 8 genera
suborder Haemosporina - all genera in this suborder
•
suborder Eimeriorina - 2 genera (Lankesterella and Schellackia)
Blood parasites belonging to the suborder Adeleorina are collectively known as
haemogregarines. Currently their sister group is thought to be the piroplasms.
Suborder Adeleorina has ~400 species and has been organised into four large and 4
small genera.
The larger genera are:
•
family Haemogregarinidae - taxon created by Neveu-Lemaire in 1901
genera:
•
•
Haemogregarina - taxon created by Danilewsky in 1885
Cyrilia - taxon created by Lainson in 1981
•
family Karyolysidae - taxon created by Wenyon in 1926
genera:
•
Karyolysus - taxon created by Labbe in 1894
•
family Hepatozoidae - taxon created by Wenyon in 1926
genera:
•
Hepatozoon - taxon created by Miller in 1908
The smaller genera are :
•
•
Hemolivia - taxon created by Petit et al in 1990
Desseria - taxon created by Siddall in 1995
•
family Dactylosomatidae
genera:
•
•
Dactylosoma
Babesiosoma
Notes:
Species of the genus Desseria infect fish and lack erythrocytic merogony.
The species of the genera Dactylosoma and Babesiosoma infect fish and reptiles.
Leeches are the only known vectors for these species and their vertebrate hosts are
aquatic.
[edit] Disease Genomics
As noted above, many of the apicomplexan parasites are important pathogens of
human and domestic animals. In contrast to bacterial pathogens, these apicomplexan
parasites are eukaryotes and share many metabolic pathways with their animal hosts.
This fact makes therapeutic target development extremely difficult – a drug that
harms an apicomplexan parasite is also likely to harm its human host. Currently there
are no effective vaccines or treatments available for most diseases caused by these
parasites. Biomedical research on these parasites is challenging because it is often
difficult, if not impossible, to maintain live parasite cultures in the laboratory and to
genetically manipulate these organisms. In the recent years, several of the
apicomplexan species have been selected for genome sequencing. The availability of
genome sequences provides a new opportunity for scientists to learn more about the
evolution and biochemical capacity of these parasite. A NIH-funded database,
ApiDB.org, provides public access to currently available genomic data sets.
[edit] References
1. ^ Duszynski1, Donald W.; Steve J. Upton and Lee Couch (2004-02-21). The
Coccidia of the World (Online database). Department of Biology, University of New
Mexico, and Division of Biology, Kansas State University.
[edit] External links
•
The Taxonomicon & Systema Naturae (Website database). Taxon: Genus
Cryptosporidium. Universal Taxonomic Services, Amsterdam, The
Netherlands (2000).
Retrieved from "http://en.wikipedia.org/wiki/Apicomplexa"
Categories: Parasitic protists | Apicomplexa
Views
•
•
•
•
Article
Discussion
Edit this page
History
Personal tools
•
Log in / create account
Navigation
•
•
•
•
•
Main Page
Contents
Featured content
Current events
Random article
Interaction
•
•
•
•
•
•
About Wikipedia
Community portal
Recent changes
Contact Wikipedia
Donate to Wikipedia
Help
Search
Toolbox
•
•
•
•
•
•
•
What links here
Related changes
Upload file
Special pages
Printable version
Permanent link
Cite this page
Languages
•
•
•
•
•
•
•
•
•
•
•
•
•
Català
Česky
Deutsch
Español
Français
עברית
Nederlands
日本語
Plattdüütsch
Polski
Português
Српски / Srpski
Svenska
•
Türkçe
http://en.wikipedia.org/wiki/Bacterial_growth
Bacterial growth
From Wikipedia, the free encyclopedia
Jump to: navigation, search
Growth is shown as L = log(numbers) where numbers is the number of colony
forming units per ml, versus T (time.)
Bacterial growth is the division of one bacterium into two idential daughter cells
during a process called binary fission. Hence, local doubling of the bacterial
population occurs. Both daughter cells from the division do not necessarily survive.
However, if the number surviving exceeds unity on average, the bacterial population
undergoes exponential growth. The measurement of an exponential bacterial growth
curve in batch culture was traditionally a part of the training of all microbiologists; the
basic means requires bacterial enumeration (cell counting) by direct and individual
(microscopic, flow cytometry[1]), direct and bulk (biomass), indirect and individual
(colony counting), or indirect and bulk (most probable number, turbidity, nutrient
uptake) methods. Models reconcile theory with the measurements [2].
In autecological studies, bacterial growth in batch culture can be modeled with four
different phases: lag phase (A), exponential or log phase (B), stationary phase (C),
and death phase (D).
1. During lag phase, bacteria adapt themselves to growth conditions. It is the
period where the individual bacteria are maturing and not yet able to divide.
During the lag phase of the bacterial growth cycle, synthesis of RNA, enzymes
and other molecules occurs.
2. During the exponential phase (sometimes called the log phase), the number of
new bacteria appearing per unit time is proportional to the present population.
This gives rise to the classic exponential growth curve, in which the logarithm
of the population density rises linearly with time (see figure). The actual rate
of this growth (i.e. the slope of the line in the figure) depends upon the growth
conditions, which affect the frequency of cell division events and the
probability of both daughter cells surviving. Exponential growth cannot
continue indefinitely, however, because the medium is soon depleted of
nutrients and enriched with wastes.
3. During stationary phase, the growth rate slows as a result of nutrient depletion
and accumulation of toxic products. This phase is reached as the bacteria
begin to exhaust the resources that are available to them.
4. At death phase, bacteria run out of nutrients and die.
This basic batch culture growth model draws out and emphasizes aspects of bacterial
growth which may differ from the growth of macrofauna. It emphasizes clonality,
asexual binary division, the short development time relative to replication itself, the
seemingly low death rate, the need to move from a dormant state to a reproductive
state or to condition the media, and finally, the tendency of lab adapted strains to
exhaust their nutrients.
In reality, even in batch culture, the four phases are not well defined. The cells do not
reproduce in synchrony without explicit and continual prompting (as in experiments
with stalked bacteria [3]) and their logarithmic phase growth is often not ever a
constant rate, but instead a slowly decaying rate, a constant stochastic response to
pressures both to reproduce and to go dormant in the face of declining nutrient
concentrations and increasing waste concentrations.
Batch culture is the most common laboratory growth environment in which bacterial
growth is studied, but it is only one of many. It is ideally spatially unstructured and
temporally structured. The bacterial culture is incubated in a closed vessel with a
single batch of medium. In some experimental regimes, some of the bacterial culture
is periodically removed to a fresh sterile media is added. In the extreme case, this
leads to the continual renewal of the nutrients. This is a chemostat also known as
continuous culture. It is ideally spatially unstructured and temporally unstructured, in
an equilibrium state defined by the nutrient supply rate and the reaction of the
bacteria. In comparison to batch culture, bacteria are maintained in expodential
growth phase and the grow growth rate of the bacteria is known. Related devices
include turbidostats and auxostats.
Bacterial growth can be suppressed with bacteriostats, without necessarily
killing the bacteria. In a synecological, a true-to-nature situation, where more than
one bacterial species is present, the growth of microbes is more dynamic and
continual.
Liquid is not the only laboratory environment for bacterial growth. Spatially
structured environments such as biofilms or agar surfaces present additional complex
growth models.
[edit] References
1. ^ Skarstad K, Steen HB, Boye E (1983). "Cell cycle parameters of slowly growing
Escherichia coli B/r studied by flow cytometry". J. Bacteriol. 154 (2): 656–62. PMID
6341358.
2. ^ Zwietering M H, Jongenburger I, Rombouts F M, van 'T Riet K (1990). "Modeling
of the Bacterial Growth Curve". Applied and Environmental Microbiology 56 (6):
1875-1881.
3. ^ Novick A (1955). "Growth of Bacteria". Annual Review of Microbiology 9: 97110.
[edit] External links
•
•
•
An examination of the exponential growth of bacterial populations
Science aid: Microbial Populations
Microbial Growth, BioMineWiki
This article includes material from an article posted on 26 April 2003 on Nupedia;
written by Nagina Parmar; reviewed and approved by the Biology group; editor,
Gaytha Langlois; lead reviewer, Gaytha Langlois ; lead copyeditors, Ruth Ifcher. and
Jan Hogle.
Retrieved from "http://en.wikipedia.org/wiki/Bacterial_growth"
Categories: Bacteriology | Population
Views
•
•
•
•
Article
Discussion
Edit this page
History
Personal tools
•
Log in / create account
Navigation
•
•
•
•
•
Main Page
Contents
Featured content
Current events
Random article
Interaction
•
•
About Wikipedia
Community portal
•
•
•
•
Recent changes
Contact Wikipedia
Donate to Wikipedia
Help
Search
Toolbox
•
•
•
•
•
•
•
What links here
Related changes
Upload file
Special pages
Printable version
Permanent link
Cite this page
Languages
•
•
Polski
Українська
•
•
This page was last modified on 12 March 2008, at 23:31.
All text is available under the terms of the GNU Free Documentation License.
(See Copyrights for details.)
Wikipedia® is a registered trademark of the Wikimedia Foundation, Inc., a
U.S. registered 501(c)(3) tax-deductible nonprofit charity.
Privacy policy
About Wikipedia
Disclaimers
•
•
•
Bacteria
http://en.wikipedia.org/wiki/Bacteria
From Wikipedia, the free encyclopedia
Jump to: navigation, search
For other uses, see Bacteria (disambiguation).
Bacteria
Fossil range: Archean or earlier Recent
Escherichia coli cells magnified
25,000 times
Scientific classification
Domain: Bacteria
Phyla
Acidobacteria
Actinobacteria
Aquificae
Bacteroidetes
Chlamydiae
Chlorobi
Chloroflexi
Chrysiogenetes
Cyanobacteria
Deferribacteres
Deinococcus-Thermus
Dictyoglomi
Fibrobacteres
Firmicutes
Fusobacteria
Gemmatimonadetes
Nitrospirae
Planctomycetes
Proteobacteria
Spirochaetes
Thermodesulfobacteria
Thermomicrobia
Thermotogae
Verrucomicrobia
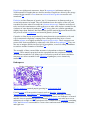
Bacteria (singular: bacterium) are unicellular microorganisms. Typically a few
micrometres in length, bacteria have a wide range of shapes, ranging from spheres to
rods to spirals. Bacteria are ubiquitous in every habitat on Earth, growing in soil,
acidic hot springs, radioactive waste,[1] seawater, and deep in the Earth's crust. There
are typically 40 million bacterial cells in a gram of soil and a million bacterial cells in
a millilitre of fresh water; in all, there are approximately five nonillion (5×1030)
bacteria on Earth,[2] forming much of the world's biomass.[3] Bacteria are vital in
recycling nutrients, and many important steps in nutrient cycles depend on bacteria,
such as the fixation of nitrogen from the atmosphere. However, most of these bacteria
have not been characterized, and only about half of the phyla of bacteria have species
that can be cultured in the laboratory.[4] The study of bacteria is known as
bacteriology, a branch of microbiology.
There are approximately ten times as many bacterial cells as human cells in the
human body, with large numbers of bacteria on the skin and in the digestive tract.[5]
Although the vast majority of these bacteria are rendered harmless or beneficial by the
protective effects of the immune system, a few are pathogenic bacteria and cause
infectious diseases, including cholera, syphilis, anthrax, leprosy and bubonic plague.
The most common fatal bacterial diseases are respiratory infections, with tuberculosis
alone killing about 2 million people a year, mostly in sub-Saharan Africa.[6] In
developed countries, antibiotics are used to treat bacterial infections and in various
agricultural processes, so antibiotic resistance is becoming common. In industry,
bacteria are important in processes such as sewage treatment, the production of cheese
and yoghurt, and the manufacture of antibiotics and other chemicals.[7]
Bacteria are prokaryotes. Unlike cells of animals and other eukaryotes, bacterial cells
do not contain a nucleus and rarely harbour membrane-bound organelles. Although
the term bacteria traditionally included all prokaryotes, the scientific classification
changed after the discovery in the 1990s that prokaryotic life consists of two very
different groups of organisms that evolved independently from an ancient common
ancestor. These evolutionary domains are called Bacteria and Archaea.[8]
Contents
[hide]
•
•
•
•
•
•
•
•
•
•
1 History of bacteriology
2 Origin and early evolution
3 Morphology
4 Cellular structure
o 4.1 Intracellular structures
o 4.2 Extracellular structures
o 4.3 Endospores
5 Metabolism
6 Growth and reproduction
7 Genetics
8 Movement
9 Classification and identification
10 Interactions with other organisms
o 10.1 Mutualists
o 10.2 Pathogens
•
•
•
•
•
11 Significance in technology and industry
12 See also
13 References
14 Further reading
15 External links
History of bacteriology
Further information: Microbiology
Antonie van Leeuwenhoek, the first microbiologist and the first person to observe
bacteria using a microscope.
Bacteria were first observed by Antonie van Leeuwenhoek in 1676, using a singlelens microscope of his own design.[9] He called them "animalcules" and published his
observations in a series of letters to the Royal Society.[10][11][12] The name bacterium
was introduced much later, by Christian Gottfried Ehrenberg in 1828, and is derived
from the Greek word βακτήριον -α , bacterion -a , meaning "small staff".[13]
Louis Pasteur demonstrated in 1859 that the fermentation process is caused by the
growth of microorganisms, and that this growth is not due to spontaneous generation.
(Yeasts and molds, commonly associated with fermentation, are not bacteria, but
rather fungi.) Along with his contemporary, Robert Koch, Pasteur was an early
advocate of the germ theory of disease.[14] Robert Koch was a pioneer in medical
microbiology and worked on cholera, anthrax and tuberculosis. In his research into
tuberculosis, Koch finally proved the germ theory, for which he was awarded a Nobel
Prize in 1905.[15] In Koch's postulates, he set out criteria to test if an organism is the
cause of a disease; these postulates are still used today.[16]
Though it was known in the nineteenth century that bacteria are the cause of many
diseases, no effective antibacterial treatments were available.[17] In 1910, Paul Ehrlich
developed the first antibiotic, by changing dyes that selectively stained Treponema
pallidum—the spirochaete that causes syphilis—into compounds that selectively
killed the pathogen.[18] Ehrlich had been awarded a 1908 Nobel Prize for his work on
immunology, and pioneered the use of stains to detect and identify bacteria, with his
work being the basis of the Gram stain and the Ziehl-Neelsen stain.[19]
A major step forward in the study of bacteria was the recognition in 1977 by Carl
Woese that archaea have a separate line of evolutionary descent from bacteria.[20] This
new phylogenetic taxonomy was based on the sequencing of 16S ribosomal RNA, and
divided prokaryotes into two evolutionary domains, as part of the three-domain
system.[21]
Origin and early evolution
Further information: Timeline of evolution
The ancestors of modern bacteria were single-celled microorganisms that were the
first forms of life to develop on earth, about 4 billion years ago. For about 3 billion
years, all organisms were microscopic, and bacteria and archaea were the dominant
forms of life.[22][23] Although bacterial fossils exist, such as stromatolites, their lack of
distinctive morphology prevents them from being used to examine the past history of
bacterial evolution, or to date the time of origin of a particular bacterial species.
However, gene sequences can be used to reconstruct the bacterial phylogeny, and
these studies indicate that bacteria diverged first from the archaeal/eukaryotic
lineage.[24] The most recent common ancestor of bacteria and archaea was probably a
hyperthermophile that lived about 2.5 billion–3.2 billion years ago.[25][26]
Bacteria were also involved in the second great evolutionary divergence, that of the
archaea and eukaryotes. Here, eukaryotes resulted from ancient bacteria entering into
endosymbiotic associations with the ancestors of eukaryotic cells, which were
themselves possibly related to the Archaea.[27][28] This involved the engulfment by
proto-eukaryotic cells of alpha-proteobacterial symbionts to form either mitochondria
or hydrogenosomes, which are still being found in all known Eukarya (sometimes in
highly reduced form, e.g. in ancient "amitochondrial" protozoa). Later on, an
independent second engulfment by some mitochondria-containing eukaryotes of
cyanobacterial-like organisms led to the formation of chloroplasts in algae and plants.
There are even some algal groups known that clearly originated from subsequent
events of endosymbiosis by heterotrophic eukaryotic hosts engulfing a eukaryotic
algae that developed into "second-generation" plastids.[29][30]
Morphology
Bacteria display a large diversity of cell morphologies and arrangements
Bacteria display a wide diversity of shapes and sizes, called morphologies. Bacterial
cells are about 10 times smaller than eukaryotic cells and are typically 0.5–
5.0 micrometres in length. However, a few species–for example Thiomargarita
namibiensis and Epulopiscium fishelsoni–are up to half a millimetre long and are
visible to the unaided eye.[31] Among the smallest bacteria are members of the genus
Mycoplasma, which measure only 0.3 micrometres, as small as the largest viruses.[32]
Most bacterial species are either spherical, called cocci (sing. coccus, from Greek
kókkos, grain, seed) or rod-shaped, called bacilli (sing. bacillus, from Latin baculus,
stick). Some rod-shaped bacteria, called vibrio, are slightly curved or comma-shaped;
others, can be spiral-shaped, called spirilla, or tightly coiled, called spirochaetes. A
small number of species even have tetrahedral or cuboidal shapes.[33] This wide
variety of shapes is determined by the bacterial cell wall and cytoskeleton, and is
important because it can influence the ability of bacteria to acquire nutrients, attach to
surfaces, swim through liquids and escape predators.[34][35]
Many bacterial species exist simply as single cells, others associate in characteristic
patterns: Neisseria form diploids (pairs), Streptococcus form chains, and
Staphylococcus group together in "bunch of grapes" clusters. Bacteria can also be
elongated to form filaments, for example the Actinobacteria. Filamentous bacteria are
often surrounded by a sheath that contains many individual cells; certain types, such
as species of the genus Nocardia, even form complex, branched filaments, similar in
appearance to fungal mycelia.[36]
The range of sizes shown by prokaryotes, relative to those of other organisms and
biomolecules
Bacteria often attach to surfaces and form dense aggregations called biofilms or
bacterial mats. These films can range from a few micrometers in thickness to up to
half a meter in depth, and may contain multiple species of bacteria, protists and
archaea. Bacteria living in biofilms display a complex arrangement of cells and
extracellular components, forming secondary structures such as microcolonies,
through which there are networks of channels to enable better diffusion of
nutrients.[37][38] In natural environments, such as soil or the surfaces of plants, the
majority of bacteria are bound to surfaces in biofilms.[39] Biofilms are also important
for chronic bacterial infections and infections of implanted medical devices, as
bacteria protected within these structures are much harder to kill than individual
bacteria.[40]
Even more complex morphological changes are sometimes possible. For example,
when starved of amino acids, Myxobacteria detect surrounding cells in a process
known as quorum sensing, migrate towards each other, and aggregate to form fruiting
bodies up to 500 micrometres long and containing approximately 100,000 bacterial
cells.[41] In these fruiting bodies, the bacteria perform separate tasks; this type of
cooperation is a simple type of multicellular organisation. For example, about one in
10 cells migrate to the top of these fruiting bodies and differentiate into a specialised
dormant state called myxospores, which are more resistant to desiccation and other
adverse environmental conditions than are ordinary cells.[42]
Cellular structure
Further information: Bacterial cell structure
Diagram of the cellular structure of a typical bacterial cell
Intracellular structures
The bacterial cell is surrounded by a lipid membrane, or cell membrane, which
encompasses the contents of the cell and acts as a barrier to hold nutrients, proteins
and other essential components of the cytoplasm within the cell. As they are
prokaryotes, bacteria do not tend to have membrane-bound organelles in their
cytoplasm and thus contain few intracellular structures. They consequently lack a
nucleus, mitochondria, chloroplasts and the other organelles present in eukaryotic
cells, such as the Golgi apparatus and endoplasmic reticulum.[43] However, recent
research is identifying increasing amounts of structural complexity in bacteria, such as
the discovery of the prokaryotic cytoskeleton.[44][45]
Many important biochemical reactions, such as energy generation, occur due to
concentration gradients across membranes, creating a potential difference analogous
to a battery. The absence of internal membranes in bacteria means these reactions,
such as electron transport, occur across the cell membrane, between the cytoplasm
and the periplasmic space.[46] Additionally, while some transporter proteins consume
chemical energy, others harness concentration gradients to import nutrients across the
cell membrane or to expel undesired molecules from the cytoplasm.
Bacteria do not have a membrane-bound nucleus, and their genetic material is
typically a single circular chromosome located in the cytoplasm in an irregularly
shaped body called the nucleoid.[47] The nucleoid contains the chromosome with
associated proteins and RNA. Like all living organisms, bacteria contain ribosomes
for the production of proteins, but the structure of the bacterial ribosome is different
from those of eukaryotes and Archaea.[48] The order Planctomycetes are an exception
to the general absence of internal membranes in bacteria, because they have a
membrane around their nucleoid and contain other membrane-bound cellular
structures.[49]
Some bacteria produce intracellular nutrient storage granules, such as glycogen,[50]
polyphosphate,[51] sulfur[52] or polyhydroxyalkanoates.[53] These granules enable
bacteria to store compounds for later use. Certain bacterial species, such as the
photosynthetic Cyanobacteria, produce internal gas vesicles, which they use to
regulate their buoyancy - allowing them to move up or down into water layers with
different light intensities and nutrient levels.[54]
Extracellular structures
Further information: Cell envelope
Around the outside of the cell membrane is the bacterial cell wall. Bacterial cell walls
are made of peptidoglycan (called murein in older sources), which is made from
polysaccharide chains cross-linked by unusual peptides containing D-amino acids.[55]
Bacterial cell walls are different from the cell walls of plants and fungi, which are
made of cellulose and chitin, respectively.[56] The cell wall of bacteria is also distinct
from that of Archaea, which do not contain peptidoglycan. The cell wall is essential to
the survival of many bacteria, and the antibiotic penicillin is able to kill bacteria by
inhibiting a step in the synthesis of peptidoglycan.[56]
There are broadly speaking two different types of cell wall in bacteria, called Grampositive and Gram-negative. The names originate from the reaction of cells to the
Gram stain, a test long-employed for the classification of bacterial species.[57]
Gram-positive bacteria possess a thick cell wall containing many layers of
peptidoglycan and teichoic acids. In contrast, Gram-negative bacteria have a relatively
thin cell wall consisting of a few layers of peptidoglycan surrounded by a second lipid
membrane containing lipopolysaccharides and lipoproteins. Most bacteria have the
Gram-negative cell wall, and only the Firmicutes and Actinobacteria (previously
known as the low G+C and high G+C Gram-positive bacteria, respectively) have the
alternative Gram-positive arrangement.[58] These differences in structure can produce
differences in antibiotic susceptibility; for instance, vancomycin can kill only Grampositive bacteria and is ineffective against Gram-negative pathogens, such as
Haemophilus influenzae or Pseudomonas aeruginosa.[59]
In many bacteria an S-layer of rigidly arrayed protein molecules covers the outside of
the cell.[60] This layer provides chemical and physical protection for the cell surface
and can act as a macromolecular diffusion barrier. S-layers have diverse but mostly
poorly understood functions, but are known to act as virulence factors in
Campylobacter and contain surface enzymes in Bacillus stearothermophilus.[61]
Helicobacter pylori electron micrograph, showing multiple flagella on the cell surface
Flagella are rigid protein structures, about 20 nanometres in diameter and up to
20 micrometres in length, that are used for motility. Flagella are driven by the energy
released by the transfer of ions down an electrochemical gradient across the cell
membrane.[62]
Fimbriae are fine filaments of protein, just 2–10 nanometres in diameter and up to
several micrometers in length. They are distributed over the surface of the cell, and
resemble fine hairs when seen under the electron microscope. Fimbriae are believed
to be involved in attachment to solid surfaces or to other cells and are essential for the
virulence of some bacterial pathogens.[63] Pili (sing. pilus) are cellular appendages,
slightly larger than fimbriae, that can transfer genetic material between bacterial cells
in a process called conjugation (see bacterial genetics, below).[64]
Capsules or slime layers are produced by many bacteria to surround their cells, and
vary in structural complexity: ranging from a disorganised slime layer of extracellular polymer, to a highly structured capsule or glycocalyx. These structures can
protect cells from engulfment by eukaryotic cells, such as macrophages.[65] They can
also act as antigens and be involved in cell recognition, as well as aiding attachment
to surfaces and the formation of biofilms.[66]
The assembly of these extracellular structures is dependent on bacterial secretion
systems. These transfer proteins from the cytoplasm into the periplasm or into the
environment around the cell. Many types of secretion systems are known and these
structures are often essential for the virulence of pathogens, so are intensively
studied.[67]
Endospores
Further information: Endospores
Bacillus anthracis (stained purple) growing in cerebrospinal fluid
Certain genera of Gram-positive bacteria, such as Bacillus, Clostridium,
Sporohalobacter, Anaerobacter and Heliobacterium, can form highly resistant,
dormant structures called endospores.[68] In almost all cases, one endospore is formed
and this is not a reproductive process, although Anaerobacter can make up to seven
endospores in a single cell.[69] Endospores have a central core of cytoplasm containing
DNA and ribosomes surrounded by a cortex layer and protected by an impermeable
and rigid coat.
Endospores show no detectable metabolism and can survive extreme physical and
chemical stresses, such as high levels of UV light, gamma radiation, detergents,
disinfectants, heat, pressure and desiccation.[70] In this dormant state, these organisms
may remain viable for millions of years,[71][72] and endospores even allow bacteria to
survive exposure to the vacuum and radiation in space.[73] Endospore-forming bacteria
can also cause disease: for example, anthrax can be contracted by the inhalation of
Bacillus anthracis endospores, and contamination of deep puncture wounds with
Clostridium tetani endospores causes tetanus.[74]
Metabolism
Further information: Microbial metabolism
Filaments of photosynthetic cyanobacteria
In contrast to higher organisms, bacteria exhibit an extremely wide variety of
metabolic types.[75] The distribution of metabolic traits within a group of bacteria has
traditionally been used to define their taxonomy, but these traits often do not
correspond with modern genetic classifications.[76] Bacterial metabolism is classified
on the basis of three major criteria: the kind of energy used for growth, the source of
carbon, and the electron donors used for growth. An additional criterion of respiratory
microorganisms are the electron acceptors used for aerobic or anaerobic
respiration.[77]
Carbon metabolism in bacteria is either heterotrophic, where organic carbon
compounds are used as carbon sources, or autotrophic, meaning that cellular carbon is
obtained by fixing carbon dioxide. Typical autotrophic bacteria are phototrophic
cyanobacteria, green sulfur-bacteria and some purple bacteria, but also many
chemolithotrophic species, such as nitrifying or sulfur-oxidising bacteria.[78] Energy
metabolism of bacteria is either based on phototrophy, the use of light through
photosynthesis, or on chemotrophy, the use of chemical substances for energy, which
are mostly oxidised at the expense of oxygen or alternative electron acceptors
(aerobic/anaerobic respiration).
Finally, bacteria are further divided into lithotrophs that use inorganic electron donors
and organotrophs that use organic compounds as electron donors. Chemotrophic
organisms use the respective electron donors for energy conservation (by
aerobic/anaerobic respiration or fermentation) and biosynthetic reactions (e.g. carbon
dioxide fixation), whereas phototrophic organisms use them only for biosynthetic
purposes. Respiratory organisms use chemical compounds as a source of energy by
taking electrons from the reduced substrate and transferring them to a terminal
electron acceptor in a redox reaction. This reaction releases energy that can be used to
synthesise ATP and drive metabolism. In aerobic organisms, oxygen is used as the
electron acceptor. In anaerobic organisms other inorganic compounds, such as nitrate,
sulfate or carbon dioxide are used as electron acceptors. This leads to the ecologically
important processes of denitrification, sulfate reduction and acetogenesis,
respectively.
Another way of life of chemotrophs in the absence of possible electron acceptors is
fermentation, where the electrons taken from the reduced substrates are transferred to
oxidised intermediates to generate reduced fermentation products (e.g. lactate,
ethanol, hydrogen, butyric acid). Fermentation is possible, because the energy content
of the substrates is higher than that of the products, which allows the organisms to
synthesise ATP and drive their metabolism.[79][80]
These processes are also important in biological responses to pollution; for example,
sulfate-reducing bacteria are largely responsible for the production of the highly toxic
forms of mercury (methyl- and dimethylmercury) in the environment.[81] Nonrespiratory anaerobes use fermentation to generate energy and reducing power,
secreting metabolic by-products (such as ethanol in brewing) as waste. Facultative
anaerobes can switch between fermentation and different terminal electron acceptors
depending on the environmental conditions in which they find themselves.
Lithotrophic bacteria can use inorganic compounds as a source of energy. Common
inorganic electron donors are hydrogen, carbon monoxide, ammonia (leading to
nitrification), ferrous iron and other reduced metal ions, and several reduced sulfur
compounds. Unusually, the gas methane can be used by methanotrophic bacteria as
both a source of electrons and a substrate for carbon anabolism.[82] In both aerobic
phototrophy and chemolithotrophy, oxygen is used as a terminal electron acceptor,
while under anaerobic conditions inorganic compounds are used instead. Most
lithotrophic organisms are autotrophic, whereas organotrophic organisms are
heterotrophic.
In addition to fixing carbon dioxide in photosynthesis, some bacteria also fix nitrogen
gas (nitrogen fixation) using the enzyme nitrogenase. This environmentally important
trait can be found in bacteria of nearly all the metabolic types listed above, but is not
universal.[83]
Growth and reproduction
Further information: Bacterial growth
Unlike multicellular organisms, increases in the size of bacteria (cell growth) and
their reproduction by cell division are tightly linked in unicellular organisms. Bacteria
grow to a fixed size and then reproduce through binary fission, a form of asexual
reproduction.[84] Under optimal conditions, bacteria can grow and divide extremely
rapidly, and bacterial populations can double as quickly as every 9.8 minutes.[85] In
cell division, two identical clone daughter cells are produced. Some bacteria, while
still reproducing asexually, form more complex reproductive structures that help
disperse the newly-formed daughter cells. Examples include fruiting body formation
by Myxobacteria and arial hyphae formation by Streptomyces, or budding. Budding
involves a cell forming a protrusion that breaks away and produces a daughter cell.
A growing colony of Escherichia coli cells[86]
In the laboratory, bacteria are usually grown using solid or liquid media. Solid growth
media such as agar plates are used to isolate pure cultures of a bacterial strain.
However, liquid growth media are used when measurement of growth or large
volumes of cells are required. Growth in stirred liquid media occurs as an even cell
suspension, making the cultures easy to divide and transfer, although isolating single
bacteria from liquid media is difficult. The use of selective media (media with specific
nutrients added or deficient, or with antibiotics added) can help identify specific
organisms.[87]
Most laboratory techniques for growing bacteria use high levels of nutrients to
produce large amounts of cells cheaply and quickly. However, in natural
environments nutrients are limited, meaning that bacteria cannot continue to
reproduce indefinitely. This nutrient limitation has led the evolution of different
growth strategies (see r/K selection theory). Some organisms can grow extremely
rapidly when nutrients become available, such as the formation of algal (and
cyanobacterial) blooms that often occur in lakes during the summer.[88] Other
organisms have adaptations to harsh environments, such as the production of multiple
antibiotics by Streptomyces that inhibit the growth of competing microorganisms.[89]
In nature, many organisms live in communities (e.g. biofilms) which may allow for
increased supply of nutrients and protection from environmental stresses.[39] These
relationships can be essential for growth of a particular organism or group of
organisms (syntrophy).[90]
Bacterial growth follows three phases. When a population of bacteria first enter a
high-nutrient environment that allows growth, the cells need to adapt to their new
environment. The first phase of growth is the lag phase, a period of slow growth when
the cells are adapting to the high-nutrient environment and preparing for fast growth.
The lag phase has high biosynthesis rates, as proteins necessary for rapid growth are
produced.[91] The second phase of growth is the logarithmic phase (log phase), also
known as the exponential phase. The log phase is marked by rapid exponential
growth. The rate at which cells grow during this phase is known as the growth rate
(k), and the time it takes the cells to double is known as the generation time (g).
During log phase, nutrients are metabolised at maximum speed until one of the
nutrients is depleted and starts limiting growth. The final phase of growth is the
stationary phase and is caused by depleted nutrients. The cells reduce their metabolic
activity and consume non-essential cellular proteins. The stationary phase is a
transition from rapid growth to a stress response state and there is increased
expression of genes involved in DNA repair, antioxidant metabolism and nutrient
transport.[92]
Genetics
Further information: Plasmid, Genome
Most bacteria have a single circular chromosome that can range in size from only
160,000 base pairs in the endosymbiotic bacteria Candidatus Carsonella ruddii,[93] to
12,200,000 base pairs in the soil-dwelling bacteria Sorangium cellulosum.[94]
Spirochaetes of the genus Borrelia are a notable exception to this arrangement, with
bacteria such as Borrelia burgdorferi, the cause of Lyme disease, containing a single
linear chromosome.[95] The genes in bacterial genomes are usually a single continuous
stretch of DNA and although several different types of introns do exist in bacteria,
these are much more rare than in eukaryotes.[96]
Bacteria may also contain plasmids, which are small extra-chromosomal DNAs that
may contain genes for antibiotic resistance or virulence factors. Another type of
bacterial DNA are integrated viruses (bacteriophages). Many types of bacteriophage
exist, some simply infect and lyse their host bacteria, while others insert into the
bacterial chromosome. A bacteriophage can contain genes that contribute to its host's
phenotype: for example, in the evolution of Escherichia coli O157:H7 and
Clostridium botulinum, the toxin genes in an integrated phage converted a harmless
ancestral bacteria into a lethal pathogen.[97]
Bacteria, as asexual organisms, inherit identical copies of their parent's genes (i.e.,
they are clonal). However, all bacteria can evolve by selection on changes to their
genetic material DNA caused by genetic recombination or mutations. Mutations come
from errors made during the replication of DNA or from exposure to mutagens.
Mutation rates vary widely among different species of bacteria and even among
different clones of a single species of bacteria.[98] Genetic changes in bacterial
genomes come from either random mutation during replication or "stress-directed
mutation", where genes involved in a particular growth-limiting process have an
increased mutation rate.[99]
Some bacteria also transfer genetic material between cells. This can occur in three
main ways. Firstly, bacteria can take up exogenous DNA from their environment, in a
process called transformation. Genes can also be transferred by the process of
transduction, when the integration of a bacteriophage introduces foreign DNA into the
chromosome. The third method of gene transfer is bacterial conjugation, where DNA
is transferred through direct cell contact. This gene acquisition from other bacteria or
the environment is called horizontal gene transfer and may be common under natural
conditions.[100] Gene transfer is particularly important in antibiotic resistance as it
allows the rapid transfer of resistance genes between different pathogens.[101]
Movement
Further information: Chemotaxis, Flagella, Pilus
The different arrangements of bacterial flagella: A-Monotrichous; B-Lophotrichous;
C-Amphitrichous and D-Peritrichous
Motile bacteria can move using flagella, bacterial gliding, twitching motility or
changes of buoyancy.[102] In twitching motility, bacterial use their type IV pili as a
grappling hook, repeatedly extending it, anchoring it and then retracting it with
remarkable force (>80 pN).[103]
Bacterial species differ in the number and arrangement of flagella on their surface;
some have a single flagellum (monotrichous), a flagellum at each end
(amphitrichous), clusters of flagella at the poles of the cell (lophotrichous), while
others have flagella distributed over the entire surface of the cell (peritrichous). The
bacterial flagella is the best-understood motility structure in any organism and is made
of about 20 proteins, with approximately another 30 proteins required for its
regulation and assembly.[102] The flagellum is a rotating structure driven by a motor at
the base that uses the electrochemical gradient across the membrane for power. This
motor drives the motion of the filament, which acts as a propeller. Many bacteria
(such as E. coli) have two distinct modes of movement: forward movement
(swimming) and tumbling. The tumbling allows them to reorient and makes their
movement a three-dimensional random walk.[104] (See external links below for link to
videos.) The flagella of a unique group of bacteria, the spirochaetes, are found
between two membranes in the periplasmic space. They have a distinctive helical
body that twists about as it moves.[102]
Motile bacteria are attracted or repelled by certain stimuli in behaviors called taxes:
these include chemotaxis, phototaxis and magnetotaxis.[105][106] In one peculiar group,
the myxobacteria, individual bacteria move together to form waves of cells that then
differentiate to form fruiting bodies containing spores.[107] The myxobacteria move
only when on solid surfaces, unlike E. coli which is motile in liquid or solid media.
Several Listeria and Shigella species move inside host cells by usurping the
cytoskeleton, which is normally used to move organelles inside the cell. By promoting
actin polymerization at one pole of their cells, they can form a kind of tail that pushes
them through the host cell's cytoplasm.[108]
Classification and identification
Streptococcus mutans visualized with a Gram stain
Further information: Scientific classification, Systematics and Clinical
pathology
Classification seeks to describe the diversity of bacterial species by naming and
grouping organisms based on similarities. Bacteria can be classified on the basis of
cell structure, cellular metabolism or on differences in cell components such as DNA,
fatty acids, pigments, antigens and quinones.[87] While these schemes allowed the
identification and classification of bacterial strains, it was unclear whether these
differences represented variation between distinct species or between strains of the
same species. This uncertainty was due to the lack of distinctive structures in most
bacteria, as well as lateral gene transfer between unrelated species.[109] Due to lateral
gene transfer, some closely related bacteria can have very different morphologies and
metabolisms. To overcome this uncertainty, modern bacterial classification
emphasizes molecular systematics, using genetic techniques such as guanine cytosine
ratio determination, genome-genome hybridization, as well as sequencing genes that
have not undergone extensive lateral gene transfer, such as the rRNA gene.[110]
Classification of bacteria is determined by publication in the International Journal of
Systematic Bacteriology,[111] and Bergey's Manual of Systematic Bacteriology.[112]
The term "bacteria" was traditionally applied to all microscopic, single-celled
prokaryotes. However, molecular systematics showed prokaryotic life to consist of
two separate domains, originally called Eubacteria and Archaebacteria, but now
called Bacteria and Archaea that evolved independently from an ancient common
ancestor.[113] The archaea and eukaryotes are more closely-related to each other than
either is to the bacteria. These two domains, along with Eukarya, are the basis of the
three-domain system, which is currently the most widely used classification system in
microbiolology.[114] However, due to the relatively recent introduction of molecular
systematics and a rapid increase in the number of genome sequences that are
available, bacterial classification remains a changing and expanding field.[4][115] For
example, a few biologists argue that the Archaea and Eukaryotes evolved from Grampositive bacteria.[116]
Identification of bacteria in the laboratory is particularly relevant in medicine, where
the correct treatment is determined by the bacterial species causing an infection.
Consequently, the need to identify human pathogens was a major impetus for the
development of techniques to identify bacteria.
Phylogenetic tree showing the diversity of bacteria, compared to other organisms.[117]
Eukaryotes are colored red, archaea green and bacteria blue.
The Gram stain, developed in 1884 by Hans Christian Gram, characterises bacteria
based on the structural characteristics of their cell walls.[57] The thick layers of
peptidoglycan in the "Gram-positive" cell wall stain purple, while the thin "Gramnegative" cell wall appears pink. By combining morphology and Gram-staining, most
bacteria can be classified as belonging to one of four groups (Gram-positive cocci,
Gram-positive bacilli, Gram-negative cocci and Gram-negative bacilli). Some
organisms are best identified by stains other than the Gram stain, particularly
mycobacteria or Nocardia, which show acid-fastness on Ziehl–Neelsen or similar
stains.[118] Other organisms may need to be identified by their growth in special
media, or by other techniques, such as serology.
Culture techniques are designed to promote the growth and identify particular
bacteria, while restricting the growth of the other bacteria in the sample. Often these
techniques are designed for specific specimens; for example, a sputum sample will be
treated to identify organisms that cause pneumonia, while stool specimens are
cultured on selective media to identify organisms that cause diarrhoea, while
preventing growth of non-pathogenic bacteria. Specimens that are normally sterile,
such as blood, urine or spinal fluid, are cultured under conditions designed to grow all
possible organisms.[119][87] Once a pathogenic organism has been isolated, it can be
further characterised by its morphology, growth patterns such as (aerobic or anaerobic
growth, patterns of hemolysis) and staining.
As with bacterial classification, identification of bacteria is increasingly using
molecular methods. Diagnostics using such DNA-based tools, such as polymerase
chain reaction, are increasingly popular due to their specificity and speed, compared
to culture-based methods.[120] These methods also allow the detection and
identification of "viable but nonculturable" cells that are metabolically active but nondividing.[121] However, even using these improved methods, the total number of
bacterial species is not known and cannot even be estimated with any certainty.
Attempts to quantify bacterial diversity have ranged from 107 to 109 total species, but
even these diverse estimates may be out by many orders of magnitude.[122][123]
Interactions with other organisms
Despite their apparent simplicity, bacteria can form complex associations with other
organisms. These symbiotic associations can be divided into parasitism, mutualism
and commensalism. Due to their small size, commensal bacteria are ubiquitous and
grow on animals and plants exactly as they will grow on any other surface. However,
their growth can be increased by warmth and sweat, and large populations of these
organisms in humans are the cause of body odor.
Mutualists
Certain bacteria form close spatial associations that are essential for their survival.
One such mutualistic association, called interspecies hydrogen transfer, occurs
between clusters of anaerobic bacteria that consume organic acids such as butyric acid
or propionic acid and produce hydrogen, and methanogenic Archaea that consume
hydrogen.[124] The bacteria in this association are unable to consume the organic acids
as this reaction produces hydrogen that accumulates in their surroundings. Only the
intimate association with the hydrogen-consuming Archaea keeps the hydrogen
concentration low enough to allow the bacteria to grow.
In soil, microorganisms which reside in the rhizosphere (a zone that includes the root
surface and the soil that adheres to the root after gentle shaking) carry out nitrogen
fixation, converting nitrogen gas to nitrogenous compounds.[125] This serves to
provide an easily absorbable form of nitrogen for many plants, which cannot fix
nitrogen themselves. Many other bacteria are found as symbionts in humans and other
organisms. For example, the presence of over 1,000 bacterial species in the normal
human gut flora of the intestines can contribute to gut immunity, synthesise vitamins
such as folic acid, vitamin K and biotin, convert milk protein to lactic acid (see
Lactobacillus), as well as fermenting complex undigestible carbohydrates.[126][127][128]
The presence of this gut flora also inhibits the growth of potentially pathogenic
bacteria (usually through competitive exclusion) and these beneficial bacteria are
consequently sold as probiotic dietary supplements.[129]
Pathogens
Main article: Pathogenic bacteria
Color-enhanced scanning electron micrograph showing Salmonella typhimurium (red)
invading cultured human cells
If bacteria form a parasitic association with other organisms, they are classed as
pathogens. Pathogenic bacteria are a major cause of human death and disease and
cause infections such as tetanus, typhoid fever, diphtheria, syphilis, cholera,
foodborne illness, leprosy and tuberculosis. A pathogenic cause for a known medical
disease may only be discovered many years after, as was the case with Helicobacter
pylori and peptic ulcer disease. Bacterial diseases are also important in agriculture,
with bacteria causing leaf spot, fire blight and wilts in plants, as well as Johne's
disease, mastitis, salmonella and anthrax in farm animals.
Each species of pathogen has a characteristic spectrum of interactions with its human
hosts. Some organisms, such as Staphylococcus or Streptococcus, can cause skin
infections, pneumonia, meningitis and even overwhelming sepsis, a systemic
inflammatory response producing shock, massive vasodilation and death.[130] Yet
these organisms are also part of the normal human flora and usually exist on the skin
or in the nose without causing any disease at all. Other organisms invariably cause
disease in humans, such as the Rickettsia, which are obligate intracellular parasites
able to grow and reproduce only within the cells of other organisms. One species of
Rickettsia causes typhus, while another causes Rocky Mountain spotted fever.
Chlamydia, another phylum of obligate intracellular parasites, contains species that
can cause pneumonia, or urinary tract infection and may be involved in coronary heart
disease.[131] Finally, some species such as Pseudomonas aeruginosa, Burkholderia
cenocepacia, and Mycobacterium avium are opportunistic pathogens and cause
disease mainly in people suffering from immunosuppression or cystic fibrosis.[132][133]
Bacterial infections may be treated with antibiotics, which are classified as
bacteriocidal if they kill bacteria, or bacteriostatic if they just prevent bacterial
growth. There are many types of antibiotics and each class inhibits a process that is
different in the pathogen from that found in the host. An example of how antibiotics
produce selective toxicity are chloramphenicol and puromycin, which inhibit the
bacterial ribosome, but not the structurally different eukaryotic ribosome.[134]
Antibiotics are used both in treating human disease and in intensive farming to
promote animal growth, where they may be contributing to the rapid development of
antibiotic resistance in bacterial populations.[135] Infections can be prevented by
antiseptic measures such as sterilizating the skin prior to piercing it with the needle of
a syringe, and by proper care of indwelling catheters. Surgical and dental instruments
are also sterilized to prevent contamination and infection by bacteria. Disinfectants
such as bleach are used to kill bacteria or other pathogens on surfaces to prevent
contamination and further reduce the risk of infection.
Significance in technology and industry
Further information: Economic importance of bacteria
Bacteria, often Lactobacillus in combination with yeasts and molds, have been used
for thousands of years in the preparation of fermented foods such as cheese, pickles,
soy sauce, sauerkraut, vinegar, wine and yoghurt.[136][137]
The ability of bacteria to degrade a variety of organic compounds is remarkable and
has been used in waste processing and bioremediation. Bacteria capable of digesting
the hydrocarbons in petroleum are often used to clean up oil spills.[138] Fertilizer was
added to some of the beaches in Prince William Sound in an attempt to promote the
growth of these naturally occurring bacteria after the infamous 1989 Exxon Valdez oil
spill. These efforts were effective on beaches that were not too thickly covered in oil.
Bacteria are also used for the bioremediation of industrial toxic wastes.[139] In the
chemical industry, bacteria are most important in the production of enantiomerically
pure chemicals for use as pharmaceuticals or agrichemicals.[140]
Bacteria can also be used in the place of pesticides in the biological pest control. This
commonly involves Bacillus thuringiensis (also called BT), a Gram-positive, soil
dwelling bacterium. Subspecies of this bacteria are used as a Lepidopteran-specific
insecticides under trade names such as Dipel and Thuricide.[141] Because of their
specificity, these pesticides are regarded as environmentally friendly, with little or no
effect on humans, wildlife, pollinators and most other beneficial insects.[142][143]
Because of their ability to quickly grow and the relative ease with which they can be
manipulated, bacteria are the workhorses for the fields of molecular biology, genetics
and biochemistry. By making mutations in bacterial DNA and examining the resulting
phenotypes, scientists can determine the function of genes, enzymes and metabolic
pathways in bacteria, then apply this knowledge to more complex organisms.[144] This
aim of understanding the biochemistry of a cell reaches its most complex expression
in the synthesis of huge amounts of enzyme kinetic and gene expression data into
mathematical models of entire organisms. This is achievable in some well-studied
bacteria, with models of Escherichia coli metabolism now being produced and
tested.[145][146] This understanding of bacterial metabolism and genetics allows the use
of biotechnology to bioengineer bacteria for the production of therapeutic proteins,
such as insulin, growth factors, or antibodies.[147][148]
See also
•
•
•
•
•
Human flora
Bioaerosol
Biotechnology
Contamination control
Denitrification
•
•
•
Desulforudis audaxviator
Extremophiles
Transgenic bacteria
References
1. ^ Fredrickson J, Zachara J, Balkwill D, et al (2004). "Geomicrobiology of high-level
nuclear waste-contaminated vadose sediments at the Hanford site, Washington state".
Appl Environ Microbiol 70 (7): 4230–41. PMID 15240306.
2. ^ Whitman W, Coleman D, Wiebe W (1998). "Prokaryotes: the unseen majority".
Proc Natl Acad Sci U S A 95 (12): 6578–83. PMID 9618454.
3. ^ Whitman W, Coleman D, Wiebe W (1998). "Prokaryotes: the unseen majority".
Proc Natl Acad Sci U S A 95 (12): 6578–83. PMID 9618454.
4. ^ a b Rappé MS, Giovannoni SJ (2003). "The uncultured microbial majority". Annu.
Rev. Microbiol. 57: 369-94. doi:10.1146/annurev.micro.57.030502.090759. PMID
14527284.
5. ^ Sears CL (2005). "A dynamic partnership: celebrating our gut flora". Anaerobe 11
(5): 247-51. doi:10.1016/j.anaerobe.2005.05.001. PMID 16701579.
6. ^ 2002 WHO mortality data. Retrieved on 2007-01-20.
7. ^ Ishige T, Honda K, Shimizu S (2005). "Whole organism biocatalysis". Curr Opin
Chem Biol 9 (2): 174–80. PMID 15811802.
8. ^ Woese C, Kandler O, Wheelis M (1990). "Towards a natural system of organisms:
proposal for the domains Archaea, Bacteria, and Eucarya". Proc Natl Acad Sci U S A
87 (12): 4576–9. PMID 2112744.
9. ^ Porter JR (1976). "Antony van Leeuwenhoek: Tercentenary of his discovery of
bacteria". Bacteriological reviews 40 (2): 260-9. PMID 786250. Retrieved on 200708-19.
10. ^ van Leeuwenhoek A (1684). "An abstract of a letter from Mr. Anthony
Leevvenhoek at Delft, dated Sep. 17, 1683, Containing Some Microscopical
Observations, about Animals in the Scurf of the Teeth, the Substance Call'd Worms in
the Nose, the Cuticula Consisting of Scales". Philosophical Transactions (1683–
1775) 14: 568-74. Retrieved on 2007-08-19.
11. ^ van Leeuwenhoek A (1700). "Part of a Letter from Mr Antony van Leeuwenhoek,
concerning the Worms in Sheeps Livers, Gnats, and Animalcula in the Excrements of
Frogs". Philosophical Transactions (1683–1775) 22: 509–18. Retrieved on 2007-0819.
12. ^ van Leeuwenhoek A (1702). "Part of a Letter from Mr Antony van Leeuwenhoek,
F. R. S. concerning Green Weeds Growing in Water, and Some Animalcula Found
about Them". Philosophical Transactions (1683–1775) 23: 1304–11. Retrieved on
2007-08-19.
13. ^ Etymology of the word "bacteria". Online Etymology dictionary. Retrieved on
2006-11-23.
14. ^ Pasteur's Papers on the Germ Theory. LSU Law Center's Medical and Public
Health Law Site, Historic Public Health Articles. Retrieved on 2006-11-23.
15. ^ The Nobel Prize in Physiology or Medicine 1905. Nobelprize.org. Retrieved on
2006-11-22.
16. ^ O'Brien S, Goedert J (1996). "HIV causes AIDS: Koch's postulates fulfilled". Curr
Opin Immunol 8 (5): 613–18. PMID 8902385.
17. ^ Thurston A (2000). "Of blood, inflammation and gunshot wounds: the history of
the control of sepsis". Aust N Z J Surg 70 (12): 855-61. PMID 11167573.
18. ^ Schwartz R (2004). "Paul Ehrlich's magic bullets". N Engl J Med 350 (11): 1079–
80. PMID 15014180.
19. ^ Biography of Paul Ehrlich. Nobelprize.org. Retrieved on 2006-11-26.
20. ^ Woese C, Fox G (1977). "Phylogenetic structure of the prokaryotic domain: the
primary kingdoms". Proc Natl Acad Sci U S A 74 (11): 5088–90. PMID 270744.
21. ^ Woese C, Kandler O, Wheelis M (1990). "Towards a natural system of organisms:
proposal for the domains Archaea, Bacteria, and Eucarya". Proc Natl Acad Sci U S A
87 (12): 4576–79. PMID 2112744.
22. ^ Schopf J (1994). "Disparate rates, differing fates: tempo and mode of evolution
changed from the Precambrian to the Phanerozoic". Proc Natl Acad Sci U S A 91
(15): 6735–42. PMID 8041691.
23. ^ DeLong E, Pace N (2001). "Environmental diversity of bacteria and archaea". Syst
Biol 50 (4): 470–78. PMID 12116647.
24. ^ Brown JR, Doolittle WF (1997). "Archaea and the prokaryote-to-eukaryote
transition". Microbiol. Mol. Biol. Rev. 61 (4): 456-502. PMID 9409149.
25. ^ Di Giulio M (2003). "The universal ancestor and the ancestor of bacteria were
hyperthermophiles". J Mol Evol 57 (6): 721–30. PMID 14745541.
26. ^ Battistuzzi F, Feijao A, Hedges S. "A genomic timescale of prokaryote evolution:
insights into the origin of methanogenesis, phototrophy, and the colonization of
land.". BMC Evol Biol 4: 44. PMID 15535883.
27. ^ Poole A, Penny D (2007). "Evaluating hypotheses for the origin of eukaryotes".
Bioessays 29 (1): 74–84. PMID 17187354.
28. ^ Dyall S, Brown M, Johnson P (2004). "Ancient invasions: from endosymbionts to
organelles". Science 304 (5668): 253–7. PMID 15073369.
29. ^ Lang B, Gray M, Burger G. "Mitochondrial genome evolution and the origin of
eukaryotes". Annu Rev Genet 33: 351-97. PMID 10690412.
30. ^ McFadden G (1999). "Endosymbiosis and evolution of the plant cell". Curr Opin
Plant Biol 2 (6): 513-9. PMID 10607659.
31. ^ Schulz H, Jorgensen B. "Big bacteria". Annu Rev Microbiol 55: 105–37. PMID
11544351.
32. ^ Robertson J, Gomersall M, Gill P. (1975). "Mycoplasma hominis: growth,
reproduction, and isolation of small viable cells". J Bacteriol. 124 (2): 1007–18.
PMID 1102522.
33. ^ Fritz I, Strömpl C, Abraham W (2004). "Phylogenetic relationships of the genera
Stella, Labrys and Angulomicrobium within the 'Alphaproteobacteria' and description
of Angulomicrobium amanitiforme sp. nov". Int J Syst Evol Microbiol 54 (Pt 3): 6517. PMID 15143003.
34. ^ Cabeen M, Jacobs-Wagner C (2005). "Bacterial cell shape". Nat Rev Microbiol 3
(8): 601–10. PMID 16012516.
35. ^ Young K (2006). "The selective value of bacterial shape". Microbiol Mol Biol Rev
70 (3): 660–703. PMID 16959965.
36. ^ Douwes K, Schmalzbauer E, Linde H, Reisberger E, Fleischer K, Lehn N,
Landthaler M, Vogt T (2003). "Branched filaments no fungus, ovoid bodies no
bacteria: Two unusual cases of mycetoma". J Am Acad Dermatol 49 (2 Suppl Case
Reports): S170–3. PMID 12894113.
37. ^ Donlan R (2002). "Biofilms: microbial life on surfaces". Emerg Infect Dis 8 (9):
881–90. PMID 12194761.
38. ^ Branda S, Vik S, Friedman L, Kolter R (2005). "Biofilms: the matrix revisited".
Trends Microbiol 13 (1): 20–26. PMID 15639628.
39. ^ a b Davey M, O'toole G (2000). "Microbial biofilms: from ecology to molecular
genetics". Microbiol Mol Biol Rev 64 (4): 847–67. PMID 11104821.
40. ^ Donlan RM, Costerton JW (2002). "Biofilms: survival mechanisms of clinically
relevant microorganisms". Clin Microbiol Rev 15 (2): 167–93. PMID 11932229.
41. ^ Shimkets L. "Intercellular signaling during fruiting-body development of
Myxococcus xanthus.". Annu Rev Microbiol 53: 525–49. PMID 10547700.
42. ^ Kaiser D. "Signaling in myxobacteria". Annu Rev Microbiol 58: 75–98. PMID
15487930.
43. ^ Berg JM, Tymoczko JL Stryer L (2002). Molecular Cell Biology, 5th ed., WH
Freeman. ISBN 0-7167-4955-6.
44. ^ Gitai Z (2005). "The new bacterial cell biology: moving parts and subcellular
architecture". Cell 120 (5): 577–86. PMID 15766522.
45. ^ Shih YL, Rothfield L (2006). "The bacterial cytoskeleton". Microbiol. Mol. Biol.
Rev. 70 (3): 729–54. PMID 16959967.
46. ^ Harold F (1972). "Conservation and transformation of energy by bacterial
membranes". Bacteriol Rev 36 (2): 172–230. PMID 4261111.
47. ^ Thanbichler M, Wang S, Shapiro L (2005). "The bacterial nucleoid: a highly
organized and dynamic structure". J Cell Biochem 96 (3): 506–21. PMID
15988757.
48. ^ Poehlsgaard J, Douthwaite S (2005). "The bacterial ribosome as a target for
antibiotics". Nat Rev Microbiol 3 (11): 870–81. PMID 16261170.
49. ^ Fuerst J (2005). "Intracellular compartmentation in planctomycetes". Annu Rev
Microbiol 59: 299–328. PMID 15910279.
50. ^ Yeo M, Chater K (2005). "The interplay of glycogen metabolism and
differentiation provides an insight into the developmental biology of Streptomyces
coelicolor". Microbiology 151 (Pt 3): 855–61. PMID 15758231.
51. ^ Shiba T, Tsutsumi K, Ishige K, Noguchi T (2000). "Inorganic polyphosphate and
polyphosphate kinase: their novel biological functions and applications".
Biochemistry (Mosc) 65 (3): 315–23. PMID 10739474.
52. ^ Brune DC. (1995). "Isolation and characterization of sulfur globule proteins from
Chromatium vinosum and Thiocapsa roseopersicina". Arch Microbiol 163 (6): 391–
99. PMID 7575095.
53. ^ Kadouri D, Jurkevitch E, Okon Y, Castro-Sowinski S. (2005). "Ecological and
agricultural significance of bacterial polyhydroxyalkanoates". Crit Rev Microbiol 31
(2): 55–67. PMID 15986831.
54. ^ Walsby A (1994). "Gas vesicles". Microbiol Rev 58 (1): 94–144. PMID 8177173.
55. ^ van Heijenoort J (2001). "Formation of the glycan chains in the synthesis of
bacterial peptidoglycan". Glycobiology 11 (3): 25R–36R. PMID 11320055.
56. ^ a b Koch A (2003). "Bacterial wall as target for attack: past, present, and future
research". Clin Microbiol Rev 16 (4): 673–87. PMID 14557293.
57. ^ a b Gram, HC (1884). "Über die isolierte Färbung der Schizomyceten in Schnitt- und
Trockenpräparaten". Fortschr. Med. 2: 185–189.
58. ^ Hugenholtz P (2002). "Exploring prokaryotic diversity in the genomic era".
Genome Biol 3 (2): REVIEWS0003. PMID 11864374.
59. ^ Walsh F, Amyes S (2004). "Microbiology and drug resistance mechanisms of fully
resistant pathogens.". Curr Opin Microbiol 7 (5): 439-44. PMID 15451497.
60. ^ Engelhardt H, Peters J (1998). "Structural research on surface layers: a focus on
stability, surface layer homology domains, and surface layer-cell wall interactions". J
Struct Biol 124 (2–3): 276–302. PMID 10049812.
61. ^ Beveridge T, Pouwels P, Sára M, Kotiranta A, Lounatmaa K, Kari K, Kerosuo E,
Haapasalo M, Egelseer E, Schocher I, Sleytr U, Morelli L, Callegari M, Nomellini J,
Bingle W, Smit J, Leibovitz E, Lemaire M, Miras I, Salamitou S, Béguin P, Ohayon
H, Gounon P, Matuschek M, Koval S (1997). "Functions of S-layers". FEMS
Microbiol Rev 20 (1–2): 99–149. PMID 9276929.
62. ^ Kojima S, Blair D. "The bacterial flagellar motor: structure and function of a
complex molecular machine". Int Rev Cytol 233: 93–134. PMID 15037363.
63. ^ Beachey E (1981). "Bacterial adherence: adhesin-receptor interactions mediating
the attachment of bacteria to mucosal surface". J Infect Dis 143 (3): 325–45. PMID
7014727.
64. ^ Silverman P (1997). "Towards a structural biology of bacterial conjugation". Mol
Microbiol 23 (3): 423–9. PMID 9044277.
65. ^ Stokes R, Norris-Jones R, Brooks D, Beveridge T, Doxsee D, Thorson L (2004).
"The glycan-rich outer layer of the cell wall of Mycobacterium tuberculosis acts as an
66.
67.
68.
69.
70.
71.
72.
73.
74.
75.
76.
77.
78.
79.
80.
81.
82.
83.
84.
85.
86.
antiphagocytic capsule limiting the association of the bacterium with macrophages".
Infect Immun 72 (10): 5676–86. PMID 15385466.
^ Daffé M, Etienne G (1999). "The capsule of Mycobacterium tuberculosis and its
implications for pathogenicity". Tuber Lung Dis 79 (3): 153–69. PMID 10656114.
^ Finlay B, Falkow S (1997). "Common themes in microbial pathogenicity revisited".
Microbiol Mol Biol Rev 61 (2): 136–69. PMID 9184008.
^ Nicholson W, Munakata N, Horneck G, Melosh H, Setlow P (2000). "Resistance of
Bacillus endospores to extreme terrestrial and extraterrestrial environments".
Microbiol Mol Biol Rev 64 (3): 548–72. PMID 10974126.
^ Siunov A, Nikitin D, Suzina N, Dmitriev V, Kuzmin N, Duda V. "Phylogenetic
status of Anaerobacter polyendosporus, an anaerobic, polysporogenic bacterium". Int
J Syst Bacteriol 49 Pt 3: 1119–24. PMID 10425769.
^ Nicholson W, Fajardo-Cavazos P, Rebeil R, Slieman T, Riesenman P, Law J, Xue
Y (2002). "Bacterial endospores and their significance in stress resistance". Antonie
Van Leeuwenhoek 81 (1–4): 27–32. PMID 12448702.
^ Vreeland R, Rosenzweig W, Powers D (2000). "Isolation of a 250 million-year-old
halotolerant bacterium from a primary salt crystal". Nature 407 (6806): 897–900.
PMID 11057666.
^ Cano R, Borucki M (1995). "Revival and identification of bacterial spores in 25- to
40-million-year-old Dominican amber". Science 268 (5213): 1060–4. PMID
7538699.
^ Nicholson W, Schuerger A, Setlow P (2005). "The solar UV environment and
bacterial spore UV resistance: considerations for Earth-to-Mars transport by natural
processes and human spaceflight". Mutat Res 571 (1–2): 249–64. PMID 15748651.
^ Hatheway C (1990). "Toxigenic clostridia". Clin Microbiol Rev 3 (1): 66–98. PMID
2404569.
^ Nealson K (1999). "Post-Viking microbiology: new approaches, new data, new
insights". Orig Life Evol Biosph 29 (1): 73–93. PMID 11536899.
^ Xu J (2006). "Microbial ecology in the age of genomics and metagenomics:
concepts, tools, and recent advances". Mol Ecol 15 (7): 1713–31. PMID 16689892.
^ Zillig W (1991). "Comparative biochemistry of Archaea and Bacteria". Curr Opin
Genet Dev 1 (4): 544-51. PMID 1822288.
^ Hellingwerf K, Crielaard W, Hoff W, Matthijs H, Mur L, van Rotterdam B (1994).
"Photobiology of bacteria". Antonie Van Leeuwenhoek 65 (4): 331–47. PMID
7832590.
^ Zumft W (1997). "Cell biology and molecular basis of denitrification". Microbiol
Mol Biol Rev 61 (4): 533–616. PMID 9409151.
^ Drake H, Daniel S, Küsel K, Matthies C, Kuhner C, Braus-Stromeyer S (1997).
"Acetogenic bacteria: what are the in situ consequences of their diverse metabolic
versatilities?". Biofactors 6 (1): 13–24. PMID 9233536.
^ Morel, FMM; Kraepiel AML, Amyot M (1998). "The chemical cycle and
bioaccumulation of mercury". Annual Review of Ecological Systems 29: 543—566.
^ Dalton H (2005). "The Leeuwenhoek Lecture 2000 the natural and unnatural
history of methane-oxidizing bacteria". Philos Trans R Soc Lond B Biol Sci 360
(1458): 1207–22. PMID 16147517.
^ Zehr J, Jenkins B, Short S, Steward G (2003). "Nitrogenase gene diversity and
microbial community structure: a cross-system comparison". Environ Microbiol 5
(7): 539–54. PMID 12823187.
^ Koch A (2002). "Control of the bacterial cell cycle by cytoplasmic growth". Crit
Rev Microbiol 28 (1): 61–77. PMID 12003041.
^ Eagon R. "Pseudomonas natriegens, a marine bacterium with a generation time of
less than 10 minutes". J Bacteriol 83: 736–7. PMID 13888946.
^ Stewart EJ, Madden R, Paul G, Taddei F (2005). "Aging and death in an organism
that reproduces by morphologically symmetric division". PLoS Biol. 3 (2): e45.
PMID 15685293.
87. ^ a b c Thomson R, Bertram H (2001). "Laboratory diagnosis of central nervous
system infections". Infect Dis Clin North Am 15 (4): 1047–71. PMID 11780267.
88. ^ Paerl H, Fulton R, Moisander P, Dyble J. "Harmful freshwater algal blooms, with
an emphasis on cyanobacteria". ScientificWorldJournal 1: 76–113. PMID
12805693.
89. ^ Challis G, Hopwood D. "Synergy and contingency as driving forces for the
evolution of multiple secondary metabolite production by Streptomyces species".
Proc Natl Acad Sci U S A 100 Suppl 2: 14555–61. PMID 12970466.
90. ^ Kooijman S, Auger P, Poggiale J, Kooi B (2003). "Quantitative steps in
symbiogenesis and the evolution of homeostasis". Biol Rev Camb Philos Soc 78 (3):
435–63. PMID 14558592.
91. ^ Prats C, López D, Giró A, Ferrer J, Valls J (2006). "Individual-based modelling of
bacterial cultures to study the microscopic causes of the lag phase". J Theor Biol 241
(4): 939–53. PMID 16524598.
92. ^ Hecker M, Völker U. "General stress response of Bacillus subtilis and other
bacteria". Adv Microb Physiol 44: 35–91. PMID 11407115.
93. ^ Nakabachi A, Yamashita A, Toh H, Ishikawa H, Dunbar H, Moran N, Hattori M
(2006). "The 160-kilobase genome of the bacterial endosymbiont Carsonella".
Science 314 (5797): 267. PMID 17038615.
94. ^ Pradella S, Hans A, Spröer C, Reichenbach H, Gerth K, Beyer S (2002).
"Characterisation, genome size and genetic manipulation of the myxobacterium
Sorangium cellulosum So ce56". Arch Microbiol 178 (6): 484-92. PMID 12420170.
95. ^ Hinnebusch J, Tilly K (1993). "Linear plasmids and chromosomes in bacteria". Mol
Microbiol 10 (5): 917-22. PMID 7934868.
96. ^ Belfort M, Reaban ME, Coetzee T, Dalgaard JZ (1995). "Prokaryotic introns and
inteins: a panoply of form and function". J. Bacteriol. 177 (14): 3897–903. PMID
7608058.
97. ^ Brüssow H, Canchaya C, Hardt W (2004). "Phages and the evolution of bacterial
pathogens: from genomic rearrangements to lysogenic conversion". Microbiol Mol
Biol Rev 68 (3): 560–602. PMID 15353570.
98. ^ Denamur E, Matic I (2006). "Evolution of mutation rates in bacteria". Mol
Microbiol 60 (4): 820–7. PMID 16677295.
99. ^ Wright B (2004). "Stress-directed adaptive mutations and evolution". Mol
Microbiol 52 (3): 643–50. PMID 15101972.
100.
^ Davison J (1999). "Genetic exchange between bacteria in the
environment". Plasmid 42 (2): 73–91. PMID 10489325.
101.
^ Hastings P, Rosenberg S, Slack A (2004). "Antibiotic-induced lateral
transfer of antibiotic resistance". Trends Microbiol 12 (9): 401–4. PMID 15337159.
102.
^ a b c Bardy S, Ng S, Jarrell K (2003). "Prokaryotic motility structures".
Microbiology 149 (Pt 2): 295–304. PMID 12624192.
103.
^ Merz A, So M, Sheetz M (2000). "Pilus retraction powers bacterial
twitching motility". Nature 407 (6800): 98–102. PMID 10993081.
104.
^ Wu M, Roberts J, Kim S, Koch D, DeLisa M (2006). "Collective bacterial
dynamics revealed using a three-dimensional population-scale defocused particle
tracking technique". Appl Environ Microbiol 72 (7): 4987–94. PMID 16820497.
105.
^ Lux R, Shi W (2004). "Chemotaxis-guided movements in bacteria". Crit
Rev Oral Biol Med 15 (4): 207-20. PMID 15284186.
106.
^ Frankel R, Bazylinski D, Johnson M, Taylor B (1997). "Magneto-aerotaxis
in marine coccoid bacteria". Biophys J 73 (2): 994–1000. PMID 9251816.
107.
^ Kaiser D. "Signaling in myxobacteria". Annu Rev Microbiol 58: 75–98.
PMID 15487930.
108.
^ Goldberg MB (2001). "Actin-based motility of intracellular microbial
pathogens". Microbiol Mol Biol Rev 65 (4): 595–626. PMID 11729265.
109.
^ Boucher Y, Douady CJ, Papke RT, Walsh DA, Boudreau ME, Nesbo CL,
Case RJ, Doolittle WF (2003). "Lateral gene transfer and the origins of prokaryotic
groups.". Annu Rev Genet 37: 283–328. PMID 14616063.
110.
^ Olsen G, Woese C, Overbeek R (1994). "The winds of (evolutionary)
change: breathing new life into microbiology". J Bacteriol 176 (1): 1–6. PMID
8282683.
111.
^ IJSEM - Home
112.
^ Bergey's Manual Trust
113.
^ Woese C, Kandler O, Wheelis M (1990). "Towards a natural system of
organisms: proposal for the domains Archaea, Bacteria, and Eucarya". Proc Natl
Acad Sci U S A 87 (12): 4576–9. PMID 2112744.
114.
^ Gupta R (2000). "The natural evolutionary relationships among
prokaryotes.". Crit Rev Microbiol 26 (2): 111-31. PMID 10890353.
115.
^ Doolittle RF (2005). "Evolutionary aspects of whole-genome biology".
Curr Opin Struct Biol 15 (3): 248–253. PMID 11837318.
116.
^ Cavalier-Smith T (2002). "The neomuran origin of archaebacteria, the
negibacterial root of the universal tree and bacterial megaclassification.". Int J Syst
Evol Microbiol 52 (Pt 1): 7–76. PMID 11837318.
117.
^ Ciccarelli FD, Doerks T, von Mering C, Creevey CJ, Snel B, Bork P
(2006). "Toward automatic reconstruction of a highly resolved tree of life". Science
311 (5765): 1283-7. PMID 16513982.
118.
^ Woods G, Walker D (1996). "Detection of infection or infectious agents by
use of cytologic and histologic stains". Clin Microbiol Rev 9 (3): 382–404. PMID
8809467.
119.
^ Weinstein M (1994). "Clinical importance of blood cultures". Clin Lab
Med 14 (1): 9–16. PMID 8181237.
120.
^ Louie M, Louie L, Simor AE (2000). "The role of DNA amplification
technology in the diagnosis of infectious diseases". CMAJ 163 (3): 301–309. PMID
10951731.
121.
^ Oliver J. "The viable but nonculturable state in bacteria". J Microbiol 43
Spec No: 93–100. PMID 15765062.
122.
^ Curtis T, Sloan W, Scannell J (2002). "Estimating prokaryotic diversity and
its limits". Proc Natl Acad Sci U S A 99 (16): 10494-9. PMID 12097644.
123.
^ Schloss P, Handelsman J (2004). "Status of the microbial census".
Microbiol Mol Biol Rev 68 (4): 686-91. PMID 15590780.
124.
^ Stams A, de Bok F, Plugge C, van Eekert M, Dolfing J, Schraa G (2006).
"Exocellular electron transfer in anaerobic microbial communities". Environ
Microbiol 8 (3): 371–82. PMID 16478444.
125.
^ Barea J, Pozo M, Azcón R, Azcón-Aguilar C (2005). "Microbial cooperation in the rhizosphere". J Exp Bot 56 (417): 1761–78. PMID 15911555.
126.
^ O'Hara A, Shanahan F (2006). "The gut flora as a forgotten organ". EMBO
Rep 7 (7): 688–93. PMID 16819463.
127.
^ Zoetendal E, Vaughan E, de Vos W (2006). "A microbial world within us".
Mol Microbiol 59 (6): 1639–50. PMID 16553872.
128.
^ Gorbach S (1990). "Lactic acid bacteria and human health". Ann Med 22
(1): 37–41. PMID 2109988.
129.
^ Salminen S, Gueimonde M, Isolauri E (2005). "Probiotics that modify
disease risk". J Nutr 135 (5): 1294–8. PMID 15867327.
130.
^ Fish D. "Optimal antimicrobial therapy for sepsis". Am J Health Syst
Pharm 59 Suppl 1: S13–9. PMID 11885408.
131.
^ Belland R, Ouellette S, Gieffers J, Byrne G (2004). "Chlamydia
pneumoniae and atherosclerosis". Cell Microbiol 6 (2): 117–27. PMID 14706098.
132.
^ Heise E. "Diseases associated with immunosuppression". Environ Health
Perspect 43: 9–19. PMID 7037390.
133.
^ Saiman, L. "Microbiology of early CF lung disease". Paediatr Respir
Rev.volume=5 Suppl A: S367–369. PMID 14980298
134.
^ Yonath A, Bashan A (2004). "Ribosomal crystallography: initiation,
peptide bond formation, and amino acid polymerization are hampered by antibiotics".
Annu Rev Microbiol 58: 233–51. PMID 15487937.
135.
^ Khachatourians G (1998). "Agricultural use of antibiotics and the evolution
and transfer of antibiotic-resistant bacteria". CMAJ 159 (9): 1129–36. PMID
9835883.
136.
^ Johnson M, Lucey J (2006). "Major technological advances and trends in
cheese". J Dairy Sci 89 (4): 1174–8. PMID 16537950.
137.
^ Hagedorn S, Kaphammer B (1994). "Microbial biocatalysis in the
generation of flavor and fragrance chemicals". Annu. Rev. Microbiol. 48: 773-800.
doi:10.1146/annurev.mi.48.100194.004013. PMID 7826026.
138.
^ Cohen Y (2002). "Bioremediation of oil by marine microbial mats". Int
Microbiol 5 (4): 189–93. PMID 12497184.
139.
^ Neves LC, Miyamura TT, Moraes DA, Penna TC, Converti A (2006).
"Biofiltration methods for the removal of phenolic residues". Appl. Biochem.
Biotechnol. 129-132: 130-52. PMID 16915636.
140.
^ Liese A, Filho M (1999). "Production of fine chemicals using biocatalysis".
Curr Opin Biotechnol 10 (6): 595–603. PMID 10600695.
141.
^ Aronson AI, Shai Y (2001). "Why Bacillus thuringiensis insecticidal toxins
are so effective: unique features of their mode of action". FEMS Microbiol. Lett. 195
(1): 1-8. PMID 11166987.
142.
^ Bozsik A (2006). "Susceptibility of adult Coccinella septempunctata
(Coleoptera: Coccinellidae) to insecticides with different modes of action". Pest
Manag Sci 62 (7): 651–4. PMID 16649191.
143.
^ Chattopadhyay A, Bhatnagar N, Bhatnagar R (2004). "Bacterial insecticidal
toxins". Crit Rev Microbiol 30 (1): 33–54. PMID 15116762.
144.
^ Serres M, Gopal S, Nahum L, Liang P, Gaasterland T, Riley M (2001). "A
functional update of the Escherichia coli K-12 genome". Genome Biol 2 (9):
RESEARCH0035. PMID 11574054.
145.
^ Almaas E, Kovács B, Vicsek T, Oltvai Z, Barabási A (2004). "Global
organization of metabolic fluxes in the bacterium Escherichia coli". Nature 427
(6977): 839–43. PMID 14985762.
146.
^ Reed JL, Vo TD, Schilling CH, Palsson BO (2003). "An expanded
genome-scale model of Escherichia coli K-12 (iJR904 GSM/GPR)". Genome Biol. 4
(9): R54. doi:10.1186/gb-2003-4-9-r54. PMID 12952533.
147.
^ Walsh G (2005). "Therapeutic insulins and their large-scale manufacture".
Appl Microbiol Biotechnol 67 (2): 151–9. PMID 15580495.
148.
^ Graumann K, Premstaller A (2006). "Manufacturing of recombinant
therapeutic proteins in microbial systems". Biotechnol J 1 (2): 164–86. PMID
16892246.
Further reading
•
•
•
•
Alcamo IE (2001). Fundamentals of microbiology. Boston: Jones and Bartlett.
ISBN 0-7637-1067-9.
Atlas RM (1995). Principles of microbiology. St. Louis: Mosby. ISBN 08016-7790-4.
Martinko JM, Madigan MT (2005). Brock Biology of Microorganisms, 11th
ed., Englewood Cliffs, N.J: Prentice Hall. ISBN 0-13-144329-1.
Holt JC, Bergey DH (1994). Bergey's manual of determinative bacteriology,
9th ed., Baltimore: Williams & Wilkins. ISBN 0-683-00603-7.
•
•
Hugenholtz P, Goebel BM, Pace NR (1998). "Impact of culture-independent
studies on the emerging phylogenetic view of bacterial diversity". J Bacteriol
180 (18): 4765–74. PMID 9733676.
Funke BR, Tortora GJ, Case CL (2004). Microbiology: an introduction, 8th
ed,, San Francisco: Benjamin Cummings. ISBN 0-8053-7614-3.
External links
Find more about Bacteria on
Wikipedia's sister projects:
Dictionary definitions
Textbooks
Quotations
Source texts
Images and media
News stories
Learning resources
•
•
•
•
•
•
•
Bacterial Nomenclature Up-To-Date from DSMZ
The largest bacteria
Tree of Life: Eubacteria
Videos of bacteria swimming and tumbling, use of optical tweezers and other
videos.
Planet of the Bacteria by Stephen Jay Gould
On-line text book on bacteriology
Animated guide to bacterial cell structure.
Chemotaxis
http://en.wikipedia.org/wiki/Chemotaxis
From Wikipedia, the free encyclopedia
Jump to: navigation, search
Chemotaxis, a kind of taxis, is the phenomenon in which bodily cells, bacteria, and
other single-cell or multicellular organisms direct their movements according to
certain chemicals in their environment. This is important for bacteria to find food (for
example, glucose) by swimming towards the highest concentration of food molecules,
or to flee from poisons (for example, phenol). In multicellular organisms, chemotaxis
is critical to early (e.g. movement of sperm towards the egg during fertilization) and
subsequent phases of development (e.g. migration of neurons or lymphocytes) as well
as in normal function. In addition, it has been recognized that mechanisms that allow
chemotaxis in animals can be subverted during cancer metastasis.
Chemotaxis is called positive if movement is in the direction of a higher concentration
of the chemical in question, and negative if the direction is opposite.
Contents
[hide]
•
•
•
•
•
•
•
•
•
1 History of chemotaxis research
2 Phylogeny and chemotactic signalling
3 Bacterial chemotaxis
o 3.1 Behavior
o 3.2 Signal transduction
3.2.1 Flagellum regulation
3.2.2 Receptor regulation
4 Eukaryotic chemotaxis
o 4.1 Motility
4.1.1 Chemotaxis related migratory responses
o 4.2 Receptors
4.2.1 Chemotactic selection
o 4.3 Chemotactic ligands
4.3.1 Chemotactic range fitting (CRF)
5 Clinical significance
6 In the mirror of publications
7 Measurement of chemotaxis
8 References
9 External links
[edit] History of chemotaxis research
Milestones of chemotaxis research
Although migration of cells was detected from the early days of the development of
microscopy (Leeuwenhoek), erudite description of chemotaxis was first made by T.W.
Engelmann (1881) and W.F. Pfeffer (1884) in bacteria and H.S. Jennings (1906) in
ciliates. The Nobel Prize Laureate E. Metchnikoff also contributed to the study of the
field with investigations of the process as an initial step of phagocytosis. The
significance of chemotaxis in biology and clinical pathology was widely accepted in
the 1930s. The most fundamental definitions belonging to the phenomenon were also
drafted by this time. The most important aspects in quality control of chemotaxis
assays were described by H. Harris in the 1950s. In the 1960s and 1970s, the
revolution of modern cell biology and biochemistry provided a series of novel
techniques which became available to investigate the migratory responder cells and
subcellular fractions responsible for chemotactic activity. The pioneering works of J.
Adler represented a significant turning point in understanding the whole process of
intracellular signal transduction of bacteria.[1]
On November 3, 2006, Dr. Dennis Bray of University of Cambridge was awarded the
Microsoft European Science Award for his work on chemotaxis on E. coli.[2][3]
[edit] Phylogeny and chemotactic signalling
Chemotaxis is one of the most basic cell physiological responses. Development of
receptor systems for the detection of harmful and favorable substances in the
environment was most essential to unicellular organisms from the very early stages of
phylogeny. Comprehensive analysis of chemotactic activity of the eukaryotic
protozoon Tetrahymena pyriformis and consensus sequences of appearance of amino
acids in the primordial soup suggest that there was a good correlation between the
chemotactic character of these relative simple organic molecules and their
development on the Earth. In this way the earliest molecules are suggested to be
highly chemoattractant (e.g. Gly, Glu, Pro), while latter ones are thought to be
strongly chemorepellent (e.g. Tyr, Trp, Phe) amino acids.[4]
[edit] Bacterial chemotaxis
Some bacteria, such as E. coli, have several flagella per cell (4–10 typically). These
can rotate in two ways :
1. Counter-clockwise rotation aligns the flagella into a single rotating bundle,
causing the bacterium to swim in a straight line.
2. Clockwise rotation breaks the flagella bundle apart such that each flagellum
points in a different direction, causing the bacterium to tumble in place.
The directions of rotation are given for an observer outside the cell looking down the
flagella toward the cell.
[edit] Behavior
The overall movement of a bacterium is the result of alternating tumble and swim
phases. If one watches a bacterium swimming in a uniform environment, its
movement will look like a random walk with relatively straight swims interrupted by
random tumbles that reorient the bacterium. Bacteria such as E. coli are unable to
choose the direction in which they swim, and are unable to swim in a straight line for
more than a few seconds due to rotational diffusion. In other words, bacteria "forget"
the direction in which they are going. Given these limitations, it is remarkable that
bacteria can direct their motion to find favorable locations with high concentrations of
attractants (usually food) and avoid repellents (usually poisons).
In the presence of a chemical gradient bacteria will chemotax, or direct their overall
motion based on the gradient. If the bacterium senses that it is moving in the correct
direction (toward attractant/away from repellent), it will keep swimming in a straight
line for a longer time before tumbling. If it is moving in the wrong direction, it will
tumble sooner and try a new direction at random. In other words, bacteria like E. coli
use temporal sensing to decide whether life is getting better or worse. In this way, it
finds the location with the highest concentration of attractant (usually the source)
quite well. Even under very high concentrations, it can still distinguish very small
differences in concentration. Fleeing from a repellent works with the same efficiency.
It seems remarkable that this purposeful random walk is a result of simply choosing
between two methods of random movement; namely tumbling and straight swimming.
In fact, chemotactic responses such as forgetting direction and choosing movements
resemble the decision-making abilities of higher lifeforms with brains that process
sensory data.
The helical nature of the individual flagellar filament is critical for this movement to
occur. As such, the protein that makes up the flagellar filament, flagellin, is quite
similar among all flagellated bacteria. Vertebrates seem to have taken advantage of
this fact by possessing an immune receptor (TLR5) designed to recognize this
conserved protein.
As in many instances in biology, there are bacteria that do not follow this rule. Many
bacteria, such as Vibrio, are monoflagellated and have a single flagellum at one pole
of the cell. Their method of chemotaxis is different. Others possess a single flagellum
that is kept inside the cell wall. These bacteria move by spinning the whole cell,
which is shaped like a corkscrew.[5]
[edit] Signal transduction
Chemical gradients are sensed through multiple transmembrane receptors, called
methyl accepting chemotaxis proteins (MCPs), which vary in the molecules that they
detect. These receptors may bind attractants or repellents directly or indirectly through
interaction with proteins of periplasmatic space. The signals from these receptors are
transmitted across the plasma membrane into the cytosol, where Che proteins are
activated. The Che proteins alter the tumbling frequency, and alter the receptors.
[edit] Flagellum regulation
The proteins CheW and CheA bind to the receptor. The activation of the receptor by
an external stimulus causes autophosphorylation in the histidine kinase, CheA, at a
single highly conserved histidine residue. CheA in turn transfers phosphoryl groups to
conserved aspartate residues in the response regulators CheB and CheY [ note: CheA
is a histidine kinase and it does not actively transfer the phosphoryl group. The
response regulator CheB takes the phosphoryl group from CheA]. This mechanism of
signal transduction is called a 'Two Component System' and is a common form of
signal transduction in bacteria. CheY induces tumbling by interacting with the
flagellar switch protein FliM, inducing a change from counter-clockwise to clockwise
rotation of the flagellum. Change in the rotation state of a single flagellum can disrupt
the entire flagella bundle and cause a tumble.
[edit] Receptor regulation
CheB, when activated by CheA, acts as a methylesterase, removing methyl groups
from glutamate residues on the cytosolic side of the receptor. It works antagonistically
with CheR, a methyltransferase, which adds methyl residues to the same glutamate
residues. The more methyl residues are attached to the receptor, the more sensitive the
receptor. As the signal from the receptor induces demethylation of the receptor in a
feedback loop, the system is continuously adjusted to environmental chemical levels,
remaining sensitive for small changes even under extreme chemical concentrations.
This regulation allows the bacterium to 'remember' chemical concentrations from the
recent past and compare them to those it is currently experiencing, thus 'know'
whether it is traveling up or down a gradient. However, the methylation system alone
cannot account for the wide range of sensitivity that bacteria have to chemical
gradients. Additional regulatory mechanisms such as receptor clustering and receptorreceptor interactions also modulate the signalling pathway.
http://en.wikipedia.org/wiki/Coccidia
Coccidia
From Wikipedia, the free encyclopedia
Jump to: navigation, search
Coccidia
Coccidia oocysts
Scientific classification
Kingdom: Protista
Phylum:
Apicomplexa
Class:
Conoidasida
Subclass: Coccidiasina
Order:
Eucoccidiorida
Suborder, Family, Genera & Species
Adeleorina
•
•
•
•
•
•
•
Adeleidae
Dactylosomatidae
Haemogregarinidae
Hepatozoidae
o Hepatozoon
Karyolysidae
Klossiellidae
Legerellidae
Eimeriorina
•
•
•
•
•
•
Aggregatidae
o Aggregata
o Merocystis
o Selysina
Calyptosporiidae
o Calyptospora
Cryptosporidiidae
o Cryptosporidium
Eimeriidae
o Atoxoplasma
o Barrouxia
o Caryospora
o Caryotropha
o Cyclospora
o Diaspora
o Dorisa
o Dorisiella
o Eimeria
o Grasseella
o Isospora
o Mantonella
o Ovivora
o Pfeifferinella
o Pseudoklossia
o Tyzzeria
o Wenyonella
Elleipsisomatidae
o Elleipsisoma
Lankesterellidae
Lankesterella
Schellackia
Sarcocystidae
o Sarcocystinae
Frenkelia
Sarcocystis
o Toxoplasmatinae
Besnoitia
Hammondia
Neospora
Toxoplasma
Selenococcidiidae
o Selenococcidium
Spirocystidae
o Spirocystis
o
o
•
•
•
Coccidia are microscopic, spore-forming, single-celled parasites belonging to the
apicomplexan class Conoidasida.[1] Coccidian parasites infect the intestinal tracts of
animals[2], and are the largest group of apicomplexan protozoa.
Coccidia are obligate, intracellular parasites, which means that they must live and
reproduce within an animal cell.
Contents
[hide]
•
•
•
•
•
1 Coccidiosis
o 1.1 Coccidia in dogs
2 Genera and species that cause coccidiosis
3 References
4 See also
5 External links
[edit] Coccidiosis
Coccidiosis is the disease caused by coccidian infection. Coccidiosis is a parasitic
disease of the intestinal tract of animals, caused by coccidian protozoa. The disease
spreads from one animal to another by contact with infected feces, or ingestion of
infected tissue. Diarrhea, which may become bloody in severe cases, is the primary
symptom. Most animals infected with coccidia are asymptomatic; however, young or
immuno-compromised animals may suffer severe symptoms, including death.
While coccidian organisms can infect a wide variety of animals, including humans,
birds, and livestock, they are usually species-specific. One well-known exception is
toxoplasmosis, caused by Toxoplasma gondii.
[edit] Coccidia in dogs
People often first encounter coccidia when they acquire a young puppy who is
infected. The infectious organisms are canine-specific and are not contagious to
humans (compare to zoonotic diseases).
Young puppies are frequently infected with coccidia and often develop active
Coccidiosis -- even puppies obtained from diligent professional breeders. Infected
puppies almost always have received the parasite from their mother's feces. Typically,
healthy adult animals shedding the parasite's oocysts in their feces will be
asymptomatic because of their developed immune systems. However, undeveloped
immune systems make puppies more susceptible. Further, stressors such as new
owners, travel, weather changes, and unsanitary conditions are believed to activate
infections in susceptible animals.
Symptoms in young dogs are universal: at some point around 2-3 months of age, an
infected dog develops persistently loose stools. This diarrhea proceeds to stool
containing liquid, thick mucus, and light colored fecal matter. As the infection
progresses, spots of blood may become apparent in the stool, and sudden bowel
movements may surprise both dog and owner alike. Coccidia infection is so common
that any pup under 4 months old with these symptoms can almost surely be assumed
to have coccidiosis.
Fortunately, the treatment is inexpensive, extremely effective, and routine. A
veterinarian can easily diagnose the disease through low-powered microscopic
examination of an affected dog's feces, which usually will be replete with oocysts.
One of many easily administered and inexpensive drugs will be prescribed, and, in the
course of just a few days, an infection will be eliminated or perhaps reduced to such a
level that the dog's immune system can make its own progress against the infection.
Even when an infection has progressed sufficiently that blood is present in feces,
permanent damage to the gastrointestinal system is rare, and the dog will most likely
make a complete recovery without long-lasting negative effects.
If one dog of a litter has coccidiosis, then most certainly all dogs at a breeder's
kennels have active coccidia infections. Breeders should be notified if a newlyacquired pup is discovered to be infected with coccidia. Breeders can take steps to
eradicate the organism from their kennels, including applying medications in bulk to
an entire facility.
[edit] Genera and species that cause coccidiosis
•
•
Genus Isospora is the most common cause of intestinal coccidiosis in dogs
and cats and is usually what is meant by coccidiosis. Species of Isospora are
species specific, meaning they only infects one type of species. Species that
infect dogs include I. canis, I. ohioensis, I. burrowsi, and I. neorivolta. Species
that infect cats include I. felis and I. rivolta. The most common symptom is
diarrhea. Sulfonamides are the most common treatment. [3]
Genus Cryptosporidium contains two species known to cause
cryptosporidiosis, C. parvum and C. muris. Cattle are most commonly affected
•
•
•
•
•
•
by Cryptosporidium, and their feces are often assumed to be a source of
infection for other mammals including humans. Recent genetic analyses of
Cryptosporidium in humans have identified Cryptosporidium hominis as a
new species specific for humans. Infection occurs most commonly in
individuals that are immunocompromised, e.g. dogs with canine distemper,
cats with feline leukemia virus infection, and humans with AIDS. Very young
puppies and kittens can also become infected with Cryptosporidium, but the
infection is usually eliminated without treatment.[3]
Genus Hammondia is transmitted by ingestion of cysts found in the tissue of
grazing animals and rodents. Dogs and cats are the definitive hosts, with the
species H. heydorni infecting dogs and the species H. hammondi and H.
pardalis infecting cats. Hammondia usually does not cause disease.[3]
Genus Besnoitia infect cats that ingest cysts found in the tissue of rodents and
opossum, but usually does not cause disease.[3]
Genus Sarcocystis infect carnivores that ingest cysts from various intermediate
hosts. It is possible for Sarcocystis to cause disease in dogs and cats.[3]
Genus Toxoplasma has one important species, Toxoplasma gondii. Cats are
the definitive host, but all mammals and some fish, reptiles, and amphibians
can be intermediate hosts. Therefore, only cat feces will hold infective
oocysts, but infection through ingestion of cysts can occur with the tissue of
any intermediate host. Toxoplasmosis occurs in humans usually as low-grade
fever or muscle pain for a few days. A normal immune system will suppress
the infection but the tissue cysts will persist in that animal or human for years
or the rest of its life. In immunocompromised individuals, those dormant cysts
can be reactivated and cause many lesions in the brain, heart, lungs, eyes, etc.
Without a competent immune system, the animal or human will most likely
die from the infection. For pregnant women, the fetus is at risk if the pregnant
woman becomes infected for the first time during pregnancy. If the woman
had been infected during childhood or adolescence, she will have an immunity
that will protect her developing fetus during pregnancy. The most important
misconception about the transmission of toxoplasmosis comes from statements
like 'ingestion of raw or undercooked meat, or cat feces.' Kitchen hygiene is
much more important because people do tend to taste marinades or sauces
before being cooked, or chop meat then vegetables without properly cleaning
the knife and cutting board. Many physicians mistakenly put panic in their
pregnant clients and advise them to get rid of their cat without really warning
them of the likely sources of infection. Adult cats are very unlikely to shed
infective oocysts. Symptoms in cats include fever, weight loss, diarrhea,
vomiting, uveitis, and central nervous system signs. Disease in dogs includes a
rapidly progressive form seen in dogs also infected with distemper, and a
neurological form causing paralysis, tremors, and seizures. Dogs and cats are
usually treated with clindamycin.[3]
Genus Neospora has one important species, Neospora caninum, that affects
dogs in a manner similar to toxoplasmosis. Neosporosis is difficult to treat.[3]
Genus Hepatozoon contains one species that causes hepatozoonosis in dogs
and cats, Hepatozoon canis. Animals become infected by ingesting an infected
Rhipicephalus sanguineus, also known as the brown dog tick. Symptoms
include fever, weight loss, and pain of the spine and limbs.
The most common medications used to treat coccidian infections are in the
sulphonamide family. Although unusual, sulphonamides can damage the tear glands
in some dogs, causing keratoconjunctivitis sicca, or "dry eye", which may have a lifelong impact. Some veterinarians recommend measuring tear production prior to
sulphonamide administration, and at various intervals after administration. Other
veterinarians will simply avoid using sulphonamides, instead choosing another
product effective against coccidia.
Left untreated, the infection may clear of its own accord, or in some cases may
continue to ravage an animal and cause permanent damage or, occasionally, death.
[edit] References
1. ^ The Taxonomicon & Systema Naturae (Website database). Taxon: Genus
Cryptosporidium. Universal Taxonomic Services, Amsterdam, The
Netherlands (2000).
2. ^ Biodiversity explorer: Apicomplexa (apicomplexans, sporozoans). Iziko
Museums of Cape Town.
3. ^ a b c d e f g Ettinger, Stephen J.; Feldman, Edward C. (1995). Textbook of
Veterinary Internal Medicine, 4th ed., W.B. Saunders Company. ISBN 07216-6795-3.
[edit] See also
•
•
cryptosporidiosis
Zoalene is a fodder additive for poultry, used to prevent infections from
coccidia.
[edit] External links
•
•
•
•
•
Mar Vista Animal Medical Center.
The Coccidia of the World, Donald W. Duszynski, Steve J. Upton, Lee Couch,
Feb. 21, 2004.
Life Cycle EIMERIA, Andreas Weck-Heimann, 1996-2005
FarmingUK, Information about Coccidiosis
Lillehoj, Hyun S. (October 1996). "Two Strategies for Protecting Poultry
From Coccidia". Agricultural Research magazine (October 1996). United
States Department of Agriculture: Agrigultural Research Service. Describes
using live-parasite vaccine versus a monoclonal antibody to block the
sporozoite from invading a host's cell.
Retrieved from "http://en.wikipedia.org/wiki/Coccidia"
Categories: Apicomplexa | Dog diseases | Cat diseases | Animal diseases | Veterinary
protozoology
http://en.wikipedia.org/wiki/Deer_Island_Waste_Water_Treatment_Plant
http://ludb.clui.org/ex/i/MA3134/
Deer Island Waste Water Treatment
Plant
From Wikipedia, the free encyclopedia
Jump to: navigation, search
The Deer Island Waste Water Treatment Plant (also known as Deer Island Sewage
Treatment Plant) run and operated by The Massachusetts Water Resources Authority
is located on Deer Island, one of the Boston Harbor Islands in Boston Harbor.
It is the second largest sewage treatment plant in the United States.[1] [2] [3]
It is a key part of the program to protect Boston Harbor against pollution from sewer
systems in eastern Massachusetts.
The plant removes human, household, business and industrial pollutants from
wastewater that originates in homes and businesses in forty three greater Boston
communities. It complies with all federal and state environmental standards and
subject to the discharge permit issued for it by EPA and DEP. Its treated wastewater
can safely be released into the marine environment.
It has an array of 150 foot tall egg-like sludge digesters and these are major harbor
landmarks.[4][5]
[edit] Notes
1. ^ Deer Island Sewage Treatment Plant. The Center for Land Use
Interpretation.
2. ^ Jardine Water Purification Plant article
3. ^ 1867 "The First Tunnel Under the Lake". Chicago Public Library.
4. ^ Islands You Can Visit - Deer Island. Boston Harbor Islands Partnership.
Retrieved on August 21, 2006.
5. ^ Deer Island Factsheet. Boston Harbor Islands Partnership. Retrieved on
August 21, 2006.
[edit] Bibliography
•
Baldwin, Sandy, "Boston Harbor Pipe Dreams Come True!": USGS Visits the
Deer Island Sewage Treatment Plant and a Cleaner Harbor, USGS Sound
Waves, April 2006.
[edit] External links
•
•
MWRA article on The Deer Island Sewage Treatment Plant
A History of the sewer system in Boston
Retrieved from
"http://en.wikipedia.org/wiki/Deer_Island_Waste_Water_Treatment_Plant"
Categories: Buildings and structures in Boston, Massachusetts | Sewage treatment
plants
Views
•
•
•
•
Article
Discussion
Edit this page
History
Personal tools
•
Log in / create account
Navigation
•
•
•
•
•
Main Page
Contents
Featured content
Current events
Random article
Interaction
•
•
•
•
•
•
About Wikipedia
Community portal
Recent changes
Contact Wikipedia
Donate to Wikipedia
Help
Search
Toolbox
•
•
•
What links here
Related changes
Upload file
•
•
•
•
Special pages
Printable version
Permanent link
Cite this page
•
•
This page was last modified on 19 October 2007, at 15:11.
All text is available under the terms of the GNU Free Documentation License.
(See Copyrights for details.)
Wikipedia® is a registered trademark of the Wikimedia Foundation, Inc., a
U.S. registered 501(c)(3) tax-deductible nonprofit charity.
Privacy policy
About Wikipedia
Disclaimers
•
•
•
http://en.wikipedia.org/wiki/Flagellate
Flagellate
From Wikipedia, the free encyclopedia
Jump to: navigation, search
"Flagellata" from Ernst Haeckel's Artforms of Nature, 1904
Parasitic excavate (Giardia lamblia)
Green alga (Chlamydomonas)
Flagellates are cells with one or more whip-like organelles called flagella. Some cells
in animals may be flagellate, for instance the spermatozoa of most phyla. Higher
plants and fungi do not produce flagellate cells, but the closely related green algae and
chytrids do. Many protists take the form of single-celled flagellates.
[edit] Form and behavior
Flagellates r protozoans (animal-like protists). Eukaryotic flagella are supported by
microtubules in a characteristic arrangement, with nine fused pairs surrounding two
central singlets. These arise from a basal body or kinetosome, with microtubule roots
that are an important part of the cell's brain. In some, for instance, they support a
cytostome or mouth, where food is ingested. The flagella often support hairs, called
mastigonemes, or contain rods. Their ultrastructure plays an important role in
classifying eukaryotes.
In protists and microscopic animals, flagella are generally used for propulsion. They
may also be used to create a current that brings in food. In most things, one or more
flagella are located at or near the anterior of the cell eg Euglena. Often there is one
directed forwards and one trailing behind. Among animals, fungi, and Choanozoa,
which make up a group called the opisthokonts, there is a single posterior flagellum.
They are from the phylum Mastigophora. They can cause diseases and they can make
their own food. For example, Trypanosome which causes the African sleeping
sickness.
[edit] Groups of flagellates
Originally the flagellated protozoa were treated as a single class of phylum, the
Mastigophora. This was divided into the Phytomastigina or phytoflagellates, which
have chloroplasts or are closely related to such forms, and the Zoomastigina or
zooflagellates, which do not. Most phytoflagellates were given a separate
classification by botanists, treating them in several divisions of algae.
This scheme has generally been abandoned or is retained only for convenience.
However, the relationships among the flagellates are still mostly unknown, and their
higher classification is confused. Some argue that the Linnaean ranks are not
appropriate for such a diverse set of organisms.
Phytoflagellates are found in most groups of algae. Both the green algae and
heterokonts include a variety of flagellates in addition to non-motile and multicellular
forms. The dinoflagellates, cryptomonads, haptophytes, and euglenids are almost
entirely single-celled flagellates.
Many of the other flagellates make up what are called the excavate taxa. These
include the euglenids and a number of important parasites, such as trypanosomes and
Giardia. The excavates generally show similarities in the structure of their flagella
and typically have a cytostome. However, they may be a paraphyletic group, and in
particular may have been ancestral to most or all other eukaryotes. Aprils fools day
yaeaahhhhh
Other notable groups including flagellates are the Cercozoa, alveolates (including
dinoflagellates), ebriids, and Apusozoa.
[edit] External links
•
MeSH Flagellata
Retrieved from "http://en.wikipedia.org/wiki/Flagellate"
http://en.wikipedia.org/wiki/Fungus#With_plants
accessed 03/04/08
Fungus
From Wikipedia, the free encyclopedia
Jump to: navigation, search
For the fictional character, see Fungus the Bogeyman. For the music genre, see
Fungi (music).
Fungi
Fossil range: Early Silurian - Recent
Clockwise from top left: Amanita muscaria, a
basidiomycete; Sarcoscypha coccinea, an
ascomycete; black bread mold, a zygomycete; a
chytrid; a Penicillium conidiophore.
Scientific classification
Domain:
Eukarya
(unranked) Opisthokonta
Kingdom: Fungi
(L., 1753) R.T. Moore, 1980[1]
Subkingdoms/Phyla
Chytridiomycota
Blastocladiomycota
Neocallimastigomycota
Glomeromycota
Zygomycota
Dikarya (inc. Deuteromycota)
Ascomycota
Basidiomycota
A fungus (pronounced /f
ŋgәs/) is any eukaryotic organism that is a member of the
kingdom Fungi (pronounced /f
nd a /).[2] The fungi are heterotrophic organisms
characterized by a chitinous cell wall, and in the majority of species, filamentous
growth as multicellular hyphae forming a mycelium; some fungal species also grow
as single cells. Sexual and asexual reproduction is commonly via spores, often
produced on specialized structures or in fruiting bodies. Some fungal species have lost
the ability to form specialized reproductive structures, and propagate solely by
vegetative growth. Yeasts, molds, and mushrooms are examples of fungi. The fungi
are a monophyletic group that is phylogenetically clearly distinct from the
morphologically similar slime molds (myxomycetes) and water molds (oomycetes).
The fungi are more closely related to animals than plants, yet the discipline of biology
devoted to the study of fungi, known as mycology, often falls under a branch of
botany.
Occurring worldwide, most fungi are largely invisible to the naked eye, living for the
most part in soil, dead matter and as symbionts of plants, animals, or other fungi.
They perform an essential role in all ecosystems in decomposing matter and are
indispensable in nutrient cycling and exchange. Some fungi become noticeable when
fruiting, either as mushrooms or molds. Many fungal species have long been used as a
direct source of food, such as mushrooms and truffles and in fermentation of various
food products, such as wine, beer, and soy sauce. More recently, fungi are being used
as sources for antibiotics and various enzymes, such as cellulases, pectinases, and
proteases, important for industrial use or as active ingredients of detergents. Many
fungi produce bioactive compounds, such as alkaloids and polyketides that are toxic
to animals including humans and are, therefore, called mycotoxins. Some fungi are
used recreationally or in traditional ceremonies as a source of psychotropic
compounds. Several species of the fungi are significant pathogens of humans and
other animals, and losses due to diseases of crops (e.g., rice blast disease) or food
spoilage caused by fungi can have a large impact on human food supply and local
economies.
Contents
[hide]
•
•
•
•
•
•
•
1 Etymology and definition
2 Diversity
3 Importance for human use
o 3.1 Cultured foods
o 3.2 Other human uses
o 3.3 Mycotoxins
o 3.4 Edible and poisonous fungi
o 3.5 Fungi in the biological control of pests
4 Ecology
o 4.1 Symbiosis
4.1.1 With plants
4.1.2 With insects
4.1.3 As pathogens and parasites
o 4.2 Nutrition and possible autotrophy
5 Morphology
o 5.1 Microscopic structures
o 5.2 Macroscopic structures
o 5.3 Morphological and physiological features for substrate penetration
6 Reproduction
o 6.1 Asexual reproduction
o 6.2 Sexual reproduction
o 6.3 Spore dispersal
o 6.4 Other sexual processes
7 Phylogeny and classification
o 7.1 Physiological and morphological traits
o 7.2 Evolutionary history
7.2.1 Cladogram
o 7.3 The taxonomic groups of fungi
•
•
•
•
o 7.4 Phylogenetic relationships with other fungus-like organisms
8 See also
9 Notes and references
10 Further reading
11 External links
Etymology and definition
The English word fungus is directly adopted from the Latin fungus, meaning
"mushroom", used in Horace and Pliny.[3] This in turn is derived from the Greek word
sphongos/σφογγος ("sponge"), referring to the macroscopic structures and
morphology of some mushrooms and molds and also used in other languages (e.g., the
German Schwamm ("sponge") or Schwammerl for some types of mushroom).
Diversity
Fungi have a worldwide distribution, and grow in a wide range of habitats, including
deserts. Most fungi grow in terrestrial environments, but several species occur only in
aquatic habitats. Fungi along with bacteria are the primary decomposers of organic
matter in most if not all terrestrial ecosystems worldwide. Based on observations of
the ratio of the number of fungal species to the number of plant species in some
environments, the fungal kingdom has been estimated to contain about 1.5 million
species. [4] Around 70,000 fungal species have been formally described by
taxonomists, but the true dimension of fungal diversity is still unknown. [5] Most fungi
grow as thread-like filaments called hyphae, which form a mycelium, while others
grow as single cells. [6][7] Until recently many fungal species were described based
mainly on morphological characteristics, such as the size and shape of spores or
fruiting structures, and biological species concepts; the application of molecular tools,
such as DNA sequencing, to study fungal diversity has greatly enhanced the
resolution and added robustness to estimates of diversity within various taxonomic
groups.[8]
Importance for human use
Sacharomyces cerevisiae cells in DIC microscopy.
Human use of fungi for food preparation or preservation and other purposes is
extensive and has a long history: yeasts are required for fermentation of beer, wine [9]
and bread, some other fungal species are used in the production of soy sauce and
tempeh. Mushroom farming and mushroom gathering are large industries in many
countries. Many fungi are producers of antibiotics, including β-lactam antibiotics such
as penicillin and cephalosporin.[10] Widespread use of these antibiotics for the
treatment of bacterial diseases, such as tuberculosis, syphilis, leprosy, and many
others began in the early 20th century and continues to play a major part in antibacterial chemotherapy. The study of the historical uses and sociological impact of
fungi is known as ethnomycology.
Cultured foods
Baker's yeast or Saccharomyces cerevisiae, a single-cell fungus, is used in the baking
of bread and other wheat-based products, such as pizza and dumplings.[11] Several
yeast species of the genus Saccharomyces are also used in the production of alcoholic
beverages through fermentation.[12] Mycelial fungi, such as the shoyu koji mold
(Aspergillus oryzae), are used in the brewing of Shoyu (soy sauce) and preparation of
tempeh.[13] Quorn is a high-protein product made from the mold, Fusarium
venenatum, and is used in vegetarian cooking.
Other human uses
Fungi are also used extensively to produce industrial chemicals like lactic acid,
antibiotics and even to make stonewashed jeans.[14] Several fungal species are
ingested for their psychedelic properties, both recreationally and religiously (see main
article, Psilocybin mushrooms).
Mycotoxins
Main article: Mycotoxins
Many fungi produce compounds with biological activity. Several of these compounds
are toxic and are therefore called mycotoxins, referring to their fungal origin and toxic
activity. Of particular relevance to humans are those mycotoxins that are produced by
moulds causing food spoilage and poisonous mushrooms (see below). Particularly
infamous are the aflatoxins, which are insidious liver toxins and highly carcinogenic
metabolites produced by Aspergillus species often growing in or on grains and nuts
consumed by humans, and the lethal amatoxins produced by mushrooms of the genus
Amanita. Other notable mycotoxins include ochratoxins, patulin, ergot alkaloids, and
trichothecenes and fumonisins, all of which have significant impact on human food
supplies or animal livestock. [15]
Mycotoxins belong to the group of secondary metabolites (or natural products).
Originally, this group of compounds had been thought to be mere byproducts of
primary metabolism, hence the name "secondary" metabolites. However, recent
research has shown the existence of biochemical pathways solely for the purpose of
producing mycotoxins and other natural products in fungi. [16] Mycotoxins provide a
number of fitness benefits to the fungi that produce them in terms of physiological
adaptation, competition with other microbes and fungi, and protection from
fungivory. [17][18] These fitness benefits and the existence of dedicated biosynthetic
pathways for mycotoxin production suggest that the mycotoxins are important for
fungal persistence and survival.
Edible and poisonous fungi
Asian mushrooms, clockwise from left, enokitake, buna-shimeji, bunapi-shimeji, king
oyster mushroom and shiitake.
Black Périgord Truffle (Tuber melanosporum), cut in half.
Stilton cheese veined with Penicillium roqueforti.
Some of the best known types of fungi are the edible and the poisonous mushrooms.
Many species are commercially raised, but others must be harvested from the wild.
Agaricus bisporus, sold as button mushrooms when small or Portobello mushrooms
when larger, are the most commonly eaten species, used in salads, soups, and many
other dishes. Many Asian fungi are commercially grown and have gained in
popularity in the West. They are often available fresh in grocery stores and markets,
including straw mushrooms (Volvariella volvacea), oyster mushrooms (Pleurotus
ostreatus), shiitakes (Lentinula edodes), and enokitake (Flammulina spp.).
There are many more mushroom species that are harvested from the wild for personal
consumption or commercial sale. Milk mushrooms, morels, chanterelles, truffles,
black trumpets, and porcini mushrooms (Boletus edulis) (also known as king boletes)
all demand a high price on the market. They are often used in gourmet dishes.
For certain types of cheeses, it is also a common practice to inoculate milk curds with
fungal spores to foment the growth of specific species of mold that impart a unique
flavor and texture to the cheese. This accounts for the blue colour in cheeses such as
Stilton or Roquefort which is created using Penicillium roqueforti spores.[19] Molds
used in cheese production are usually non-toxic and are thus safe for human
consumption; however, mycotoxins (e.g., aflatoxins, roquefortine C, patulin, or
others) may accumulate due to fungal spoilage during cheese ripening or storage.[20]
Many mushroom species are toxic to humans, with toxicities ranging from slight
digestive problems or allergic reactions as well as hallucinations to severe organ
failures and death. Some of the most deadly mushrooms belong to the genera Inocybe,
Cortinarius, and most infamously, Amanita, which includes the destroying angel (A.
virosa) and the death cap (A. phalloides), the most common cause of deadly
mushroom poisoning. [21] The false morel (Gyromitra esculenta) is considered a
delicacy by some when cooked yet can be deadly when raw. Tricholoma equestre is
one which was considered edible for centuries yet recently responsible for a series of
serious poisonings in France.
Fly agaric mushrooms (A. muscaria) also cause occasional poisonings, mostly as a
result of ingestion for use as a recreational drug for its hallucinogenic properties.
Historically Fly agaric was used by Celtic Druids in Northern Europe and the Koryak
people of north-eastern Siberia for religious or shamanic purposes.[22] It is difficult to
identify a safe mushroom without proper training and knowledge, thus it is often
advised to assume that a mushroom in the wild is poisonous and not to consume it.
Fungi in the biological control of pests
In agricultural settings, fungi that actively compete for nutrients and space with, and
eventually prevail over, pathogenic microorganisms, such as bacteria or other fungi,
via the competitive exclusion principle,[23] or are parasites of these pathogens, may be
beneficial agents for human use. For example, some fungi may be used to suppress
growth or eliminate harmful plant pathogens, such as insects, mites, weeds,
nematodes and other fungi that cause diseases of important crop plants.[24] This has
generated strong interest in the use and practical application of these fungi for the
biological control of these agricultural pests. Entomopathogenic fungi can be used as
biopesticides, as they actively kill insects.[25] Examples of fungi that have been used
as bioinsecticides are Beauveria bassiana, Metarhizium anisopliae, Hirsutella spp,
Paecilomyces fumosoroseus, and Verticillium lecanii.[26] [27] Endophytic fungi of
grasses of the genus Neotyphodium, such as N. coenophialum produce alkaloids that
are toxic to a range of invertebrate and vertebrate herbivores. These alkaloids protect
the infected grass plants from herbivory, but some endophyte alkaloids can cause
poisoning of grazing animals, such as cattle and sheep. [28] Infection of grass cultivars
of turf or forage grasses with isolates of the grass endophytes that produce only
specific alkaloids to improve grass hardiness and resistance to herbivores such as
insects, while being non-toxic to livestock, is being used in grass breeding
programs.[29]
Ecology
Polypores growing on a tree in Borneo
Although often inconspicuous, fungi occur in every environment on Earth and play
very important roles in most ecosystems. Along with bacteria, fungi are the major
decomposers in most terrestrial (and some aquatic) ecosystems, and therefore play a
critical role in biogeochemical cycles and in many food webs. As decomposers, they
play an indispensable role in nutrient cycling, especially as saprotrophs and
symbionts, degrading organic matter to inorganic molecules, which can then re-enter
anabolic metabolic pathways in plants or other organisms.[30][31]
Symbiosis
Many fungi have important symbiotic relationships with organisms from most if not
all Kingdoms.[32][33][34] These interactions can be mutualistic or antagonistic in nature,
or in case of commensal fungi are of no apparent benefit or detriment to the host.
[35][36][37]
With plants
Mycorrhizal symbiosis between plants and fungi is one of the most well-known plantfungus associations and is of significant importance for plant growth and persistence
in many ecosystems; over 90% of all plant species engage in some kind of
mycorrhizal relationship with fungi and are dependent upon this relationship for
survival.[38][39][40] The mycorrhizal symbiosis is ancient, dating to at least 400 million
years ago.[41] It often increases the plant's uptake of inorganic compounds, such as
nitrate and phosphate from soils having low concentrations of these key plant
nutrients.[30] In some mycorrhizal associations, the fungal partners may mediate plantto-plant transfer of carbohydrates and other nutrients. Such mycorrhizal communities
are called "common mycorrhizal networks". [42]
Lichens are formed by a symbiotic relationship between algae or cyanobacteria
(referred to in lichens as "photobionts") and fungi (mostly various species of
ascomycetes and a few basidiomycetes), in which individual photobiont cells are
embedded in a tissue formed by the fungus.[43] As in mycorrhizas, the photobiont
provides sugars and other carbohydrates, while the fungus provides minerals and
water. The functions of both symbiotic organisms are so closely intertwined that they
function almost as a single organism.
With insects
Many insects also engage in mutualistic relationships with various types of fungi.
Several groups of ants cultivate fungi in the order Agaricales as their primary food
source, while ambrosia beetles cultivate various species of fungi in the bark of trees
that they infest.[44] Termites on the African Savannah are also known to cultivate
fungi.[45]
As pathogens and parasites
However, many fungi are parasites on plants, animals (including humans), and other
fungi. Serious fungal pathogens of many cultivated plants causing extensive damage
and losses to agriculture and forestry include the rice blast fungus Magnaporthe
oryzae,[46] tree pathogens such as Ophiostoma ulmi and Ophiostoma novo-ulmi
causing Dutch elm disease,[47] and Cryphonectria parasitica responsible for chestnut
blight, [48] and plant-pathogenic fungi in the genera Fusarium, Ustilago, Alternaria,
and Cochliobolus; [36] fungi with the potential to cause serious human diseases,
especially in persons with immuno-deficiencies, are in the genera Aspergillus,
Candida, Cryptoccocus,[49][37][50] Histoplasma,[51] and Pneumocystis. [52] Several
pathogenic fungi are also responsible for relatively minor human diseases, such as
athlete’s foot and ringworm. Some fungi are predators of nematodes, which they
capture using an array of specialized structures, such as constricting rings or adhesive
nets.[53]
Nutrition and possible autotrophy
Growth of fungi as hyphae on or in solid substrates or single cells in aquatic
environments is adapted to efficient extraction of nutrients from these environments,
because these growth forms have high surface area to volume ratios. These
adaptations in morphology are complemented by hydrolytic enzymes secreted into the
environment for digestion of large organic molecules, such as polysaccharides,
proteins, lipids, and other organic substrates into smaller molecules. [54][55][56] These
molecules are then absorbed as nutrients into the fungal cells.
Traditionally, the fungi are considered heterotrophs, organisms that rely solely on
carbon fixed by other organisms for metabolism. Fungi have evolved a remarkable
metabolic versatility that allows many of them to use a large variety of organic
substrates for growth, including simple compounds as nitrate, ammonia, acetate, or
ethanol.[57] [58] Recent research raises the possibility that some fungi utilize the
pigment melanin to extract energy from ionizing radiation, such as gamma radiation
for "radiotrophic" growth. [59] It has been proposed that this process might bear some
similarity to photosynthesis in plants, [59] but detailed biochemical data supporting the
existence of this hypothetical pathway are presently lacking.
Morphology
Microscopic structures
Mold covering a decaying peach over a period of six days. The frames were taken
approximately 12 hours apart.
Though fungi are part of the opisthokont clade, all phyla except for the chytrids have
lost their posterior flagella.[60] Fungi are unusual among the eukaryotes in having a
cell wall that, besides glucans (e.g., β-1,3-glucan) and other typical components,
contains the biopolymer chitin.[61]
Many fungi grow as thread-like filamentous microscopic structures called hyphae,
and an assemblage of intertwined and interconnected hyphae is called a mycelium. [6]
Hyphae can be septate, i.e., divided into hyphal compartments separated by a septum,
each compartment containing one or more nuclei or can be coenocytic, i.e., lacking
hyphal compartmentalization. However, septa have pores, such as the doliporus in the
basidiomycetes that allow cytoplasm, organelles, and sometimes nuclei to pass
through.[6] Coenocytic hyphae are essentially multinucleate supercells.[62] In some
cases, fungi have developed specialized structures for nutrient uptake from living
hosts; examples include haustoria in plant-parasitic fungi of nearly all divisions, and
arbuscules of several mycorrhizal fungi,[63] which penetrate into the host cells for
nutrient uptake by the fungus.
Macroscopic structures
Fungal mycelia can become visible macroscopically, for example, as concentric rings
on various surfaces, such as damp walls, and on other substrates, such as spoilt food
(see figure), and are commonly and generically called mould (American spelling,
mold); fungal mycelia grown on solid agar media in laboratory petri dishes are usually
referred to as colonies, with many species exhibiting characteristic macroscopic
growth morphologies and colours, due to spores or pigmentation.
Specialized fungal structures important in sexual reproduction are the apothecia,
perithecia, and cleistothecia in the ascomycetes, and the fruiting bodies of the
basidiomycetes, and a few ascomycetes. These reproductive structures can sometimes
grow very large, and are well known as mushrooms.
Morphological and physiological features for substrate penetration
Fungal hyphae are specifically adapted to growth on solid surfaces and within
substrates, and can exert astoundingly large penetrative mechanical forces. The plant
pathogen, Magnaporthe grisea, forms a structure called an appressorium specifically
designed for penetration of plant tissues, and the pressure generated by the
appressorium, which is directed against the plant epidermis can exceed 8 MPa (80
bars). [64] The generation of these mechanical pressures is the result of an interplay
between physiological processes to increase intracellular turgor by production of
osmolytes such as glycerol, and the morphology of the appressorium. [65]
Reproduction
Fungi on a fence post near Orosí, Costa Rica.
Reproduction of fungi is complex, reflecting the heterogeneity in lifestyles and
genetic make up within this group of organisms. [6] Many fungi reproduce both
sexually or asexually, depending on conditions in the environment. These conditions
trigger genetically determined developmental programs leading to the expression of
specialized structures for sexual or asexual reproduction. These structures aid both
reproduction and efficient dissemination of spores or spore-containing propagules.
Asexual reproduction
Asexual reproduction via vegetative spores or through mycelial fragmentation is
common in many fungal species and allows more rapid dispersal than sexual
reproduction. In the case of the "Fungi imperfecti" or Deuteromycota, which lack a
sexual cycle, it is the only means of propagation. Asexual spores, upon germination,
may found a population that is clonal to the population from which the spore
originated, and thus colonize new environments.
Sexual reproduction
Sexual reproduction with meiosis exists in all fungal phyla, except the
Deuteromycota. It differs in many aspects from sexual reproduction in animals or
plants. Many differences also exist between fungal groups and have been used to
discriminate fungal clades and species based on morphological differences in sexual
structures and reproductive strategies. Experimental crosses between fungal isolates
can also be used to identify species based on biological species concepts. The major
fungal clades have initially been delineated based on the morphology of their sexual
structures and spores; for example, the spore-containing structures, asci and basidia,
can be used in the identification of ascomycetes and basidiomycetes, respectively.
Many fungal species have elaborate vegetative incompatibility systems that allow
mating only between individuals of opposite mating type, while others can mate and
sexually reproduce with any other individual or itself. Species of the former mating
system are called heterothallic, and of the latter homothallic. [66]
Most fungi have both a haploid and diploid stage in their life cycles. In all sexually
reproducing fungi, compatible individuals combine by cell fusion of vegetative
hyphae by anastomosis, required for the initiation of the sexual cycle. Ascomycetes
and basidiomycetes go through a dikaryotic stage, in which the nuclei inherited from
the two parents do not fuse immediately after cell fusion, but remain separate in the
hyphal cells (see heterokaryosis).
In ascomycetes, dikaryotic hyphae of the hymenium form a characteristic hook at the
hyphal septum. During cell division formation of the hook ensures proper distribution
of the newly divided nuclei into the apical and basal hyphal compartments. An ascus
(plural asci) is then formed, in which karyogamy (nuclear fusion) occurs. These asci
are embedded in an ascocarp, or fruiting body, of the fungus. Karyogamy in the asci is
followed immediately by meiosis and the production of ascospores. The ascospores
are disseminated and germinate and may form a new haploid mycelium.[67]
Sexual reproduction in basidiomycetes is similar to that of the ascomycetes.
Compatible haploid hyphae fuse to produce a dikaryotic mycelium. However, the
dikaryotic phase is more extensive in the basidiomycetes, in many cases also present
in the vegetatively growing mycelium. A specialized anatomical structure, called a
clamp connection, is formed at each hyphal septum. As with the structurally similar
hook in the ascomycetes, formation of the clamp connection in the basidiomycetes is
required for controlled transfer of nuclei during cell division, to maintain the
dikaryotic stage with two genetically different nuclei in each hyphal compartment. [67]
A basidiocarp is formed in which club-like structures known as basidia generate
haploid basidiospores after karyogamy and meiosis.[68] The most commonly known
basidiocarps are mushrooms, but they may also take many other forms (see
Morphology section).
In zygomycetes, haploid hyphae of two individuals fuse, forming a zygote, which
develops into a zygospore. When the zygospore germinates, it quickly undergoes
meiosis, generating new haploid hyphae, which in turn may form asexual
sporangiospores. These sporangiospores are means of rapid dispersal of the fungus
and germinate into new genetically identical haploid fungal colonies, able to mate and
undergo another sexual cycle followed by the generation of new zygospores, thus
completing the lifecycle.
Spore dispersal
Both asexual and sexual spores or sporangiospores of many fungal species are
actively dispersed by forcible ejection from their reproductive structures. This
ejection ensures exit of the spores from the reproductive structures as well as
travelling through the air over long distances. Many fungi thereby possess specialized
mechanical and physiological mechanisms as well as spore-surface structures, such as
hydrophobins, for spore ejection. These mechanisms include, for example, forcible
discharge of ascospores enabled by the structure of the ascus and accumulation of
osmolytes in the fluids of the ascus that lead to explosive discharge of the ascospores
into the air. [69] The forcible discharge of single spores termed ballistospores involves
formation of a small drop of water (Buller's drop), which upon contact with the spore
leads to its projectile release with an initial acceleration of more than 10,000 g. [70]
Other fungi rely on alternative mechanisms for spore release, such as external
mechanical forces, exemplified by puffballs. Attracting insects, such as flies, to
fruiting structures, by virtue of their having lively colours and a putrid odour, for
dispersal of fungal spores is yet another strategy, most prominently used by the
stinkhorns.
Other sexual processes
Besides regular sexual reproduction with meiosis, some fungal species may exchange
genetic material via parasexual processes, initiated by anastomosis between hyphae
and plasmogamy of fungal cells. The frequency and relative importance of parasexual
events is unclear and may be lower than other sexual processes. However, it is known
to play a role in intraspecific hybridization [71] and is also likely required for
hybridization between fungal species, which has been associated with major events in
fungal evolution. [72]
Phylogeny and classification
The mushroom Oudemansiella nocturnum eats wood
For a long time taxonomists considered fungi to be members of the Plant Kingdom.
This early classification was based mainly on similarities in lifestyle: both fungi and
plant are mainly sessile, have similarities in general morphology and growth habitat
(like plants, fungi often grow in soil, in the case of mushrooms forming conspicuous
fruiting bodies, which sometimes bear resemblance to plants such as mosses).
Moreover, both groups possess a cell wall, which is absent in the Animal Kingdom.
However, the fungi are now considered a separate kingdom, distinct from both plants
and animals, from which they appear to have diverged approximately one billion
years ago.[73] Many studies have identified several distinct morphological,
biochemical, and genetic features in the Fungi, clearly delineating this group from the
other kingdoms. For these reasons, the fungi are placed in their own kingdom.
Physiological and morphological traits
Similar to animals and unlike most plants, fungi lack the capacity to synthesize
organic carbon by chlorophyll-based photosynthesis; whereas plants store the reduced
carbon as starch, fungi, like animals and some bacteria, use glycogen [74] for storage
of carbohydrates. A major component of the cell wall in many fungal species is the
nitrogen-containing carbohydrate, chitin,[75] also present in some animals, such as the
insects and crustaceans, while the plant cell wall consists chiefly of the carbohydrate
cellulose. The defining and unique characteristics of fungal cells include growth as
hyphae, which are microscopic filaments of between 2-10 microns in diameter and up
to several centimetres in length, and which combined form the fungal mycelium.
Some fungi, such as yeasts, grow as single ovoid cells, similar to unicellular algae and
the protists.
Unlike many plants, most fungi lack an efficient vascular system, such as xylem or
phloem for long-distance transport of water and nutrients; as an example for
convergent evolution, some fungi, such as Armillaria, form rhizomorphs or mycelial
cords,[76] resembling and functionally related to, but morphologically distinct from,
plant roots.
Some characteristics shared between plants and fungi include the presence of
vacuoles in the cell,[77] and a similar pathway in the biosynthesis of terpenes using
mevalonic acid and pyrophosphate as biochemical precursors; plants however use an
additional terpene biosynthesis pathway in the chloroplasts that is apparently absent in
fungi.[78] Ancestral traits shared among members of the fungi include chitinous cell
walls and heterotrophy by absorption.[67] A further characteristic of the fungi that is
absent from other eukaryotes, and shared only with some bacteria, is the biosynthesis
of the amino acid, L-lysine, via the α-aminoadipate pathway. [79]
Similar to plants, fungi produce a plethora of secondary metabolites functioning as
defensive compounds or for niche adaptation; however, biochemical pathways for the
synthesis of similar or even identical compounds often differ markedly between fungi
and plants. [80][81]
Evolutionary history
Even though traditionally included in many botany curricula and textbooks, fungi are
now thought to be more closely related to animals than to plants, and are placed with
the animals in the monophyletic group of opisthokonts. [67]For much of the Paleozoic
Era, the fungi appear to have been aquatic, and consisted of organisms similar to the
extant Chytrids in having flagellum-bearing spores.[82] The first land fungi probably
appeared in the Silurian, right after the first land plants appeared, even though their
fossils are fragmentary. For some time after the Permian-Triassic extinction event, a
fungal spike, detected as an extraordinary abundance of fungal spores in sediments
formed shortly after this event, indicates that they were the dominant life form during
this period—nearly 100% of the fossil record available from this period.[83]
Analyses using molecular phylogenetics support a monophyletic origin of the
Fungi.[8] The taxonomy of the Fungi is in a state of constant flux, especially due to
recent research based on DNA comparisons. These current phylogenetic analyses
often overturn classifications based on older and sometimes less discriminative
methods based on morphological features and biological species concepts obtained
from experimental matings.[84][85]
There is no unique generally accepted system at the higher taxonomic levels and there
are constant name changes at every level, from species upwards. However, efforts
among fungal researchers are now underway to establish and encourage usage of a
unified and more consistent nomenclature.[8] Fungal species can also have multiple
scientific names depending on its life cycle and mode (sexual or asexual) of
reproduction. Web sites such as Index Fungorum and ITIS define preferred up-to-date
names (with cross-references to older synonyms), but do not always agree with each
other.
Cladogram
Unikonta
Amoebozoa
Opisthokonta
Animalia
Choanozoa
Fungi
Chytridiomycota
Blastocladiomycota
Neocallimastigomycota
Zygomycota
Glomeromycota
Dikarya
Ascomycota
Basidiomycota
The taxonomic groups of fungi
The major divisions (phyla) of fungi have been classified based mainly on their sexual
reproductive structures. Currently, seven fungal divisions are proposed:[8]
Arbuscular mycorrhiza seen under microscope. Flax root cortical cells containing
paired arbuscules.
Conidiophores of molds of the genus Aspergillus, an ascomycete, seen under
microscope.
•
•
•
•
•
The Chytridiomycota are commonly known as chytrids. These fungi are
ubiquitous with a worldwide distribution; chytrids produce zoospores that are
capable of active movement through aqueous phases with a single flagellum.
Consequently, some taxonomists had earlier classified them as protists on the
basis of the flagellum. Molecular phylogenies, inferred from the rRNA-operon
sequences representing the 18S, 28S, and 5.8S ribosomal subunits, suggest
that the Chytrids are a basal fungal group divergent from the other fungal
divisions, consisting of four major clades with some evidence for paraphyly or
possibly polyphyly. [82]
The Blastocladiomycota were previously considered a taxonomic clade within
the Chytridiomycota. Recent molecular data and ultrastructural characteristics,
however, place the Blastocladiomycota as a sister clade to the Zygomycota,
Glomeromycota, and Dikarya (Ascomycota and Basiomycota). The
blastocladiomycetes are fungi that are saprotrophs and parasites of all
eukaryotic groups and undergo sporic meiosis unlike their close relatives, the
chytrids, which mostly exhibit zygotic meiosis. [82]
The Neocallimastigomycota were earlier placed in the phylum
Chytridomycota. Members of this small phylum are anaerobic organisms,
living in the digestive system of larger herbivorous mammals and possibly in
other terrestrial and aquatic environments. They lack mitochondria but contain
hydrogenosomes of mitochondrial origin. As the related chrytrids,
neocallimastigomycetes form zoospores that are posteriorly uniflagellate or
polyflagellate.[8]
The Zygomycota contain the taxa, Zygomycetes and Trichomycetes, and
reproduce sexually with meiospores called zygospores and asexually with
sporangiospores. Black bread mold (Rhizopus stolonifer) is a common species
that belongs to this group; another is Pilobolus, which is capable of ejecting
spores several meters through the air. Medically relevant genera include
Mucor, Rhizomucor, and Rhizopus. Molecular phylogenetic investigation has
shown the Zygomycota to be a polyphyletic phylum with evidence of
paraphyly within this taxonomic group. [86]
Members of the Glomeromycota are fungi forming arbuscular mycorrhizae
with higher plants. Only one species has been observed forming zygospores;
all other species solely reproduce asexually. The symbiotic association
between the Glomeromycota and plants is ancient, with evidence dating to 400
million years ago.[41]
Diagram of an apothecium (the typical cup-like reproductive structure of
Ascomycetes) showing sterile tissues as well as developing and mature asci.
•
•
The Ascomycota, commonly known as sac fungi or ascomycetes, constitute
the largest taxonomic group within the Eumycota. These fungi form meiotic
spores called ascospores, which are enclosed in a special sac-like structure
called an ascus. This division includes morels, a few mushrooms and truffles,
single-celled yeasts (e.g., of the genera Saccharomyces, Kluyveromyces,
Pichia, and Candida), and many filamentous fungi living as saprotrophs,
parasites, and mutualistic symbionts. Prominent and important genera of
filamentous ascomycetes include Aspergillus, Penicillium, Fusarium, and
Claviceps. Many ascomycetes species have only been observed undergoing
asexual reproduction (called anamorphic species), but molecular data has often
been able to identify their closest teleomorphs in the Ascomycota. Because the
products of meiosis are retained within the sac-like ascus, several ascomyctes
have been used for elucidating principles of genetics and heredity (e.g.
Neurospora crassa).
Members of the Basidiomycota, commonly known as the club fungi or
basidiomycetes, produce meiospores called basidiospores on club-like stalks
called basidia. Most common mushrooms belong to this group, as well as rust
(fungus) and smut fungi, which are major pathogens of grains. Other
important Basidiomyces include the maize pathogen,Ustilago maydis, human
commensal species of the genus Malassezia, and the opportunistic human
pathogen, Cryptococcus neoformans.
Phylogenetic relationships with other fungus-like organisms
Because of some similarities in morphology and lifestyle, the slime molds
(myxomycetes) and water molds (oomycetes) were formerly classified in the kingdom
Fungi. Unlike true fungi, however, the cell walls of these organisms contain cellulose
and lack chitin. Slime molds are unikonts like fungi, but are grouped in the
Amoebozoa. Water molds are diploid bikonts, grouped in the Chromalveolate
kingdom. Neither water molds nor slime molds are closely related to the true fungi,
and, therefore, taxonomists no longer group them in the kingdom Fungi. Nonetheless,
studies of the oomycetes and myxomycetes are still often included in mycology
textbooks and primary research literature.
It has been suggested that the nucleariids, currently grouped in the Choanozoa, may
be a sister group to the oomycete clade, and as such could be included in an expanded
fungal kingdom.[87]
See also
•
•
•
•
•
•
•
•
•
•
Bioaerosol
Carnivorous fungus
Fusicoccin
List of fungal orders
MycoBank
Mycotoxin
Plant pathology
Wood-decay fungus
Quorn
Pathogenic fungi
Notes and references
1. ^ (1980) "Taxonomic proposals for the classification of marine yeasts and other
yeast-like fungi including the smuts". Bot. Mar. 23: 371.
2. ^ These are the pronunciations listed first in most dictionaries. See, for example, the
Merriam-Webster Online entry Alternative pronunciations for fungi include
/ f ŋga /, / f nd i/, and / f ŋgi/. Funguses (/ f ŋgәsәz/) is an alternative
plural form.
3. ^ Simpson, D.P. (1979). Cassell's Latin Dictionary, 5, London: Cassell Ltd., 883.
ISBN 0-304-52257-0.
4. ^ Hawksworth DL (2006). "The fungal dimension of biodiversity: magnitude,
significance, and conservation". Mycol. Res. 95: 641–655.
5. ^ Mueller GM, Schmit JP (2006). "Fungal biodiversity: what do we know? What can
we predict?". Biodivers Conserv 16: 1–5.
6. ^ a b c d Alexopoulos CJ, Mims CW, Blackwell M (1996). Introductory Mycology.
John Wiley and Sons. ISBN 0471522295.
7. ^ Meredith Blackwell; Rytas Vilgalys, and John W. Taylor (2005-02-14). Eumycota:
mushrooms, sac fungi, yeast, molds, rusts, smuts, etc. (English). Retrieved on 200704-06.
8. ^ a b c d e Hibbett, D.S., et al. (2007). "A higher level phylogenetic classification of the
Fungi". Mycol. Res. 111 (5): 509-547. doi:doi:10.1016/j.mycres.2007.03.004.
9. ^ Strains of wine yeast
10. ^ Demain AL. (1991). "Production of beta-lactam antibiotics and its regulation.".
Proc Natl Sci Counc Repub China B. 15: 251-265. PMID 1815263.
11. ^ Kulp, Karel (2000). Handbook of Cereal Science and Technology. CRC Press.
ISBN 0824782941.
12. ^ Piskur J, Rozpedowska E, Polakova S, Merico A, Compagno C. (2006). "How did
Saccharomyces evolve to become a good brewer?". Trends Genet. 22: 183-186.
PMID 16499989.
13. ^ Kitamoto N, Yoshino S, Ohmiya K, Tsukagoshi N. (1999). "Sequence analysis,
overexpression, and antisense inhibition of a beta-xylosidase gene, xylA, from
Aspergillus oryzae KBN616.". Appl. Env. Microbiol. 65: 20-24. PMID 9872754.
14. ^ Trichoderma spp., including T. harzianum, T. viride, T. koningii, T. hamatum and
other spp. Deuteromycetes, Moniliales (asexual classification system). Biological
Control: A Guide to Natural Enemies in North America. Retrieved on 2007-07-10.
15. ^ van Egmond HP, Schothorst RC, Jonker MA (2007). "Regulations relating to
mycotoxins in food: perspectives in a global and European context". Anal Bioanal
Chem. 389: 147-157. PMID 17508207.
16. ^ Keller NP, Turner G, Bennett JW (2005). "Fungal secondary metabolism - from
biochemistry to genomics". Nat Rev Microbiol. 3: 937-497. PMID 16322742.
17. ^ Demain AL, Fang A (2000). "The natural functions of secondary metabolites". Adv
Biochem Eng Biotechnol. 69: 1-39. PMID 11036689.
18. ^ Rohlfs M, Albert M, Keller NP, Kempken F (2007). "Secondary chemicals protect
mould from fungivory". Biol Lett. 3: 523-525. PMID 17686752.
19. ^ Questions & Answers - Mold on Cheese whatscookingamerica.net. Retrieved 200704-06.
20. ^ Erdogan A, Gurses M, Sert S. (2004). "Isolation of moulds capable of producing
mycotoxins from blue mouldy Tulum cheeses produced in Turkey.". Int J Food
Microbiol. 85: 83-85. PMID 12810273.
21. ^ On the Trail of the Death Cap Mushroom Richard Harris, www.npr.org, 2007-0208. Retrieved 2007-04-06.
22. ^ Mythology and Folklore of Fly Agaric Paul Kendall, Trees for Life. Retrieved
2007-04-06.
23. ^ López-Gómez J, Molina-Meyer M (2006). "The competitive exclusion principle
versus biodiversity through competitive segregation and further adaptation to spatial
heterogeneities". Theor Popul Biol. 69: 94-109. PMID 16223517.
24. ^ Setting the Stage To Screen Biocontrol Fungi Hank Becker, July 1998. Retrieved
2007-04-06.
25. ^ WHEY-BASED FUNGAL MICROFACTORY TECHNOLOGY FOR
ENHANCED BIOLOGICAL PEST MANAGEMENT USING FUNGI Todd. S.
Keiller, Technology Transfer, University of Vermont. Retrieved 2007-04-06.
26. ^ Deshpande MV. (1999). "Mycopesticide production by fermentation: potential and
challenges.". Crit Rev Microbiol. 25: 229-243. PMID 10524330.
27. ^ Thomas MB, Read AF. (2007). "Can fungal biopesticides control malaria?". Nat
Rev Microbiol. 5: 377-383. PMID 17426726.
28. ^ Bush LP, Wilkinson HH, Schardl CL. (1997). "Bioprotective Alkaloids of GrassFungal Endophyte Symbioses". Plant Physiol. 114: 1-7. PMID 12223685.
29. ^ Bouton JH, Latch GCM, Hill NS, Hoveland CS, McCannc MA, Watson RH, Parish
JA, Hawkins LL, Thompson FN (2002). "Use of nonergot alkaloid-producing
endophytes for alleviating tall fescue toxicosis in sheep.". Agron. J. 94: 567-574.
http://agron.scijournals.org/cgi/content/full/94/3/567.
30. ^ a b Lindahl BD, Ihrmark K, Boberg J, Trumbore SE, Högberg P, Stenlid J, Finlay
RD (2007). "Spatial separation of litter decomposition and mycorrhizal nitrogen
uptake in a boreal forest". New Phytol. 173: 611-620. PMID 17244056.
31. ^ Barea JM, Pozo MJ, Azcón R, Azcón-Aguilar C (2005). "Microbial co-operation in
the rhizosphere". J. Exp. Bot. 56: 1761-1778. PMID 15911555.
32. ^ Aanen DK. (2006). "As you reap, so shall you sow: coupling of harvesting and
inoculating stabilizes the mutualism between termites and fungi.". Biol Lett. 2: 209212. PMID 17148364.
33. ^ Nikoh N, Fukatsu T. (2000). "Interkingdom host jumping underground:
phylogenetic analysis of entomoparasitic fungi of the genus Cordyceps.". Mol Biol
Evol. 17: 2629-2638. PMID 10742053.
34. ^ Perotto S, Bonfante P. (1997). "Bacterial associations with mycorrhizal fungi: close
and distant friends in the rhizosphere.". Trends Microbiol. 5: 496-501. PMID
9447662.
35. ^ Arnold AE, Mejía LC, Kyllo D, Rojas EI, Maynard Z, Robbins N, Herre EA.
(2003). "Fungal endophytes limit pathogen damage in a tropical tree.". Proc. Natl.
Acad. Sci. USA 100: 15649-15654. PMID 14671327.
36. ^ a b Paszkowski U. (2006). "Mutualism and parasitism: the yin and yang of plant
symbioses.". Curr Opin Plant Biol. 9: 364-370. PMID 16713732.
37. ^ a b Hube B. (2004). "From commensal to pathogen: stage- and tissue-specific gene
expression of Candida albicans.". Curr Opin Microbiol. 7: 336-341. PMID
15288621.
38. ^ Volk, Tom. Tom Volk's Fungi FAQ. Retrieved on 2006-09-21.
39. ^ Wong, George. Symbiosis: Mycorrhizae and Lichens. Retrieved on 2006-09-21.
40. ^ Knowledge of nitrogen transfer between plants and beneficial fungi expands
southwestfarmpress.com. 2005-06-10 Retrieved 2007-04-06.
41. ^ a b Remy W, Taylor TN, Hass H, Kerp H (1994). "4-hundred million year old
vesicular-arbuscular mycorrhizae.". Proc. Natl. Acad. Sci 91: 11841-11843. PMID
11607500.
42. ^ Selosse MA, Richard F, He X, Simard SW (2006). "Mycorrhizal networks: des
liaisons dangereuses?". Trends Ecol Evol. 21: 621-628. PMID 16843567.
43. ^ Brodo, Irwin M.; Sylvia Duran Sharnoff (2001). Lichens of North America. Yale
University Press. ISBN 0300082495.
44. ^ Fungi and Insect Symbiosis www.botany.hawaii.edu. Retrieved 2007-04-06.
45. ^ Pascal Jouquet, Virginie Tavernier, Luc Abbadie and Michel Lepage. Nests of
subterranean fungus-growing termites (Isoptera, Macrotermitinae) as nutrient
patches for grasses in savannah ecosystems. African Journal of Ecology. 2005. Vol
43, 191–196
46. ^ Talbot NJ (2003). "On the trail of a cereal killer: Exploring the biology of
Magnaporthe grisea.". Annu Rev Microbiol. 57: 177-202. PMID 14527276.
47. ^ Paoletti M, Buck KW, Brasier CM. (2006). "Selective acquisition of novel mating
type and vegetative incompatibility genes via interspecies gene transfer in the
globally invading eukaryote Ophiostoma novo-ulmi.". Mol Ecol. 15: 249-262. PMID
16367844.
48. ^ Gryzenhout M, Wingfield BD, Wingfield MJ. (2006). "New taxonomic concepts
for the important forest pathogen Cryphonectria parasitica and related fungi.". FEMS
Microbiol Lett. 258: 161-172. PMID 16640568.
49. ^ Nielsen K, Heitman J. (2007). "Sex and virulence of human pathogenic fungi.". Adv
Genet. 57: 143-173. PMID 17352904.
50. ^ Brakhage AA (2005). "Systemic fungal infections caused by Aspergillus species:
epidemiology, infection process and virulence determinants.". Curr. Drug Targets 6:
875-886. PMID 16375671.
51. ^ Kauffman CA. (2007). "Histoplasmosis: a clinical and laboratory update". Clin
Microbiol Rev. 20: 115-132. PMID 17223625.
52. ^ Cushion MT, Smulian AG, Slaven BE, Sesterhenn T, Arnold J, Staben C, Porollo
A, Adamczak R, Meller J. (2007). "Transcriptome of Pneumocystis carinii during
Fulminate Infection: Carbohydrate Metabolism and the Concept of a Compatible
Parasite.". PLoS ONE 2: e423. PMID 17487271.
53. ^ ILLUSTRATIONS for Predatory Fungi, wood Decay and the Carbon Cycle
www.uoguelph.ca. Retrieved 2007-04-06.
54. ^ Pereira JL, Noronha EF, Miller RN, Franco OL. (2007). "Novel insights in the use
of hydrolytic enzymes secreted by fungi with biotechnological potential.". Lett Appl
Microbiol. 44: 573-581. PMID 17576216.
55. ^ Schaller M, Borelli C, Korting HC, Hube B. (2007). "Hydrolytic enzymes as
virulence factors of Candida albicans.". Mycoses 48: 365-377. PMID 16262871.
56. ^ Farrar JF (1985). "Carbohydrate metabolism in biotrophic plant pathogens.".
Microbiol Sci. 2: 314-317. PMID 3939987.
57. ^ Marzluf GA (1981). "Regulation of nitrogen metabolism and gene expression in
fungi". Microbiol Rev. 45: 437-461. PMID 6117784.
58. ^ Heynes MJ (1994). "Regulatory circuits of the amdS gene of Aspergillus nidulans".
Antonie Van Leeuwenhoek. 65: 179-782. PMID 7847883.
59. ^ a b Dadachova E, Bryan RA, Huang X, Moadel T, Schweitzer AD, Aisen P,
Nosanchuk JD, Casadevall A. (2007). "Ionizing radiation changes the electronic
properties of melanin and enhances the growth of melanized fungi". PLoS ONE 2:
e457. PMID 17520016.
60. ^ The Protistan Origins of Animals and Fungi Emma T. Steenkamp, Jane Wright and
Sandra L. Baldauf. Molecular Biology and Evolution 2006 23(1):93-106;
doi:10.1093/molbev/msj011. Retrieved 2007-04-06.
61. ^ Stevens DA, Ichinomiya M, Koshi Y, Horiuchi H. (2006). "Escape of Candida
from caspofungin inhibition at concentrations above the MIC (paradoxical effect)
accomplished by increased cell wall chitin; evidence for beta-1,6-glucan synthesis
inhibition by caspofungin.". Antimicrob Agents Chemother. 50: 3160-3161.. PMID
16940118.
62. ^ Chang, Shu-ting; Philip G. Miles (2004). Mushrooms: Cultivation, Nutritional
Value, Medicinal Effect and Environmental Impact. CRC Press. ISBN 0849310431.
63. ^ “Fungal Biology” at The University of Sydney Retrieved on 26 June 2007
64. ^ Howard RJ, Ferrari MA, Roach DH, Money NP (1991). "Penetration of hard
substrates by a fungus employing enormous turgor pressures". Proc Natl Acad Sci U
S A. 88: 11281-11284. PMID 1837147.
65. ^ Wang ZY, Jenkinson JM, Holcombe LJ, Soanes DM, Veneault-Fourrey C,
Bhambra GK, Talbot NJ (2005). "The molecular biology of appressorium turgor
generation by the rice blast fungus Magnaporthe grisea". Biochem Soc Trans. 33:
384-388. PMID 15787612.
66. ^ Metzenberg RL, Glass NL. (1990). "Mating type and mating strategies in
Neurospora.". Bioessays 12: 53-59. PMID 2140508.
67. ^ a b c d P. Sitte, H. Ziegler, F. Ehrendorfer (1991). Strasburger Lehrbuch der Botanik
(Textbook of Botany), 33 ed, Urban & Fischer. ISBN 3437204475.
68. ^ Reproduction of fungi MicrobiologyBytes, 2007-01-18. Retrieved 2007-04-06.
69. ^ Trail F. (2007). "Fungal cannons: explosive spore discharge in the Ascomycota".
FEMS Microbiol Lett. 276: 12-18. PMID 17784861.
70. ^ Pringle A, Patek SN, Fischer M, Stolze J, Money NP. (2005). "The captured launch
of a ballistospore". Mycologia 97: 866-871. PMID 16457355.
71. ^ Furlaneto MC, Pizzirani-Kleiner AA. (1992). "Intraspecific hybridisation of
Trichoderma pseudokoningii by anastomosis and by protoplast fusion.". FEMS
Microbiol Lett. 69: 191-195. PMID 1537549.
72. ^ Schardl CL, Craven KD. (2003). "Interspecific hybridization in plant-associated
fungi and oomycetes: a review.". Mol. Ecol. 12: 2861-2873. PMID 14629368.
73. ^ Bruns T. (2006). "Evolutionary biology: a kingdom revised.". Nature 443: 758-761.
PMID 17051197.
74. ^ Lomako J, Lomako WM, Whelan WJ. (2004). "Glycogenin: the primer for
mammalian and yeast glycogen synthesis". Biochim Biophys Acta. 1673: 45-55.
PMID 15238248.
75. ^ Bowman SM, Free SJ. (2006). "The structure and synthesis of the fungal cell wall".
Bioessays 28: 799-808. PMID 16927300.
76. ^ Mihail JD, Bruhn JN. (2005). "Foraging behaviour of Armillaria rhizomorph
systems". Mycol. Res. 109: 1195-1207. PMID 16279413.
77. ^ Shoji JY, Arioka M, Kitamoto K (2006). "Possible involvement of pleiomorphic
vacuolar networks in nutrient recycling in filamentous fungi". Autophagy. 2: 226-227.
PMID 16874107.
78. ^ Wu S, Schalk M, Clark A, Miles RB, Coates R, Chappell J. (2007). "Redirection of
cytosolic or plastidic isoprenoid precursors elevates terpene production in plants".
Nat Biotechnol. 24: 1441-7. PMID 17057703.
79. ^ Xu H, Andi B, Qian J, West AH, Cook PF (2006). "The alpha-aminoadipate
pathway for lysine biosynthesis in fungi". Cell Biochem Biophys. 46: 43-64. PMID
16943623.
80. ^ Tudzynski B. (2005). "Gibberellin biosynthesis in fungi: genes, enzymes,
evolution, and impact on biotechnology". Appl Microbiol Biotechnol. 66: 597-611.
PMID 15578178.
81. ^ Siewers V, Smedsgaard J, Tudzynski P. (2004). "The P450 monooxygenase
BcABA1 is essential for abscisic acid biosynthesis in Botrytis cinerea.". Appl
Environ. Microbiol. 70: 3868-3876. PMID 15240257.
82. ^ a b c James TY et al (2006). "Reconstructing the early evolution of Fungi using a
six-gene phylogeny.". Nature 443: 818-822. PMID 17051209.
83. ^ Eshet, Y. et al. (1995) Fungal event and palynological record of ecological crisis
and recovery across the Permian-Triassic boundary. Geology, 23, 967-970.
84. ^ See Palaeos: Fungi for an introduction to fungal taxonomy, including recent
controversies.
85. ^ “A Higher-Level Phylogenetic Classification of the Fungi” by David S. Hibbett,
(.pdf file) Retrieved on 8 March 2007
86. ^ White MM, James TY, O'Donnell K, Cafaro MJ, Tanabe Y, Sugiyama J. (2006).
"Phylogeny of the Zygomycota based on nuclear ribosomal sequence data.".
Mycologia 98: 872-884. PMID 17486964.
87. ^ Esser, Karl; Paul A. Lemke (1994). The Mycota: A Comprehensive Treatise on
Fungi as Experimental Systems for Basic and Applied Research. Springer. ISBN
3540580085.
Further reading
•
•
•
•
Alexopoulos, C.J., Charles W. Mims, M. Blackwell et al., Introductory
Mycology, 4th ed. (John Wiley and Sons, Hoboken NJ, 2004) ISBN 0-47152229-5
Arora, David. (1986). "Mushrooms Demystified: A Comprehensive Guide to
the Fleshy Fungi". 2nd ed. Ten Speed Press. ISBN 0898151694
Deacon JW. (2005). "Fungal Biology" (4th ed). Malden, MA: Blackwell
Publishers. ISBN 1-4051-3066-0.
Kaminstein D. (2002). Mushroom poisoning.
External links
Wikimedia Commons has media related to:
Fungi
Look up fungi in
Wiktionary, the free dictionary.
Wikispecies has information related to:
fungi
•
•
•
The WWW Virtual Library: Mycology
MykoWeb
Illinois Mycological Association Mycological Glossary
Tree of Life web project: Fungi
Fungal Biology, University of Sydney, School of Biological Sciences, June,
2004. – Online textbook
The Fifth Kingdom – Online textbook
CABI Bioscience Databases - Includes Index Fungorum genus and species
names and top-down hierarchy
Comparative Analysis of Fungal Genomes (at DOE's IMG system)
•
•
•
•
•
[hide]
v•d•e
Elements of nature
Earth
History of Earth · Earth science · Structure of the Earth · Plate tectonics ·
Geological history of Earth · Geology
Weather Climate · Earth's atmosphere
Life
Biosphere · Origin of life · Microbe · Plants · Fungus · Fauna · Animals ·
Biology · Evolutionary history of life
Environment Wilderness · Ecology · Ecosystem
Universe Matter · Energy · Outer space
Category · Portal
Retrieved from "http://en.wikipedia.org/wiki/Fungus"
Categories: Fungi
Hidden category: Semi-protected
Views
•
•
•
•
Article
Discussion
View source
History
Personal tools
•
Log in / create account
Navigation
•
•
•
•
•
Main Page
Contents
Featured content
Current events
Random article
Interaction
•
•
•
•
•
•
About Wikipedia
Community portal
Recent changes
Contact Wikipedia
Donate to Wikipedia
Help
Search
Toolbox
•
•
•
•
•
•
•
What links here
Related changes
Upload file
Special pages
Printable version
Permanent link
Cite this page
Languages
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
ﺍﻝﻉﺭﺏﻱﺓ
Asturianu
Bân-lâm-gú
Български
Català
Česky
Чăвашла
Cymraeg
Dansk
Deutsch
Ελληνικά
Eesti
Español
Esperanto
Français
Gaeilge
한국어
Hornjoserbsce
Hrvatski
Bahasa Indonesia
Íslenska
Italiano
עברית
Latina
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
Latviešu
Lëtzebuergesch
Lietuvių
Magyar
Македонски
Bahasa Melayu
Nahuatl
Nederlands
日本語
(Norsk (bokmål)(
(Norsk (nynorsk)(
Occitan
Plattdüütsch
Polski
Português
Română
Runa Simi
Русский
Sicilianu
Simple English
Slovenčina
Slovenščina
Српски / Srpski
Suomi
Svenska
Türkçe
Українська
Tiếng Việt
Walon
ייִדיש
Žemaitėška
中文
Growth medium
http://en.wikipedia.org/wiki/Growth_me
dium
From Wikipedia, the free encyclopedia
Jump to: navigation, search
An Agar Plate -- an example of a bacterial growth medium. Specifically, it is a streak
plate; the orange lines and dots are formed by bacterial colonies.
A growth medium or culture medium is a liquid or gel designed to support the
growth of microorganisms or cells.[1] There are different types of media for growing
different types of cells.[2]
There are two major types of growth media: those used for cell culture, which use
specific cell types derived from plants or animals, and microbiological culture, which
are used for growing microorganisms, such as bacteria or yeast. The most common
growth media for microorganisms are nutrient broths and agar plates; specialized
media are sometimes required for microorganism and cell culture growth.[1] Some
organisms, termed fastidious organisms, require specialized environments due to
complex nutritional requirements. Viruses, for example, are obligatory intracellular
parasites and require a growth medium composed of living cells.
Contents
[hide]
•
•
•
•
•
•
1 Types of growth mediums
o 1.1 Nutrient media
o 1.2 Minimal media
o 1.3 Selective media
o 1.4 Differential media
2 Transport media
3 Enriched media
4 See also
5 References
6 External links
[edit] Types of growth mediums
This article needs additional citations for verification.
Please help improve this article by adding reliable references. Unsourced material may be
challenged and removed. (August 2007)
The most common growth mediums for microorganisms are nutrient broths (liquid
nutrient medium) or Luria Bertani medium (LB medium or Lysogeny Broth). Liquid
mediums are often mixed with agar and poured into petri dishes to solidify. These
agar plates provide a solid medium on which microbes may be cultured. Bacteria
grown in liquid cultures often form colloidal suspensions.
The differences between growth mediums used for cell culture and those used for
microbiological culture are due to the fact that cells derived from whole organisms
and grown in culture often cannot grow without the addition of, for instance,
hormones or growth factors which usually occur in vivo.[3] In the case of animal cells,
this difficulty is often addressed by the addition of blood serum to the medium. In the
case of microorganisms, there are no such limitations, as they are often unicellular
organisms. One other major difference is that animal cells in culture are often grown
on a flat surface to which they attach, and the medium is provided in a liquid form,
which covers the cells. In contrast, bacteria such as Escherichia coli may be grown on
solid media or in liquid media.
An important distinction between growth media types is that of defined versus
undefined media.[1] A defined medium will have known quantities of all ingredients.
For microorganisms, they consist of providing trace elements and vitamins required
by the microbe and especially a defined carbon source and nitrogen source. Glucose
or glycerol are often used as carbon sources, and ammonium salts or nitrates as
inorganic nitrogen sources). An undefined medium has some complex ingredients,
such as yeast extract or casein hydrolysate, which consist of a mixture of many, many
chemical species in unknown proportions. Undefined media are sometimes chosen
based on price and sometimes by necessity - some microorganisms have never been
cultured on defined media.
A good example of a growth medium is the wort used to make beer. The wort
contains all the nutrients required for yeast growth, and under anaerobic conditions,
alcohol is produced. When the fermentation process is complete, the combination of
medium and dormant microbes, now beer, is ready for consumption.
[edit] Nutrient media
Undefined media (also known as basal or complex media) is an undefined media that
contains:
•
•
•
•
a carbon source such as glucose for bacterial growth
water
various salts need for bacterial growth
a source of amino acids and nitrogen (e.g., beef, yeast extract)
This is an undefined medium because the amino acid source contains a variety of
compounds with the exact composition unknown. Nutrient media contain all the
elements that most bacteria need for growth and are non-selective, so they are used
for the general cultivation and maintenance of bacteria kept in laboratory culture
collections.
Defined media (also known as chemical defined media)
•
•
all the chemicals used are known and
does not contain any animal, yeast, plant tissue.
Differential medium
•
some sort of indicator, typically a dye, is added, that allows for the
differentiation of particular chemical reactions occurring during growth.
[edit] Minimal media
Minimal media are those that contain the minimum nutrients possible for colony
growth, generally without the presence of amino acids, and are often used by
microbiologists and geneticists to grow "wild type" microorganisms. Minimal media
can also be used to select for or against recombinants or exconjugants.
Minimal medium typically contains:
•
•
•
a carbon source for bacterial growth, which may be a sugar such as glucose, or
a less energy-rich source like succinate
various salts, which may vary among bacteria species and growing conditions;
these generally provide essential elements such as magnesium, nitrogen,
phosphorus, and sulfur to allow the bacteria to synthesize protein and nucleic
acid
water
Supplementary minimal media are a type of minimal media that also contains a single
selected agent, usually an amino acid or a sugar. This supplementation allows for the
culturing of specific lines of auxotrophic recombinants.
[edit] Selective media
Blood-free, charcoal-based selective medium agar (CSM) for isolation of
Campylobacter.
Blood agar plates are often used to diagnose infection. On the right is a positive
Streptococcus culture; on the left a positive Staphylococcus culture.
Selective mediums are used for the growth of only select microorganisms. For
example, if a microorganism is resistant to a certain antibiotic, such as ampicillin or
tetracycline, then that antibiotic can be added to the medium in order to prevent other
cells, which do not possess the resistance, from growing. Media lacking an amino acid
such as proline in conjunction with E. coli unable to synthesize it were commonly
used by geneticists before the emergence of genomics to map bacterial chromosomes.
Selective growth media are also used in cell culture to ensure the survival or
proliferation of cells with certain properties, such as antibiotic resistance or the ability
to synthesize a certain metabolite. Normally, the presence of a specific gene or an
allele of a gene confers upon the cell the ability to grow in the selective medium. In
such cases, the gene is termed a marker.
Selective growth media for eukaryotic cells commonly contain neomycin to select
cells that have been successfully transfected with a plasmid carrying the neomycin
resistance gene as a marker. Gancyclovir is an exception to the rule as it is used to
specifically kill cells that carry its respective marker, the Herpes simplex virus
thymidine kinase (HSV TK).
Four types of agar plates demonstrating differential growth depending on bacterial
metabolism.
Some examples of selective media include:
•
•
•
•
•
•
•
•
eosin-methylen blue agar (EMB) that contains methylene blue – toxic to
Gram-positive bacteria, allowing only the growth of Gram negative bacteria
YM (yeast and mold) which has a low pH, deterring bacterial growth
blood agar (used in strep tests), which contains beef heart blood that becomes
transparent in the presence of hemolytic Streptococcus
MacConkey agar for Gram-negative bacteria
Hektoen Enteric (HE) which is selective for Gram-negative bacteria
Mannitol Salt Agar (MSA) which is selective for Gram-positive bacteria and
differential for mannitol
xylose lysine desoxyscholate (XLD), which is selective for Gram-negative
bacteria
Buffered charcoal yeast extract agar, which is selective for certain gramnegative bacteria, especially Legionella pneumophila
[edit] Differential media
Differential media or indicator media distinguish one microorganism type from
another growing on the same media.[4] This type of media uses the biochemical
characteristics of a microorganism growing in the presence of specific nutrients or
indicators (such as neutral red, phenol red, eosin y, or methylene blue) added to the
medium to visibly indicate the defining characteristics of a microorganism. This type
of media is used for the detection of microorganisms and by molecular biologists to
detect recombinant strains of bacteria.
Examples of differential media include:
•
•
•
•
Eosin methylene blue (EMB), which is differential for lactose and sucrose
fermentation
MacConkey (MCK), which is differential for lactose fermentation
Mannitol Salt Agar (MSA), which is differential for mannitol fermentation
X-gal plates, which are differential for lac operon mutants
[edit] Transport media
These are used for the temporary storage of specimens being transported to the
laboratory for cultivation. Such media ideally maintain the viability of all organisms
in the specimen without altering their concentration. Transport media typically
contain only buffers and salt. The lack of carbon, nitrogen, and organic growth factors
prevents microbial multiplication. Transport media used in the isolation of anaerobes
must be free of molecular oxygen.
[edit] Enriched media
Enriched media contain the nutrients required to support the growth of a wide variety
of organisms, including some of the more fastidious ones. They are commonly used to
harvest as many different types of microbes as are present in the specimen. Blood
agar is an enriched medium in which nutritionally rich whole blood supplements the
basic nutrients. Chocolate agar is enriched with heat-treated blood (40-45°C), which
turns brown and gives the medium the color for which it is named.
[edit] See also
•
•
•
R2a agar
MRS agar
Cell biology
[edit] References
1. ^ a b c Madigan M, Martinko J (editors). (2005). Brock Biology of Microorganisms,
11th ed., Prentice Hall. ISBN 0131443291.
2. ^ Ryan KJ, Ray CG (editors) (2004). Sherris Medical Microbiology, 4th ed.,
McGraw Hill. ISBN 0838585299.
3. ^ Cooper GM (2000). "Tools of Cell Biology", The cell: a molecular approach.
Washington, D.C: ASM Press. ISBN 0-87893-106-6.
4. ^ Washington JA (1996). "Principles of Diagnosis", Baron's Medical Microbiology
(Baron S et al, eds.), 4th ed., Univ of Texas Medical Branch. ISBN 0-9631172-1-1.
INTESTINAL PROTOZOA
http://www.tulane.edu/~wiser/protozoology/notes/inte
s.html
Lumen-Dwelling Protozoa
Flagellates:
Giardia lamblia
Dientamoeba fragilis
Chilomastix mesnili
Enteromonas hominis
Retortamonas intestinalis
Trichomonas hominis
Trichomonas tenax (oral)
Trichomonas vaginalis
(urogenital)
Ameba:
Entamoeba histolytica
Entamoeba dispar
Entamoeba coli
Entamoeba hartmanni
Entamoeba polecki
Entamoeba gingivalis (oral)
Endolimax nana
Iodamoeba bütschlii
Numerous protozoa inhabit the gastrointestinal tract of humans (see Box).
Cryptosporidium parvum
This list includes representatives from
Cryptosporidium hominis
many diverse protozoan groups. The
Cyclospora cayetanensis
majority of these protozoa are nonIsospora belli
pathogenic commensals, or only result
Microsporidia:
in mild disease. Some of these
Enterocytozoon bieneusi
Encephalitozoon intestinalis organisms can cause severe disease
under certain circumstances. For
Other:
example, Giardia lamblia can cause
Blastocystis hominis
Balantidium coli
severe acute diarrhea which may lead
to a chronic diarrhea and nutritional
disorders; Entamoeba histolytica can become a highly virulent and
invasive organism that causes a potentially lethal systemic disease.
Apicomplexa and microsporidia species (discussed elsewhere),
which normally do not evoke severe disease, can cause severe and
life-threatening diarrhea in AIDS patients and other
immunocompromised individuals. Trichomonas vaginalis does not
reside within the gastro-intestinal tract, but is often discussed with
the intestinal flagellates. It infects the urogenital tract and and
causes a sexually-transmitted disease.
Apicomplexa:
Intestinal protozoa are transmitted by the fecal-oral route and tend
to exhibit similar life cycles consisting of a cyst stage and a
trophozoite stage (Figure). Fecal-oral transmission involves the
ingestion of food or water contaminated with cysts. After ingestion
by an appropriate host, the cysts transform into trophozoites which
exhibit an active metabolism and are usually motile. The parasite
takes up nutrients and undergoes asexual replication during the
trophic phase. Some of the trophozoites will develop into cysts
instead of undergoing replication. Cysts are characterized by a
resistant wall and are excreted with the feces. The cyst wall
functions to protect the organism from desiccation in the external
environment as the parasite undergoes a relatively dormant period
waiting to be ingested by the next host. Factors which increase the
likelyhood of ingesting material contaminated with fecal material
play a role in the transmission of this intestinal protozoa (see Box).
In general, situations involving close human-human contact and
unhygenic conditions promote
transmission.
TOPICS:
•
•
•
•
•
Giardiasis
o Life Cycle and
Morphology
Trophozoite
Cyst
o The Adhesive Disk
o Symptoms and
Pathogenesis
o Diagnosis
o Treatment and Control
Trichomoniasis
Balantidosis
Amebiasis
Non-Pathogenic Commensals
GIARDIASIS
Fecal-Oral Transmission Factors
poor personal hygiene
•
•
•
children (eg, day-care
centers)
institutions (eg, prisons,
mental hospitals, orphanages)
food handlers
developing countries
•
•
•
•
poor sanitation
lack of indoor plumbing
endemic
travelers' diarrhea
water-borne epidemics
•
water treatment failures
Giardia lamblia (also known as G.
male homosexuality
duodenalis, see comments on
taxonomy) is a protozoan parasite
• oral-anal contact
that colonizes the upper portions of
the small intestine. It has a
zoonosis?
worldwide distribution and is the
most common protozoan isolated
• Entamoeba = no
from human stools. The incidence is
• Cryptosporidium = yes
• Giardia = controversial
estimated at 200 million clinical
cases per year. In fact, it was
probably the first symbiotic
protozoan ever observed. It is quite likely that Van Leeuwenhoek,
the inventor of the microscope, first described Giardia in 1681in his
own stools based upon his description of its characteristic
movement. However, van Leeuwenhoek never submitted drawings
of the organisms and Lambl is usually given credit for the
identification of Giardia in the stools of pediatric patients in Praque
in 1859.
Typically Giardia is non-invasive and often results in asymptomatic
infections. Symptomatic giardiasis is characterized by acute or
chronic diarrhea and/or other gastro-intestinal manifestations.
LIFE CYCLE AND MORPHOLOGY
Giardia exhibits a typical fecal-oral transmission
cycle (see above). The infection is acquired
through the ingestion of cysts. Factors leading to
contamination of food or water with fecal
material are correlated with transmission (Box).
For example, giardiasis is especially prevalent in
children and particularly those children in
institutions or day-care centers. In developing
countries, poor sanitation contributes to the
higher levels of giardiasis, and water-borne
outbreaks due to inadequate water treatment
have also been documented. Backpackers in areas of no human
habitation are believed to acquire from drinking from streams and
some data suggest that beavers are the reservoir. However, the
zoonotic transmission of Giardia is controversial and has not been
unambiguously demonstrated. It is not clear whether Giardia
lamblia represents a single species capable of infecting a wide range
of animals, or whether each host has their own 'pet' Giardia.
Evidence indicating that Giardia transmission between dogs and
humans is quite rare favors the latter. Molecular evidence suggests
that some isolates exhibit narrow host ranges whereas others
exhibit wide host ranges (see notes on taxonomy). Regardless of
whether zoonotic transmission is possible, person-to-person
transmission is the most prevalent mode of transmission and the
risk factors are close human contact combined with unhygienic
conditions.
The ingested cyst passes through the stomach and excystation
takes place in the duodenum. Excystation can be induced in vitro by
a brief exposure of the cysts to acidic pH (~2) or other sources of
hydrogen ions. This exposure to the acidic pH mimics the conditions
of the stomach and probably functions as an environmental cue for
the parasite. Flagellar activity begins within 5-10 minutes following
the acid treatment and the trophozoite emerges through a break in
the cyst wall. The breakdown of the cyst wall is believed to be
mediated by proteases. The trophozoite will undergo cytokinesis
(cell division without nuclear replication) within 30 minutes after
emerging from the cyst resulting in two binucleated trophozoites.
The Giardia trophozoite exhibits a characteristic pear, or tear-drop,
shape with bilateral symmetry when viewed from the top (Figure).
It is typically 12-15 µm long, 5-10 µm wide, and 2-4 µm thick.
Characteristic features of the stained trophozoite include: two nuclei
(Nu) with central karyosomes (k), fibrils running the length of the
parasite, and median bodies (MB). The large karyosome and lack of
peripheral chromatin gives the nuclei a halo
appearance. The fibrils are called axonemes (Ax) and
are formed from the proximal regions of the flagella
(Fg) within the body of the trophozoite. The median
bodies are a pair of curved rod-shaped structures
which lie posterior to the nuclei. At the ultrastructural
level the median bodies contain an array of
microtubules. The function of the median bodies is not
known, but most believe they are somehow involved
with the adhesive disk and its formation. An adhesive disk (AD), not
always visible by light microscopy, occupies the ventral side of the
anterior end.
Giardia trophozoites possess four pairs of flagella and are motile.
Three pairs of flagella emerge from the dorsal surface (anterior,
posterior-lateral, caudal) and one pair emerges from the ventral
surface. Trophozoites exhibit a distinctive erratic twisting motion,
sometimes compared to that of a falling leaf. However, the
trophozoites are predominantly found attached to epithelial cells of
the small intestine (especially the duodenum and jejunum) and are
rarely found in stools, except in the cases of severe diarrhea. This
attachment to the intestinal epithelium is mediated by an organelle
on the ventral side of the parasite referred to as the adhesive disk
(see below). The trophozoite absorbs nutrients from the intestinal
lumen via pinocytosis and no specialized feeding organelles have
been described.
The trophic stage is also characterized by an asexual replication.
Both nuclei divide at about the same time and cytokinesis restores
the binucleated state. Each daughter cell receives one copy of each
nuclei. Both nuclei appear equal in regards to gene expression and
other properties.
As an alternative to replication the trophozoite can encyst. During
encystment the parasite rounds up, detaches from the intestinal
epithelium, and secretes a cyst wall. Encystation can also be carried
out in vitro. Optimal induction of encystment is obtained by
depriving the trophozoites of bile at pH 7 followed by an exposure
to high concentrations of bile at pH 7.8. The lack of bile at neutral
pH mimics the conditions under the mucus blanket adjacent to the
intestinal epithelial cells, whereas exposure to high concentrations
of bile at more alkaline pH is analogous to the intestinal lumen.
These studies highlight the extent to which Giardia has adapted to
life within the gastrointestinal tract.
Molecular and ultrastructural studies reveal the synthesis of cyst
wall proteins and the appearance of large secretory vesicles in the
parasite cytoplasm follow the induction of encystment. After cyst
wall formation the parasite undergoes one round of nuclear division
without cytokinesis resulting in four nuclei. These four nuclei (Nu)
are usually located at the anterior end of the cyst (Figure). The
flagella and adhesive disk are lost as the cyst matures, but the
axonemes (Ax) and median bodies (MB) persist. The distinctive
fibrils (ie, axonemes), which extend across the length of the cyst,
result in Giardia being relatively easy to unambiguously identify.
The cysts are oval shaped and typical measure 11-14 µm in length
and 6-10 µm wide. Other characteristics of Giardia cysts include a
well-defined wall (CW) which is often set apart from the cytoplasm
of the parasite. The cysts are passed in the feces and can survive
for up to three months under appropriate temperature and moisture
conditions. Mature cysts are infective to the next host that happens
to ingest them, thus completing the life cycle.
THE ADHESIVE DISK
A unique ultrastructural feature of Giardia is the adhesive disk (also
called ventral disk, sucking disk, sucker, or striated disk). The
adhesive disk is a concave structure which occupies approximately
two-thirds of the anterior end of the ventral surface (Figure, left
panel). As the names imply, this structure plays a role in the
attachment of the trophozoite to the intestinal epithelium and
ultrastructural studies reveal close associations between the
adhesive disk and the intestinal brush border (Figure, upper right
panel). (Click here for larger image.)
The adhesive disk appears to be
a relatively rigid structure and
striations are evident by
transmission electron
microscopy. These striations are
the result of microtubules (mT)
and a unique cytoskeletal
element called microribbons
(mR). Microribbons are long
flattened structures and each
microribbon is associated with a
microtubule (Figure, middle
right panel). The combined microtubule-microribbon structure are
arranged in concentric rows that form a flatten spiral with minimal
overlap. The outer rim of the adhesive disk, called the lateral crest,
contains components of the actin-myosin cytoskeleton.
A major component of microribbons are proteins called giardins
(aka beta-giardins). These giardins play primarily a structural role in
the formation of the microribbons. Interestingly, the giardins show
a limited homology to a protein called 'striated fibre assemblin' from
Chlamydomonas (a free-living, bi-flagellated unicellular algae). In
Chlamydomonas this protein forms filamentous structures at the
base of the flagella. The giardins have evolved to play a different
functional role in Giardia, but are still associated with microtubule
based cytoskeletal elements.
This association of proteins involved in the generation of contractile
force and other cytoskeletal elements in the adhesive disk suggests
that attachment is mediated by mechanical forces generated by the
parasite. The observation that imprints and circular dome-shaped
lesions remain in the intestinal brush border (ie, microvilli) following
detachment of trophozoites (Figure, lower right panel) is consistent
with contractile forces playing a role in attachment. Other proposed
mechanisms for the attachment of Giardia to the intestinal
epithelium include hydrodynamic forces generated by the ventral
flagella and receptor-mediated binding via lectins on the trophozoite
surface. However, flagellar movement is poorly correlated with
attachment and the surface lectins cover the entire trophozoite and
are not specifically localized to the adhesive disk.
SYMPTOMS AND PATHOGENESIS
The clinical features associated with Giardia infection range from
total latency (ie, asymptomatic), to acute self-resolving diarrhea, to
chronic syndromes associated with nutritional disorders, weight loss
and failure to thrive. Children exhibit clinical symptoms more
frequently that adults and subsequent infections tend to be less
severe than initial infections. The incubation period is generally 1-2
weeks, but ranges of 1-75 days have been reported.
The first signs of acute giardiasis include nausea, loss of appetite
and an upper gastro-intestinal uneasiness. These signs are often
followed or accompanied by a sudden onset of explosive, watery,
foul-smelling diarrhea. Stools associated with Giardia infection are
generally described as loose, bulky, frothy and/or greasy with the
absence of blood or mucus, which may help distinguish giardiasis
from other acute diarrheas. Other gastro-intestinal disturbances
associated with giardiasis include: flatulence, bloating, anorexia,
cramps, and foul sulfuric belching (sometimes called 'purple burbs').
The acute stage usually resolves spontaneously in 3-4 days and is
often not recognized as being giardiasis. Occasionally, though, an
acute infection will persist and lead to malabsorption, steatorrhea
(excessive loss of fat in the feces), debility (loss of strength) and
weight loss. Some of the individuals who resolve the acute
symptoms do not clear the infection, but become asymptomatic cyst
passers without clinical manifestations, whereas others may have a
few sporadic recurrences of the acute symptoms.
Acute infections can also develop into long-standing subacute or
chronic infections which in rare cases last for years. The typical
chronic stage patient presents with recurrent brief episodes of loose
foul stools which may be yellowish, frothy and float, accompanied
by intestinal gurgling, abdominal distention and flatulence. Between
episodes the stools are usually mushy, but normal stools or
constipation can also occur. Cramps are uncommon during chronic
infections, but sulfuric belching is frequent. Anorexia, nausea, and
epigastric uneasiness are additional frequent complaints during
chronic infections. In the majority of chronic cases the parasites and
symptoms spontaneously
disappear.
The specific mechanisms of Giardia
pathogenesis leading to diarrhea
and intestinal malabsorption are not
completely understood and no
Click for larger image
specific virulence factors have been
identified. Attachment of trophozoites to the brush border could
produce a mechanical irritation or mucosal injury. In addition,
normal villus structure is affected in some patients. For example,
villus blunting (atrophy) and crypt cell hypertrophy and an increase
in crypt depth have been observed to varying degrees. The increase
in crypt cells will lead to a repopulation of the intestinal epithelium
by relatively immature enterocytes with reduced absorptive
capacities. An increased inflammatory cell infiltration in the lamina
propria has also been observed and this inflammation may be
associated with the pathology. Giardia infection can also lead to
lactase deficiency (see lactose intolerance below) as well as other
enzyme deficiencies in the microvilli. This reduced digestion and
absorption of solutes may lead to an osmotic diarrhea and could
also explain the malabsorption syndromes. Thus far, no single
virulence factor or unifying mechanism explains the pathogenesis of
giardiasis. [See also Pathophysiology of Diarrhea for a general
discussion of diarrhea.]
Post-Giardia Lactose Intolerance. Some patients may present
with a lactose intolerence during active Giardia infections which can
persist after parasite clearance. This clinical manifestation is due to
the parasite-induced lactase deficiency and is most common in
ethnic groups with a predisposition for lactase deficiency. Lactase is
an enzyme that breaks down lactose, a sugar found in milk, to
monosaccharides which can be absorbed. This lactose intolerence
syndrome should be considered in persons who still present mushy
stools and excessive gas following treatment, but have no
detectable parasites.
DIAGNOSIS
Diagnosis is confirmed by finding cysts
or trophozoites in feces or in
duodenojejunal aspirates or biopsies.
Stool Examination
Detection of the parasites can be difficult
since Giardia does not appear
• 3 non-consecutive days
consistently in the stools of all patients.
• wet mount or stained
Some patients will express high levels of
• IFA, copro-antigens
cysts in nearly all the stools, whereas
Duodenal Aspirate or Biopsy others will only exhibit low parasite
counts in some of the stools. A mixed
• Enterotest®
pattern, in which periods of high cyst
excretion alternate with periods of low
excretion, has also been observed. In
addition, parasites are easier to find during acute infections than
chronic infections. Aspiration and biopsy may also fail to confirm the
infection due to patchy loci of infection, and some question the
usefulness of these invasive procedures.
Parasite Detection
Stool examination is the preferred method for Giardia diagnosis.
Three stools taken at intervals of at least two days should be
examined. Watery or loose stools may contain motile trophozoites
which are detectable by the immediate examination of wet smears.
Otherwise the specimen should be preserved and stained due to
trophozoite lability. The hardier cysts are relatively easy to
recognize in either direct or stained smears (see cyst morphology).
In addition, diagnostic kits based on immunofluorescence or the
detection of copro-antigens are also available.
Diagnosis can also be made by examining duodenal fluid for
trophozoites. Duodenal fluid is obtained by either intubation or the
Enterotest® (also called 'string test'). The Enterotest® consists of a
gelatin capsule containing a nylon string of the appropriate length.
The free end of the string is taped to the patient's face and the
capsule is swallowed. After four hours to overnight the string is
retrieved and the bile-stained mucus on the distal portion of the
string is scraped off and examined by both wet mount and
permanent staining. A small intestinal biopsy, preferably from
multiple duodenal and jejunal sites, may also reveal trophozoites
attached to the intestinal epithelium. [The small intestine is divided
into 3 sections: the duodenum (first or proximal portion after the
stomach); the jejunum (the middle portion); and the ileum (the
distal or last portion before the large intestine).]
TREATMENT AND CONTROL
Infected individuals should be treated since Giardia can persist and
lead to severe malabsorption syndromes and weight loss. Treatment
is effective at reducing morbidity and there are no sequelae.
Metronidazole (Flagyl®), although not licensed in the United States
for giardiasis, effectively clears the parasite (cure rates
approximately 85%) and is the drug of choice. The recommended
dosage is 750 mg three times per day for five days (or at least >3
days). For children 15 mg/kg/d in three doses is recommended.
Other effective drugs include: quinacrine (Atabrine®), tinidazole
(Fasigyn®), furazolidone (Furoxone®), and paramomycin
(Humatin®). Tinidazole is effective as a single two gram dose;
paramomycin is not absorbed and may be useful during pregnancy.
The widespread distribution of Giardia and the infectivity of the
cysts make it unlikely that human infection will be completely
eliminated. Control measures to prevent or reduce Giardia infection
will depend on the specific circumstances of transmission, but in
general involve measures which prevent the ingestion of substances
contaminated with fecal material (see fecal-oral transmission
factors). Health promotion and education aimed at improving
personal hygiene, and emphasizing hand washing, sanitation and
food handling, are effective control activities for the reduction of
person-to-person transmission. Special attention to personal
hygiene in high-risk situations such as day-care centers and other
institutions is needed. Treatment of asymptomatic household
members prevents reinfection in non-endemic areas. However, the
value of treating asymptomatic carriers in hyperendemic
communities is questionable since reinfection rates are high. The
socio-economic situation in many developing countries makes it
difficult to prevent infection. Public health measures to protect
water supplies from contamination are required to prevent
epidemics and to reduce endemicity. Tourists should not drink tap
water without additional treatment in places where purity is
questionable. Boiling or iodine treatment kills Giardia cysts, but
standard chlorination does not. There are no safe or effective
chemoprophylatic drugs for giardiasis.
TRICHOMONIASIS
•
•
•
•
Tricomonad Morphology and Species
Transmission and Life Cycle
Symptoms and Pathogenesis
Diagnosis, Treatment and Control
The trichomonads are a group of flagellated protozoa. Most of the
members of this group are parasitic and only a few free-living
species have been identified. Generally the trichomonads are nonpathogenic commensals and only a few species are of importance in
animals and humans. Four species of trichomonads infect humans
(Table). Among these only Trichomonas vaginalis is clearly
pathogenic and it is usually of low virulence. The others exhibit a
questionable pathogenicity.
The trichomonads of humans
inhabit different anatomical
Species
Location
locations. T. vaginalis is a
Trichomonas vaginalis
uro-genital tract common sexually transmitted
disease found in the uroTrichomonas tenax
oral cavity
genital tract. T. tenax, also
Pentatrichomonas hominis intestine
called T. buccalis, is a
Dientamoeba fragilis
intestine
commensal of the human oral
cavity, found particularly in
patients with poor oral hygiene and advanced periodontal disease.
T. tenax, or an organism with similar morphology is also
occasionally found in the lungs. Such cases have reported mainly in
patients with underlying cancers or other lung diseases or following
surgery. Pentatrichomonas hominis, formerly known as
Trichomonas hominis, is a non-pathogenic commensal of the large
intestine (see non-pathogenic intestinal flagellates). Some authors
divide the trichomonads into three genera based on the number of
free flagella. Species with three flagella are called Tritrichomonas,
those with four are called Trichomonas, and Pentatrichomonas
refers to trichomonads with five free anterior flagella. Dientamoeba
fragilis was originally believed to be an ameba (see non-pathogenic
intestinal ameba). Now it is know to be a flagellate—however
without flagella—related to the trichomonads.
Trichomonads of Humans
A distinctive feature of the trichomonads is an axostyle (ax) which
runs the length of the organism and appears to protrude from the
posterior end (Figure). The axostyle is a cytoskeletal element
composed of concentric rows of microtubules and is believed to
function in the attachment of the parasite to epithelial cells.
Trichomonads are also characterized by 4-6 flagella (fg) emerging
from the anterior end. One of the flagella is attached to the body of
the organism and forms a posteriorly-directed undulating
membrane (um), whereas the remaining flagella are free. The
combined basal bodies (bb) and the base of the undulating
membrane, called the costa (cs), are often seen is stained
preparations. Less frequently seen is the cytostomal groove (cy). A
single nucleus (nu) is found at the anterior end of the parasite.
Schematic representation of major structural features of trichmonads (left). Giemsa-stained trophoz
vaginalis from in vitro culture (middle). Electron micrograph of axostyle cross-section showing conce
of microtubules (right).
The trichomonads, like many other intestinal protozoa, exhibit an
anerobic metabolism and lack mitochondria. Part of energy
metabolism of trichomonads involves a unique organelle called the
hydrogenosome. The hydrogenosome has a double membrane and
is distantly related to the mitochondrion. However, it lacks DNA,
cytochromes and many typical mitochnondrial functions such as
enzymes of the tricarboxylic acid cycle and oxidative
phosphorylation. The primary function of the hydrogenosome is the
metabolism of pyruvate, produced during glycolysis within the
cytosol, to acetate and carbon dioxide with the concomitant
production of ATP. The electrons release from the oxidation of
pyruvate are transferred to hydrogen ions to produce molecular
hydrogen, hence the name hydrogenosome.
TRICHOMONAS VAGINALIS
Trichomonas vaginalis was first described from purulent vaginal
discharges in 1836 and by the early part of the twentieth century
was recognized as an etiological agent of vaginitis. Trichomoniasis is
a common sexually transmitted disease with a worldwide
distribution and an estimated 167 million people becoming infected
per year worldwide and 5 million new infections per year in the
United States. Trichomoniasis is believed to be the most common
non-viral sexually transmitted disease. Despite the frequency of
trichomoniasis it has in the past been considered more of a
nuisance parasite rather than a major pathogen. However it is now
recognized a factor in promoting HIV infection (see Box), causing
low-weight and premature births, and predisposing women to
substantial discomfort and stress.
Trichomonas and HIV
The pathology caused by Trichomonas may enhance the efficiency of HIV transmission
(1). T. vaginalis infection typically elicits a local cellular immune response with
inflammation of the vaginal epithelium and cervix in women and the urethra of men. This
inflammatory response includes the infiltration of potential HIV target cells such as CD4+
bearing lymphocytes and macrophages. In addition, T. vaginalis can cause punctate
hemorrhages on the vaginal walls and cervix. This leukocyte infiltration and the genital
lesions may increase the number of target cells for the virus and allowing direct viral
access to the bloodstream through open lesions. In addition, the hemorrhages and
inflammation can increase the level of virus in body fluids and the numbers of HIVinfected lymphocytes and macrophages present in the genital area in persons already
infected with HIV. This increase of free virus and virus-infected leukocytes can increase
the probability of HIV exposure and transmission to an uninfected partner. Increased
cervical shedding of HIV has been shown to be associated with cervical inflammation, and
substantially increased viral loads in semen have been documented in men with
trichomoniasis. Moreover, since many patients with Trichomonas infection are
asymptomatic, or only mildly symptomatic, they are likely to remain sexually active in
spite of infection.
1. Sorvillo F, Smith L, Kerndt P, Ash L. (2001) Trichomonas vaginalis, HIV, and
African-Americans. Emerg Infect Dis. 7:927-32.
T. vaginalis, despite its name, infects both men and women. In
females the organism primarily inhabits the vagina, and in males it
is usually found in the urethra, prostate or epididymis. The life cycle
consists only of a trophozoite stage which is transmitted by direct
contact during sexual intercourse. Non-venereal transmission is
rare, but possible since the trophozoites can survive 1-2 days in
urine and 2-3 hours on a wet sponge. In addition, neonatals have
been infected during the birth process. The trophozoites live closely
associated or attached to the epithelium of the urogenital tract,
where they replicate by binary fission.
SYMPTOMS AND PATHOGENESIS
T. vaginalis causes
different clinical
manifestations in men
Females
Males
and women and women
(Table) are more likely
• asymptomatic
• asymptomatic
to exhibit symptoms
(15-20%*)
(50-90%*)
• vaginal discharge
• urethral discharge which tend to persist
(50-75%*)
(50-60%**)
longer. The incubation
• dyspareunia
• dysuria (12period typically ranges
(50%*)
25%**)
from 4-28 days. In
• pruritus (25• urethral pruritus
females the infection
50%*)
(25%**)
can present as a mild
vaginitis, an acute or
chronic vulvovaginitis,
*% of infected; **% of symptomatic
or urethritis. The onset
or exacerbation of symptoms commonly occurs during or
immediately after menstration. The most common complaint
associated with T. vaginalis infection is a persistent mild vaginitis
associated with a copious, foul-smelling discharge that is often
accompanied by burning or itching. This discharge is most often
gray, but can be yellow or green and is occasionally frothy or blood
tinged. The discharge diminishes as the infection becomes more
chronic. Many women also experience painful or difficult coitus.
Urethral involvement occurs in a large number of cases and is
characterized by dysuria (painful urination) and frequent urination.
Clinical Manifestations
The vaginal epithelium is the primary site of infection. Thus the
vaginal walls are usually erythematous (i.e., red) and may show
petechial (a small non-raised spot) hemorrhages. Punctate
hemorrhages of the cervix, called strawberry cervix, are observed in
approximately 2% of the cases. This strawberry cervix is a
distinctive pathological observation associated with trichomonasis
not seen with other sexually transmitted diseases.
Males are likely to be asymptomatic (50-90%) and the infection
tends to be self-limiting. The urethra and prostate are the most
common sites of infection. Common symptoms include: urethral
discharge (ranging from scant to purulent), dysuria, and urethral
pruritus (itching). Some men experience burning immediately after
coitus.
Little is known about the pathophysiology associated with T.
vaginalis infection, but is presumably due to interactions between
the parasite and host epithelial cells. In vitro studies indicate that T.
vaginalis can destroy cells in a contact dependent manner.
Therefore adhesion of the trophozoites to the epithelium is believed
to be a major factor in the pathogenesis. Several adhesion proteins
have been identified on the surface of the trophozoites. In addition,
secreted proteases that could play a role in pathogenesis have also
been identified.
DIAGNOSIS, TREATMENT AND CONTROL
In general, the clinical manifestations are not reliable as sole means
of diagnosis since the clinical presentation is similar to other STDs
and many patients have mild or no symptoms. Diagnosis is
confirmed by the demonstration of trophozoites in vaginal, urethral,
prostatic secretions, or urine sediment (following prostate
massage). Microscopic examination of wet mounts of fresh vaginal
discharge, preferably collected with a speculum on a cotton-tipped
applicator, is the most practical method of diagnosis. Specimens
should be diluted in saline and examined immediately. T. vaginalis
is recognized by its characteristic morphological features (see
above) and its rapid jerky motility. Specimens can also be fixed and
stained with Giemsa or fluorescent dyes. However, the organism
may be difficult to recognize on stained slides.
The sensitivity of direct observation ranges from 40-80%.
Therefore, in vitro culture is considered the gold standard for
diagnosis despite some limitations. For example, access to facilities
is needed and organisms require 2-7 days of growth before they are
detected. The accessibility issue is partly resolved by the
InPouch™TV culture system (Biomed Diagnostics). This is a
commercially available self-contained system for the detection of T.
vaginalis in clinical specimens. Antibody and DNA-based tests with
high sensitivity and specificity are being developed.
Metronidazole (Flagyl®) and other nitroimidazoles, such as
tinidazole, are highly effective against trichomoniasis. The
metronidazole is activated by the hydrogensome to a nitro radical
ion intermediate. Either a single two gram dose (85-92% cure rate)
or 250 mg three time daily for 7-10 days (>95% cure rate) can be
used. Sexual partners should be treated at the same time to
prevent reinfection. Some drug resistance has been reported, but
this is not a wide-spread problem. Treatment failures are generally
due to noncompliance or reinfection.
Trichomoniasis as an STD
•
•
•
5% females attending
family planning clinics
7-32% females attending
venereal disease clinics
50-75% prostitutes
The epidemiology of trichomonasis
• 4% males attending
exhibits features similar to other
venereal disease clinics
sexually transmitted diseases (Box)
• 5-15% males with nongonococcal urethritis
and incidence correlates with the
number of sexual partners. In
addition, co-infection with other STDs
is common. It is estimated that up to 25% of sexually active women
will become infected at some point during their lives and the
disease will be transmitted to 30-70% of their male partners.
Measures used in the control of other STD, such as limiting number
of sexual partners and use of condoms, are also effective in
preventing trichomoniasis.
Reviews on Trichomoniasis:
•
•
•
Lehker, M.W. and Alderete, J.F. (2000) Biology of
trichomonosis. Current Opinion in Infectious Diseases 13, 3745.
Petrin, D., Delgaty, K., Bhatt, R., Garber, G. (1998) Clinical
and microbiological aspects of Trichomonas vaginalis. Clin.
Microbiol. Rev. 11: 300-317.
Schwebke, J.R. and Burgess, D. (2004) Trichomoniasis.
Clinical Microbiology Reviews 17, 794-803.
DIENTAMOEBA FRAGILIS
Dientamoeba fragilis was originally described as an ameba based
upon its morphology. However, later it was recognized to exhibit a
morphology more similar to the turkey parasite Histomonas
meleagridis, except for the lack of flagella. Ultrastructural studies
also suggest similarities to the trichomonads, including the
possession of hydrogenosomes and molecular studies have
confirmed a close phylogenetic relationship between Dientamoeba
and Histomonas and a possible more distal relationship to
Trichomonas.
As with other trichomonads, Dientamoeba only exhibits a
trophozoite stage (Figure). This raises some questions about the
mode of transmission in that a cyst stage is usually involved in fecal
oral transmission. In addition, the trophozoites of Dientamoeba
survive outside of the body for a very short time. H. meleagridis
also lacks a cyst stage and has been demonstrated to be
transmitted via the eggs of a nematode. Due to the close
relationship between Histomonas and Dientamoeba, it is proposed
that Dientamoeba is also transmitted via helminth eggs.
Epidemiological and experimental evidence tends to incriminate the
pinworm Enterobius vermicularis as the carrier for Dientamoeba.
Morphology of Dientamoeba fragilis from a stool sample. Trophozoites exhibit an amebalike morphology and are often bi-nucleated.
Historically Dientamoeba has been considered as a non-pathogenic
commensal. However, clinical symptoms often correlate with the
presence of large numbers of trophozoites and treatment of the
infection resolves the symptoms. The incidence of symptoms is
estimated at 15-30% of infected individuals. Clinical symptoms
associated with Dientamoeba include intermittent diarrhea,
abdominal pain, flatulence, nausea and fatigue. Little is known
about the pathogenesis and Dientamoeba probably acts as a lowgrade irritant of intestinal mucosal surfaces that may lead to some
inflammation. Iodoquinol is generally the drug of choice for the
treatment of Dientamoeba. Tetracycline, paromomycin, and
metronidazole are also effective.
For a comprehensive review of Dientamoeba see: Johnson et al,
Clin. Microbiol. Rev. 17:553, 2004.
BALANTIDOSIS
Balantidium coli is the only ciliate which infects
humans. It is found world wide, but like many other
fecal-oral transmitted diseases, it is more prevalent
in the tropics. However, prevalence rates rarely
exceed 1%. B. coli also infects a wide variety of
mammals and is especially common in monkeys and
pigs. Prevalence in pigs ranges from 20–100% and
human balantidiosis usually exhibits an increased
prevalence in communities that live in close
association with pigs. For example, in Papua New
Guinea, where pigs are the principal domestic
animals, the prevalence among swine herders and
slaughterhouse workers has been reported to be as
high as 28%. Human-to-human transmission has also been
documented and this mode of transmission is likely to occur in
environments with crowding and poor personal hygiene such as
mental hospitals and prisons. (Skip general ciliate biology)
GENERAL CILIATE BIOLOGY
Ciliates are a large and diverse group of protozoa. Most ciliates are
free-living and are found in a variety of habitats. Well-known
ciliates include Paramecium species, which are found in ponds
throughout the world, and Ichthyophthirius multifiliis, an
ectoparasite of fish that causes white spot disease (also called 'ick').
As the name implies, ciliates possess cilia at some point during their
life cycles. The cilia are generally arranged in longitudinal rows and
typically cover the surface of the organism. Ciliates are also
characterized by nuclear dimorphism in that they have two distinct
nuclei. The large kidney-shaped macronucleus is involved in the
'housekeeping' or somatic functions of the cell, whereas the smaller
spherical micronucleus contains the complete genome. The
macronucleus contains thousands of copies of transcriptionally
active 'minichromosomes' representing 10-20,000 different DNA
molecules. This large number of telomeres (chromosome ends)
resulted in ciliates being an early model system for the study of
telomeres and telomerase (the enzyme that synthesizes telomeres).
Ciliates undergo both an asexual reproduction (ie, binary fission)
and a sexual reproduction involving conjugation (Figure above).
During conjugation, two ciliates of opposite mating types pair and
exchange genetic material. Conjugal contact triggers meiosis in the
micronuclei resulting in 4 haploid micronuclei. Concurrently, the
macronucleus breaks down and disappears. Three of the micronuclei
disintegrate and the remaining micronucleus divides again. Each of
the conjugating organisms donates a micronucleus (gametic or
male) to its mate via a cytoplasmic bridge that connects them. The
gametic micronucleus fuses with the stationary (or female)
micronucleus forming the diploid zygotic micronucleus. The
conjucating pair separates and the zygotic nucluei undergo another
round of division. One of these micronuclei develops into the the
macronucleus, thus completing the cycle. Formation of the
macronucleus involves fragmentation of the chromosomes and loss
of some DNA sequences. The remaining minichromosomes are then
amplified. (See diagram of DNA processing during macronucleus
formation.)
BALANTIDOSIS
B. coli usually lives as a non-pathogenic commensal in the large
intestine and produces no symptoms. Superficial inflammation of
the colonic mucosa may occur which can result in diarrhea and
colicky pain. Mild or chronic infections are characterized by
intermittent diarrhea and constipation, weight loss, and abdominal
pain. On rare occasions the trophozoites will invade the intestinal
epithelium and produce ulceration. Clinically this results in an acute
diarrhea with mucus and blood (ie, dysentery). This balantidial
dysentery is similar to the dysentery produced by Entameoba
histolytica (see below). Rare extra-intestinal infections involving
lungs, vagina, ureter and urinary bladder and intestinal perforations
leading to peritonitis have been reported.
Laboratory diagnosis is made by identifying the organism in feces.
Balantidium exhibits a typical fecal-oral life cycle consisting of
trophozoite and cyst stages. The large size and unique
morphological features of Balantidium (Figure) precludes its
confusion with any other protozoa found in human feces. The
trophozoite is ovoid and has an average size of 70 x 45 µm, but can
range upwards to 150-200 µm. The cyst has a distinctive cyst wall
(CW) and is more spherical with an average diameter of 55 µm. In
stained specimens the most obvious internal structure is the large
macronucleus (maN). The micronucleus (miN) may not always be
apparent because of its close association with the macronucleus.
Contractile vacuoles (CV), which function in osmotic regulation, are
often visible and occasionally the cytostome (Cy) is detectable.
Similar to many other ciliates, Balantidium is covered by rows of
cilia. The cilia give the parasite surface a fuzzy appearance and are
less pronounced in the cyst stage.
The treatment of choice is tetracycline given at 500 mg four times
per day for 10 days. Iodoquinol is the recommended alternate drug.
Metronidazole has not produced consistent results. Preventive
measures are the same as other diseases transmitted by the fecaloral route (see fecal-oral transmission factors or discussion of
Giardia prevention). In addition, pig sewerage should be kept away
from supplies of drinking water and food.
AMEBIASIS
Several members of the genus Entamoeba infect humans (see
below). Among these only E. histolytica is considered pathogenic
and the disease it causes is called amebiasis or amebic dysentery.
E. dispar is morphologically identical to E. histolytica and the two
were previously considered to be the same species. However,
genetic and biochemical data indicate that the non-pathogenic E.
histolytica is a distinct species (see discussion of criteria). The two
species are found throughout the world, but like many other
intestinal protozoa, they are more common in tropical countries or
other areas with poor sanitary conditions. It is estimated that up to
10% of the world's population may be infected with either E.
histolytica or E. dispar and in many tropical countries the
prevalence may approach 50%. There are an estimated 50 million
cases of amebiasis per year and up to 100,000 deaths.
•
•
•
•
•
Life Cycle and Morphology
Pathogenesis
Possible Mechanisms of Pathogenisis
o Schematic Figure of Trophozoite
Invasion
Clinical Presentation
Diagnosis, Treatment and
Control
LIFE CYCLE AND MORPHOLOGY
E. histolytica exhibits a typical fecal-oral life cycle consisting of
infectious cysts passed in the feces and trophozoites which replicate
within the large intestine. The infection is acquired through the
ingestion of cysts and the risk factors are similar to other diseases
transmitted by the fecal-oral route (see Table). Contaminated food
and water are probably the primary sources of infection. The higher
prevalence in areas of lower socioeconomic status is likely due to
poor sanitation and a lack of indoor plumbing. However, E.
histolytica is rarely the cause of travelers' diarrhea and is usually
associated with a long-term (>1 month) stay in an endemic area. A
higher prevalence of E. histolytica infection is also observed in
institutions, such as mental hospitals, orphanages and prisons,
where crowding and problems with fecal contamination are
contributing factors. A high prevalence among male homosexuals
has also been noted. Humans are the only host of E. histolytica and
there are no animal reservoirs.
Upon ingestion the cysts pass through the stomach and excyst in
the lower portion of the small intestine. Excystation involves a
disruption of the cyst wall and the quadranucleated ameba emerges
through the opening. The ameba undergoes another round of
nuclear division followed by three successive rounds of cytokinesis
(ie, cell division) to produce eight small uninucleated trophozoites,
sometimes called amebulae. These immature trophozoites colonize
the large intestine, especially the cecal and sigmoidorectal regions,
where they feed on bacteria and cellular debris and undergo
repeated rounds of binary fission.
E. histolytica trophozoites have an amorphous shape and are
generally 15-30 µm in diameter. The trophozoites move by
extending a finger-like pseudopodium (psd) and pulling the rest of
the body forward (called ameboid movement). The pseudopodia,
and sometimes the outer edge of the trophozoite, have a clear
refractile appearance and is referred to as the ectoplasm (ecto). The
rest of the cytoplasm has a granular appearance and is called the
endoplasm (endo). Occasionally a glycogen vacuole (vac) is evident.
Nuclear (Nu) morphology in stained specimens
is characterized by a finely granular ring of
peripheral chromatin and a centrally located
karyosome (ka).
As an alternative to asexual replication
trophozoites can also encyst. The factors
responsible for the induction of encystation
are not known. Encystation begins with the
trophozoites become more spherical and the
appearance of chromatoid bodies in the
cytoplasm. Chromatoid bodies (cb) are stained
elongated structures with round ends and
represent the aggregation of ribosomes. The cyst wall is composed
of chitin and has a smooth refractile appearance. Cyst maturation
involves two rounds of nuclear replication without cell division and
cysts with 1-4 nuclei (Nu) are found in feces. The nuclear
morphology of the cyst is similar to that of the trophozoite except
that the nuclei become progressively smaller following each
division. Sometimes the young cysts (ie, 1-2 nuclei) will have a
glycogen vacuole (vac) which will appear as a clear area in stained
specimens. This vacuole will sometimes displace and alter the
morphology of the nuclei. The chromatoid bodies tend to disappear
as the cyst matures. The cysts are generally 12-15 µm in diameter.
Cysts are immediately infective upon excretion with the feces and
will be viable for weeks-to-months depending on environmental
conditions.
PATHOGENESIS
Amebiasis Progression
non-invasive
•
ameba colony on mucosa surface
o asymptomatic cyst passer
o non-dysenteric diarrhea
invasive
•
•
•
necrosis of mucosa → ulcer
o dysentery
o hematophagous trophozoites
ulcer enlargement → peritonitis
o occasional ameboma
metastasis → extraintestinal
amebiasis
o via blood-stream or direct
E. histolytica frequently lives as
extension
a commensal within the large
o primarily liver → amebic
intestine with no overt clinical
abscess
manifestations. However,
o other sites infrequent
trophozoites can invade the
o ameba-free stools common
colonic epithelium and produce
ulcers and dysentery (see Box).
This invasive disease can become progressively worse and lead to a
more serious disease. The amebas can also metastasize to other
organs and produce anextraintestinal amebiasis. In other words, E.
histolytica is a facultative pathogen that exhibits a wide range of
virulence.
The non-invasive disease is often asymptomatic, but can cause
diarrhea or other gastro-intestinal symptoms such as abdominal
pain or cramps. This non-invasive infection can persist or progress
to an invasive disease in which trophozoites penetrate the intestinal
mucosa and kill the epithelial cells. The early lesion is a small area
of necrosis, or ulcer, characterized by raised edges and virtually no
inflammation between lesions (Figure). The ameba will spread
laterally and downward in the submucosa (beneath the epithelium)
and kill host cells as they progress. This results in the classic 'flaskshaped' ulcer with a small opening and a wide base. Trophozoites
are most numerous at the boundary between the healthy tissue and
the necrotic tissue. These invasive ameba are ingesting host cells
and trophozoites with ingested erythrocytes are often evident.
These hematophagous trophozoites are sometimes found in the
dysenteric feces. Cyst production decreases during the invasive
stage of the infection and cysts are never found in the tissue
lesions.
Left: The lumenal side of the colon from fulminating amebiasis case showing several ulcers. Note
raised edges (arrow). Middle: Histological preparation showing cross-section of ulcer. Note the
high degree of necrosis in center of ulcer. The amebas are advancing laterally under the intact
mucosa as indicated by the microvilli. Right: Higher magnification of ulcer showing several
hematophagous trophozoites. The nucleus (arrow) is evident in one of the amebas. Pictures from
Peters and Gilles (1989), A Colour Atlas of Tropical Medicine and Parasitology (3rd edition).
The ulcerative process may continue
to expand laterally or downward. If
large numbers of ulcers are present,
they may coalesce which could lead
to a localized sloughing off of the
intestinal wall. Ulcer expansion can
also penetrate the serous layer and
lead to perforation of the intestinal
wall. This perforation can lead to
local abscesses or a generalized
peritonitis. (See also schematic
representation of tissue invasion.)
Amebic ulcers can also become
secondarily infected with bacteria
which may confuse the clinical
picture. In addition, E. histolytica
infection can occasionally lead to the
formation of an amebic granuloma,
also called an ameboma. The
ameboma is an inflammatory
thickening of the intestinal wall
around the ulcer which can be
confused with a tumor.
Amebiasis can also progress to a
systemic, or extraintestinal infection.
Dissemination from the primary
E. histolytica is found primarily in
intestinal lesion is predominantly via
the colon where it can live as a
the blood stream, but can also occur
non-pathogenic commensal or
by direct extension of the lesion. The
invade the intestinal mucosa
(green). The ameba can
liver is the most commonly affected
metastasize to other organs via a
organ and this is probably due to the
hematogenous route (purple);
direct transport of trophozoites from
primarily involving the portal vein
the large intestine to the liver via the
and liver. The ameba can also
hepatic portal vein (Figure). Initially
spread via a direct expansion
(blue) causing a pulmonary
the lesions are small foci of necrosis
infection, cutaneous lesions or
which tend to coalesce into a single
perianal ulcers.
abscess as they expand. This hepatic
abscess will continue to enlarge as the trophozoites progressively
destroy and ingest host cells. The center of the abscess, consisting
of lysed hepatocytes, erythrocytes, bile and fat, may liquefy and
this necrotic material (sometimes incorrectly called pus) will range
in color from yellowish to reddish brown. Secondary bacterial
infections in the liver abscess are not common (~2%).
Hematogenous spread of trophozoites to other sites, such as the
lungs or brain, is rare, but does occur. The second most common
extraintestinal site after the liver is the lungs. Pulmonary infections
generally result from a direct extension of the hepatic lesion across
the diaphragm and into the pleura and lungs. Cutaneous lesions
formed as a result of hepatic or intestinal fistula can also occur,
although extremely rare. Other cutaneous lesions include perianal
ulcers and involvement of the genitalia, including the penis of
homosexuals. These later manifestations are likely due to the skin
or mucous membranes coming in contact with invasive
trophozoites.
POSSIBLE MECHANISMS OF
PATHOGENESIS
As discussed above, E. histolytica is
Entamoeba Prevalences
pathogen that exhibits a wide
spectrum of virulence, ranging from
• E. dispar ~10-fold > E.
an avirulent commensal to a highly
histolytica
invasive and destructive organism
• discrete endemic pockets of
(see discussion of pathogenicity vs.
E. histolytica observed
virulence). Some of this difference in
• ~25% seropositive for E.
virulence is explained by the
histolytica in endemic areas
• ~10% infected with E.
existence of the morphologically
histolytica will develop
identical, but avirulent, E. dispar. E.
invasive amebiasis
dispar has never been associated with
a symptomatic invasive disease and
infection does not elicit serum
antibodies. In contrast, anti-ameba humoral responses are
observed in both asymptomatic and symptomatic E. histolytica
infections. This suggests that even in asymptomatic cases there is a
limited amount of invasion. However, infection with E. histolytica
does not always lead to invasive disease, though, in that only about
10% of the infected individuals will develop symptomatic invasive
amebiasis. The factors responsible for the pathogenesis of E.
histolytica are not well understood. One approach to understanding
the pathogenesis is to compare possible virulence factors between
these two closely related species.
Possible Virulence Factors
host factors
•
•
ineffective innate immunity
inflammatory response
Pathology results from host-parasite
interactions, and therefore, host
factors, parasite factors or a
• resistance to host response
(eg, complement resistance) combination of both may contribute
to the disease state. For example, the
• adherence properties (eg,
development of invasive disease
'Eh-lectin')
could be due to quantitative or
• cytolytic properties (eg,
qualitative aspects of the host
adherence + 'amebapore')
• ability to breakdown tissues immune response. Recruitment of
(eg, secreted proteases)
neutrophils and intense inflammation
are noted in the early phases of
amebic invasion. However,
inflammation surrounding established ulcers and abscesses if often
minimal given the degree of tissue damage.
parasite factors
The nature of protective immune responses is not clear. Innate or
nonspecific immunity, as well as acquired immunity, are probably
both important for the prevention of invasive disease. The mucous
layer covering the epitheilial cells can prevent contact between
trophozoite and host cells. In addition, mucosal IgA responses do
occur as a result of infection and fecal IgA against a trophozoite
surface lectin (see Eh-lectin) are associated with a lower incidence
of new E. histolytica infections. High titers of serum antibodies also
develop in patients with liver abscesses. However, since the
invasive disease is often progressive and unremitting, the role of
these anti-ameba antibodies is in question. Cell-mediated responses
appear to play a role in limiting the extent of invasive amebiasis
and protecting the host from recurrence following successful
treatment.
Resistance to the host immune response is another possible
virulence factor which could contribute to the development and
exacerbation of invasive disease. For example, one phenotypic
difference between E. dispar and E. histolytica is the resistance of
the latter to complement mediated lysis (see E. dispar). In addition,
E. histolytica rapidly degrades secretory IgA and possibly
suppresses T-cell responses to E. histolytica antigens. E. histolytica
is also able to kill cells, including neutrophils and other immune
effector cells, in a contact dependent manner. Lysis of neutrophils
could also release toxic products which contribute to the destruction
of host tissue. However, the role of these various phenomena in
pathogenesis is not known.
Invasion of intestinal mucosa by E. histolytica is an active process
mediated by the parasite and distinct steps can be recognized
(Figure, click here for larger image and detailed legend).
Trophozoites adhere to the mucus layer (step 1). This adherence
per se probably does not contribute to pathogenesis and is simply a
mechanism for the ameba to crawl along the substratum. Depletion
of the mucus barrier allows for the trophozoite to come in contact
with epithelial cells. Epithelial cells are killed in a contact dependent
manner leading to a disruption of the intestinal mucosa (step 2).
The trophozoites will continue to kill host cells in the submucosa
and further disrupt the tissue as they advance (step 3). Disruption
of the intestinal wall (step 4) or metastasis via the circulatory
system (step 5) is also possible. Adherence, cytotoxicity, and
disruption of the tissues are important factors in the pathogenesis
of E. histolytica. Parasite proteins which could play a role in these
processes include: the Eh-lectin, amebapore, and proteases.
(Skip detailed discussions of Eh-lectin, amebapore, and proteases and go to
clinical symptoms.)
Eh-lectin. E. histolytica can kill cells within minutes of adhering to
them in the presence of extracellular calcium. Adherence of E.
histolytica trophozoites to host cells and colonic mucins is mediated
by a lectin-activity expressed on the ameba's surface. This lectin
binds galactose or N-acetyl-D-galactosamine (GalNAc) with a high
affinity and is also called the galactose-inhibitable adherence
protein (GIAP) or the Gal/GalNAc lectin. The contact-dependent
killing of target cells is almost completely inhibited by galactose or
GalNAc and target cells lacking terminal galactose residues on their
surface glycoproteins are resistant to trophozoite adherence and
cytotoxicity. This suggests that the Gal/GalNAc lectin is an
important virulence factor. In addition, the Eh-lectin is involved in
resistance to complement mediated lysis. Because of its potential
role in adherence and virulence and since fecal IgA against it
protect against amebic colitis, the Gal/GalNAc is a vaccine candidate
(Petri et al, 2006, Arch. Med. Res. 37:288).
The Eh-lectin is a heterodimer consisting of a 170 kDa heavy chain
and a 31-35 kDa light chain joined by disulfide bonds. An
intermediate subunit of 150 kDa is noncovalently associated with
the heterodimer. The heavy chain has a transmembrane domain
and a carbohydrate binding domain. All of subunits are encoded by
multigene families. There are five members of the heavy chain
family, 6-7 members of the light chain family and 30 members of
the intermediate chain family. The members of the heavy chain
gene family exhibit 89-95% sequence identity at the amino acid
level whereas the light chain family members are less conserved
sharing only 79-85% sequence identity.
E. dispar also expresses Gal/GalNAc lectin on its surface. Both E.
dispar and E. histolytica need to adhere to the mucous layer which
is medicated by the Gal/GalNAc lectin. Mucus is composed of
glycoproteins called mucins. The predominant mucin found on the
intestinal mucosa is Muc2 which is extensively glycosylated with Olinked GalNAc residues. The sequence of the light and heavy chain
genes from E. dispar are homologous, but not identical, to those of
E. histolytica. Antigenic differences between the GIAP of E. dispar
and E. histolytica have also been described in that only two epitopes
out of six are shared between the two species (see E. dispar). It is
not known whether these sequence differences can account for the
differences in virulence between E. dispar and E. histolytica.
Adherence is obviously important for both species, but it is possible
that the adherence is qualitatively or quantitatively different
between the two species.
[Review on the Eh-lectin: Petri et al (2002) Annu. Rev. Microbiol.
56:39.]
Amebapore. A family of pore-forming polypeptides has been
identified in E. histolytica and E. dispar. The three family members
are designated as amebapore A, B and C with amebapore A being
predominant expressed. The mature polypeptide is 77 amino acids
long and forms dimers at low pH (4-6). Three of these dimers then
assemble into a hollow ring-shaped structure. This hexamer then
can intercalate into membranes and introduce 2 nm pores (i.e.,
holes) which results in cell death. The pore-forming activity is
dependent on this assembly process beginning with the
dimerization. Amebabpore A is 95% identical (i.e., four residues are
different) between E. histolytica and E. dispar. In addition, the E.
dispar amebapore has approximately half of the pore-forming
activity as the E. histolytica amebapore. This difference in poreforming activity has been attributed to a glutamate residue at
position 2 in the E. histolytica amebapore, as compared to a proline
residue in the E. dispar amebapore. This particular amino acid
residue is important for the formation of the dimers and it is
believed that the dimers of E. dispar amebapore are less stable.
Amebapore is localized to vacuolar compartments (eg, food
vacuoles) within the trophozoite and is most active at acidic pH
suggesting that the major function of amebapore is to lyse ingested
bacteria. Nonetheless, amebapore is implicated as a virulence factor
in that genetic manipulation of E. histolytica resulting in decreased
expression of amebapore leads to a reduction in pathogenicity
(ability to form liver abscesses) as well as a reduction in
bacteriocidal activity (Bracha et al Mol. Microbiol. 34:363, 1999).
Similarly, modified E. histolytica completely devoid of amebapore
production are unable to form liver abscesses in model systems
(Zhang et al, Inf. Imm. 72:678, 2004). However, these amebas are
able to cause inflammation and tissue damage in models for amebic
colitis.
[Review on amebapore: Leippe et al, Tr. Parasitol. 21:5, 2005.]
Proteases. Proteases are enzymes that degrade other proteins and
could contribute to the pathogenesis cause by E. histolytica. In this
regard, E. histolytica expresses and secretes higher levels of
cysteine proteases, a particular class of protease, than E. dispar.
Cysteine proteases have been shown to disrupt the polymerization
of MUC2, the major component of colonic mucus. This degraded
mucus is less efficient at blocking adherence of trophozoites to
epithelial cells. Destruction of the extracellular matrix (ECM) by
proteases may also facilitate trophozoite invasion. Inhibitors of
cysteine proteases can decrease liver abscess size in experimental
models.
Twenty different cysteine
protease genes have been
identified in E. histolytica.
Orthologs of two of the E.
histolytica cysteine protease
genes are not found in E.
dispar. One of these,
designated CP5, is expressed at
high levels on the trophozoite
surface. Mutants expressing
lower levels of CP5 had a
reduced ability to generate liver
abscesses in a hamster
amebiasis model. However,
these mutants also had a
reduced growth rate and lower
erythrophagocytic activity, thus
Figure from Horstmann et al (1992) Trop. Med.
Parasitol. 43, 213.
Factor
histolytica vs dispar
Eh-lectin
sequence and epitope
differences
amebapore Ed has less activity (Pro/Glu)
proteases
Eh has unique genes and
expresses more activity
Figure from Horstmann et al (1992) Trop. Med.
Parasitol. 43, 213.
it is not clear whether CP5 directly participates in the invasiveness
of E. histolytica. Furthermore inhibition of 90% of CP5 activity did
not affect the ability of E. histolytica trophozoites to destroy cell
monolayers in vitro. CP1, CP2, and CP5 are the most abundantly
expressed cysteine proteases in E. histolytica, whereas CP3 is the
most abundant in E. dispar. Interestingly, over expression of CP2 in
E. dispar increased the ability of trophozoites to destroy cell
monolayers in vitro. However, the over expression of CP2 did not
lead to the ability of E. dispar to form liver abscesses in gerbils.
Therefore, it is not clear the precise roles proteases may play in
pathogenesis.
In summary, the pathogenesis associated with E. histolytica
infection is primarily due to its ability to invade tissues and kill host
cells. Several potential virulence factors have been identified (see
Table). However, it is not clear the exact role these various
virulence factors play in the development of invasive disease. One
approach to understanding the pathogenesis is to compare these
factors from E. histolytica and E. dispar. These two species are
closely related and the potential virulence factors are found in both
species. Adherence, cytolytic activity and proteolytic activity are
inherent biological features of both species and these activities do
not necessarily lead to pathology. However, there are qualitative
and quantitative differences between E. histolytica and E. dispar
which may account for the differences in virulence. These genetic
differences between E. histolytica and E. dispar indicate that
pathogenesis is in part an inherent feature of the parasite.
However, pathogenesis is probably due to the combined effects of
several host and parasite factors, and the virulence may represent
the degree to which the host can control trophozoite invasion and
replication.
[See Huston, 2004, Tr. Parasitol. 20:23 for review of
pathogenesis.]
CLINICAL PRESENTATION
Amebiasis presents a wide range of clinical syndromes (Table)
which reflects the potential for E. histolytica to become invasive and
cause a progressive disease. The incubation period can range from
a few days to months or years with 2-4 weeks being the most
common. Transitions from one type of intestinal syndrome to
another can occur and intestinal infections can give rise to
extraintestinal infections.
Clinical Syndromes
Clinical Syndromes
Associated with Amebiasis
Intestinal Disease
•
•
•
•
•
•
asymptomatic cyst passer
symptomatic nondysenteric
infection
amebic dysentery (acute)
fulminant colitis
o + perforation
(peritonitis)
ameboma (amebic
granuloma)
perianal ulceration
Extraintestinal Disease
The majority of individuals diagnosed
with E. histolytica (or E. dispar)
exhibit no symptoms or have vague
and nonspecific abdominal
symptoms. This state can persist or
progress to a symptomatic infection.
Symptomatic nondysenteric
infections exhibit variable symptoms
ranging from mild and transient to
intense and long lasting. Typical
symptoms include: diarrhea, cramps,
flatulence, nausea, and anorexia. The
diarrhea frequently alternates with
periods of constipation or soft stools.
Stools sometimes contain mucus, but
there is no visible blood.
Amebic dysentery usually starts
slowly over several days with
abdominal cramps, tenesmus, and
occassional loose stools, but
progresses to diarrhea with blood
and mucus. Blood, mucus and pieces of necrotic tissue become
more evident as the number of stools increases (10-20 or more per
day) and stools will often contain little fecal material. A few patients
may develop fever, vomiting, abdominal tenderness, or dehydration
(especially children) as the severity of the disease increases.
Fulminant, or grangrenous, colitus is a rare but extremely severe
form of intestinal amebiasis. Patients present with severe bloody
diarrhea, fever, and diffuse abdominal tenderness. Most of the
mucosa is involved and mortality exceeds 50%. A chronic
amebiasis, characterized by recurrent attacks of dysentery with
intervening periods of mild or moderate gastrointestinal symptoms,
can also occur.
•
•
•
•
liver abscess
pleuropulmonary amebiasis
brain and other organs
cutaneous and genital
diseases
Amebomas present as painful abdominal masses which occur most
frequently in the cecum and ascending colon. Obstructive symptoms
or hemorrhages may also be associated with an ameboma.
Amebomas are infrequent and can be confused with carcinomas or
tumors. Perianal ulcers are a form of cutaneous amebiasis that
result from the direct spread of the intestinal infection.
Amebic liver abscesses are the most common form of extraintestinal
amebiasis. The onset of hepatic symptoms can be rapid or gradual.
Hepatic infections are characterized by hepatomegaly, liver
tenderness, pain in the upper right quadrant, fever and anorexia.
Fever sometimes occurs on a daily basis in the afternoon or
evening. Liver function tests are usually normal or slightly abnormal
and jaundice is unusual. Liver abscesses will occasionally rupture
into the peritoneum resulting in peritonitis.
Pulmonary amebiasis is generally results from the direct extension
of the liver abscess through the diaphragm. Clinical symptoms most
often include cough, chest pain, dyspnea (difficult breathing), and
fever. The sputum may be purulent or blood-stained and contain
trophozoites. A profuse expectoration (ie, vomica) of purulent
material can also occur. Primary metastasis to the lungs is rare, but
does occur. Similarly, infection of other organs (eg., brain, spleen,
pericardium) is also rare. Clinical symptoms are related to the
affected organ.
Cutaneous amebiasis is the result of skin or mucus membranes
being bathed in fluids containing trophozoites. This contact can be
the result of fistula (intestinal, hepatic, perineal) or an invasion of
the genitalia. Cutaneous lesions have a wet, granular, necrotic
surface with prominent borders and can be highly destructive.
Clinical diagnosis is difficult and is usually considered with
epidemiological risk factors (eg., endemic areas, male
homosexuality, etc.).
DIAGNOSIS, TREATMENT AND CONTROL
Diagnosis
Intestinal Disease
•
•
•
stool examination
o cysts and/or
trophozoites
sigmoidoscopy
o lesions, aspirate,
biopsy
antigen detection
o histolytica/dispar
Extraintestinal (hepatic) Disease
•
•
•
serology
o current or past?
imaging
o CT, MRI, ultrasound
abscess aspiration
o only select cases
o reddish brown liquid
o trophozoites at
abscess wall
Definitive diagnosis of amebiasis
requires the demonstration of E.
histolytica cysts or trophozoites in
feces or tissues. Stool specimens
should be preserved and stained and
microscopically examined. Cysts will
tend to predominate in formed stools
and trophozoites in diarrheic stools
(see morphology). Fresh stools can
also be immediately examined for
motile trophozoites which exhibit a
progressive motility. Sigmoidoscopy
may reveal the characteristic ulcers,
especially in more severe disease.
Aspirates or biopsies should also be
examined microscopically for
trophozoites.
E. histolytica and E. dispar cannot be
distinguished on morphological
criteria. Antigen detection kits are
available for the positive
identification of these species.
Serology is especially useful for the diagnosis of extraintestinal
amebiasis. Greater than 90% of patients with invasive colitis and
liver abscesses exhibit serum antibodies against E. histolytica.
However, the antibodies can persist for years and distinguishing
past and current infections may pose problems in endemic areas.
Non-invasive imaging techniques (eg., ultrasound, CT, MRI) can be
used to detect hepatic abscesses. It is also possible to aspirate
hepatic abscesses. However, this is rarely done and only indicated
in selected cases (eg., serology and imaging not available,
therapeutic purposes). The aspirate is usually a thick reddish brown
liquid that rarely contains trophozoites. Trophozoites are most likely
to be found at the abscess wall and not in the necrotic debris at the
abscess center.
Several drugs are available for the treatment of amebiasis and the
choice of drug(s) depends on the clinical stage of the infection
(Table). The prognosis following treatment is generally good in
uncomplicated cases. In cases where E. histolytica is confirmed or
the species (ie, dispar or histolytica) is unknown, asymptomatic cyst
passers should be treated to prevent the progression to severe
disease and to control the spread of the disease. However, in many
endemic areas, where the rates of reinfection are high and
treatment is expensive, the standard practice is to only treat
symptomatic cases. Metronidazole or tinidazole (if available) is
recommended for all symptomatic infections. This treatment should
be followed by or combined with lumenal antiamebic drugs as
described for asymptomatic patients.
Amebiasis Treatment
Drugs
Uses
Iodoquinol, Paromomycin, or
Diloxanide furoate
Luminal agents to treat asymptomatic cases and as a follow up
treatment after a nitroimidazole.
Metronidazole or Tinidazole
Treatment of nondysenteric colitis, dysentery, and extraintestinal infections.
Dehydroemetine or Emetine
Treatment of severe disease such as necrotic colitis, perforation
of intestinal wall, rupture of liver abscess.
In the cases of fulminant amemic colitis or perforation of the
intestinal wall a broad spectrum antibiotic can also be used to treat
intestinal bacteria in the peritoneum. Necrotic colitis requires urgent
hospitalization to restore fluid and electrolyte balance. In addition,
emetine or dehydroemetine are sometimes co-administered with
the nitroimidazole. This is only done in the most severe cases due
to the toxicity of these drugs. Surgery may also be needed to close
perforations or a partial colostomy. Abscess drainage of hepatic
lesions (ie, needle aspiration or surgical drainage) is now rarely
performed for therapeutic purposes and is only indicated in cases of
large abscesses with a high probability of rupture.
Prevention and control measures are similar to other diseases
transmitted by the fecal-oral route (see Risk Factors or discussion of
Giardia control). The major difference is that humans are the only
host for E. histolytica and there is no possibility of zoonotic
transmission. Control is based on avoiding the contamination of
food or water with fecal material. Health education in regards to
improving personal hygiene, sanitary disposal of feces, and hand
washing are particularly effective. Protecting water supplies will
lower endemicity and epidemics. Like Giardia, Entamoeba cysts are
resistant to standard chlorine treatment, but are killed by iodine or
boiling. Sedimentation and filtration processes are quite effective at
removing Entamoeba cysts. Chemoprophylaxis is not
recommended.
Recent review on amebiasis:
•
•
Haque, R. et al (2003) Amebiasis. N. Engl. J. Med. 348:1565.
Stanley, S.L. (2003) Amoebiasis. The Lancet 361:1025.
NON-PATHOGENIC COMMENSALS
Numerous protozoa can inhabit the gastro-intestinal tract of
humans. Most of these exhibit little or no overt pathology. Infection
with these protozoa is evidence of fecal contamination and indicates
a risk for more serious infections such as Giardia or E. histolytica.
These non-pathogenic species can also be confused with the
potentially pathogenic Giardia or E. histolytica and result in
unnecessary drug treatment. In addition, such a misdiagnosis is
also problematic in that the true cause of the symptoms may be
missed and the appropriate treatment will be
delayed.
• E. histolytica
• Other Entamoeba
• E. dispar
• Other Intestinal Amebae
• E. coli
• Other Intestinal Flagellates
• E. hartmanni
• E. polecki
• Blastocystis
• E. gingivalis
Entamoeba Species Infecting
Humans
Several Entamoeba species infect humans (box). E. histolytica can
cause a severe intestinal disease characterized by dysentery as well
as an invasive disease affecting primarily the liver (see Amebiasis).
E. dispar is morphologically identical to E. histolytica, but does not
produce an invasive disease (see further discussion on E. dispar). A
distinguishing feature of the Entamoeba is their nuclear morphology
which is described as having peripheral chromatin and a small
karyosome. E. histolytica/dispar, E.coli, and E. hartmanni can be
distinguished by size and minor morphological differences (see
Table).
Intestinal Entamoeba Species
E. dispar*
Trophozoites
•
•
•
15-20 µm**
extend pseudopodia
progressive movement
Cysts
•
•
•
E. coli
Trophozoites
•
•
•
20-25 µm
broad blunt pseudopodia
sluggish, non-directional
movement
Cysts
12-15 µm
4 nuclei
blunt chromatoid bodies
•
•
•
E. hartmanni
Trophozoites
•
•
8-10 µm
less progressive than E.
dispar
Cysts
15-25 µm
8 nuclei
pointed chromatoid
bodies
•
•
•
•
6-8 µm
4 nuclei
blunt chromatoid bodies
CB persist in mature
cysts
•
blunt chromatoid bodies
•
pointed chromatoid
bodies
•
•
blunt chromatoid bodies
CB persist in mature
cysts
*=E. histolytica; **invasive E. histolytica can be >20 mm
E. coli is the largest and is best distinguished by 8 nuclei in the
mature cyst. The trophozoites of E. coli can be difficult to
distinguish from E. histolytica/dispar since there is some overlap in
the size ranges. E. hartmanni is quite similar to E. histolytica and
was previously considered a 'small race' of E. histolytica. Generally
10 µm is chosen as the boundary between E. histolytica and E.
hartmanni.
E. polecki is usually associated with pigs and monkeys, but human
cases have been occasionally documented. It appears to be
geographically restricted to particular areas such a Papua, New
Guinea. The trophozoites are similar to E. coli, except a little
smaller, and the cysts are similar to E. histolytica except that the
mature cyst has a single nucleus.
E. gingivalis can be recovered from the soft tartar between teeth
and exhibits a similar morphology to E. histolytica except that it has
no cyst stage. E. gingivalis can also multiply in bronchial mucus,
and thus can appear in the sputum. In this case it could be
confused with E. histolytica from a pulmonary abscess. E. gingivalis
trophozoites will often contain ingested leukocytes which can be
used to differentiate it from E. histolytica. The trophozoites are
most often recovered from patients with periodontal disease, but an
etiology between the organism and disease has not been
established and E. gingivalis is considered to be non-pathogenic.
Other Intestinal Amebae
Other non-pathogenic amebae include Endolimax nana and
Iodoamoeba bütschlii. Historically, Dientamoeba fragilis has been
grouped with the ameba, but electron microscopy and molecular
phylogenetics suggests that it is actually a flagellate and may be
closely related to the trichomonads (see above). All three of these
organisms exhibit similar morphologies and have nuclei which do
not have peripheral chromatin and a large karyosome. Minor
morphological differences allow these organims to be distinguished
(Table).
Other Intestinal Amebae
Endolimax nana
Iodoamoeba bütschlii
Trophozoites
•
Trophozoites
8-10 µm
•
Cysts
•
•
•
•
•
Trophozoites
12-15 µm
Cysts
6-8 µm
4 nuclei
Dientamoeba fragilis*
8-10 µm
often bi-nucleated
fragmented karyosome
•
•
•
Cysts
10-12 µm
1 nuclei
glycogen vacuole
•
no cysts
*A flagellate possibly related to the trichomonads.
Other Intestinal Flagellates
Four additional non-pathogenic flagellates recovered from human
stools are: Trichomonas hominis, Chilomastix mesnili, Enteromonas
hominis, and Retortamonas intestinalis. Among these T. hominis,
also called Pentatrichomonas hominis, is the most common and is
often recovered from diarrheic stools. These flagellates exhibit
similar morphologies (Table) and can be difficult to distinguish. The
trophozoites from all of these flagellates are somewhat teardrop
shaped and contain a single nucleus and the cyst tend to be slightly
elongated or oval.
Other Intestinal Flagellates
trophozoites
cysts
Size
Flagella
Size
Nuclei
Trichomonas hominis
6-14 µm
4 anterior, 1 posterior
No cyst stage
Chilomastix mesnili
10-15 µm 3 anterior, 1 in cytostome
7-9 µm
1
Enteromonas hominis
6-8 µm
3 anterior, 1 posterior
4-8 µm
1-4
Retortamonas intestinalis
4-10 µm
1 anterior, 1 posterior
4-7 µm
1
Blastocystis hominis
Blastocystis hominis is a common organism found in human stools.
Since its initial description approximately 100 years ago, it has been
variously classified as an ameba, a yeast, a sporozoan, and the cyst
stage of a flagellate. Analysis of the small subunit rRNA sequence
indicates that Blastocystis is most closely related to the
stramenopiles, a complex assemblage of unicellular and muticellular
protists. Other stramenopiles include diatoms, brown algae, and
water molds. Many of the characteristics of Blastocystis are
unknown or controversial. The mode of transmission, mechanism of
cell replication, and other features of the life cycle have not
conclusively demonstrated.
Similarly, the status of
Blastocystis as a pathogen,
commensal, or opportunistic
organism is unknown.
Blastocystis is polymorphic in
that a variety of
morphological forms are found
in feces and in vitro culture.
The most widely recognized
form is spherical 10-15 µm in
diameter with a large central
vacuole (Figure). This large
vacuole pushes the nuclei and
other organelles to the
periphery of the cell. The vacuole is sometimes filled with a granular
material. Small resistant cyst-like forms have been identified from
in vitro cultures and occasionally observed in feces. These
presumed cysts are approximately 5 µm and surround by a
multilayered wall. Furthermore, the cysts do not lyse when placed in
water suggesting that they are resistant to environmental
conditions. Presumably Blastocystis is transmitted via a fecal-oral
route. However, this has not been conclusively demonstrated.
There have been several reports suggesting Blastocystis causes
disease, as well as many reports suggesting the opposite. Diarrhea,
cramps, nausea, vomiting and abdominal pain have been associated
with large numbers of organisms in the stool. In addition, some
studies have shown that treatment alleviates the symptoms and
clears the organisms. However, the drugs used against Blastocystis
(eg., metronidazole) also work against many other intestinal
protozoa and bacteria. The inability to rule out other organisms as
the source of symptoms and the observation that many infected
persons exhibit no symptoms makes it difficult to draw any
definitive conclusions about the pathogenesis of Blastocystis.
Furthermore, it could be that Blastocystis is primarily a commensal,
but can exhibit virulence under specific host conditions like
concomitant infections, poor nutrition, or immunosuppression.
Blastocystis is also found in a wide range of animals, including
mammals, birds, reptiles, amphibians and even insects, and exhibits
a wide range of molecular diversity. The genetic distance between
Blastocystis isolates is greater than the genetic distance between E.
histolytica and E. dispar (see discussion on E. dispar). This
complicates the designation of species and historically human
isolates have been designated as B. hominis and isolates for other
hosts as Blastocytis sp.. However, phylogenetic analysis reveals
that there are no exclusively human clades and human isolates are
found in all of the clades. This raises the possibility that Blastocystis
is not host specific and can be transmitted zoonotically. In addition,
the wide range of genetic diversity might explain the controversy
concerning the pathogenecity of Blastocystis in that some
genotypes may be more virulent than others. However, studies
addressing this issue suggest that this is not the case. Resolution of
the confusion about the taxonomy, transmission and virulence of
Blastocystis will require additional studies.
Recent reviews on Blastocystis:
•
•
•
Stenzel, D.J. and Boreham, P.F.L. (1996) Blastocystis hominis
revisited. Clinical Microbiology Reviews 9, 563-584.
Tan, K.S.W. (2004) Blastocystis in humans and animals: new
insights using modern methodologies. Veterinary Parasitology
126, 121-144.
Yoshikawa, H. Morimoto, K., Wu, Z., Singh, M. and
Hashimoto, T. (2004) Problems in speciation in the genus
Blastocystis. Trends in Parasitology 20, 251-255.
LINKS
•
•
•
•
•
•
•
•
•
•
•
Top
Contents
Giardiasis
Trichomoniasis
Balantidosis
Amebiasis
Non-pathogenic Commensals
Protozoology Home
o Study Guides
o Syllabus
Other Courses and Lectures
Wiser Home
Other Internet Sites
o Parasites, Division of Parasitic Diseases, CDC
o The Medical Letter (treatment recommendations)
http://en.wikipedia.org/wiki/Category:Laboratory_techniques
Category:Laboratory techniques
From Wikipedia, the free encyclopedia
Jump to: navigation, search
Laboratory techniques, as used in Biology, Biochemistry, Chemistry, Molecular
biology, etc.
Subcategories
This category has the following 8 subcategories, out of 8 total.
B
D
•
[+] Biochemistry
methods
M cont.
•
[+] Distillation
E
C
[+]
Chromatography
[+] Microscopy
•
[+] Polymerase chain
reaction
[+] Protein-protein
interaction assays
P
•
•
•
[+] Electrophoresis
M
•
•
[+] Microbiology
techniques
Pages in category "Laboratory techniques"
The following 132 pages are in this category, out of 132 total.
A
F cont.
•
•
•
•
•
•
•
Acid-base extraction
Air-free technique
Allele specific
oligonucleotide
Ames test
Ammonium sulfate
precipitation
Animal testing
Assay
B
•
Baby Gender Mentor
•
Fractionation
G
P cont.
•
•
•
•
•
•
•
Gas chromatographymass spectrometry
Gas-liquid
chromatography
Gel electrophoresis
Gel extraction
Gene gun
•
•
•
•
•
Plant tissue
culture
Polymerase
chain reaction
Post harvest
freshness
Protein
Misfolding
Cyclic
Amplification
Protein
electrophoresis
Protein tag
Protein-
•
•
Blot (biology)
Borax
H
C
•
•
•
•
•
•
•
•
•
•
•
Cell disruption by
nitrogen
decompression
Cell fractionation
Centrifugation
I
Chemotaxis assay
Chromosome jumping
Cooling bath
Cosmid
Cot analysis
Cot filtration
Crystallization
Cycling probe
technology
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
DNA extraction
DNA footprinting
DNA laddering
DNase footprinting
assay
Degasification
Diethylpyrocarbonate
Differential
centrifugation
Digital polymerase
chain reaction
Direct fluorescent
antibody
Distillation
Dithioerythritol
•
•
•
•
•
•
•
•
•
•
•
•
•
•
Immunohistochemistry
Immunomagnetic
separation
Immunoperoxidase
In situ hybridization
Inductively coupled
plasma atomic
emission spectroscopy
Inductively coupled
plasma mass
spectrometry
Inverse polymerase
chain reaction
Isotopic dilution
Isotopic labeling
S
L
•
•
•
•
•
•
•
•
E
Quantitative
polymerase
chain reaction
R
•
D
Hofmeister series
Homogenization
Host-Cell Reactivation Q
Hydrophilic
interaction liquid
•
chromatography
fragment
Complementatio
n Assay
•
•
•
Electrochromatography
Electropherogram
Electrophoresis
(disambiguation)
M
Electrophoretic
mobility shift assay
•
Electrophoretogram
•
Ellman's reagent
•
LIESST
Laboratory automation
Laboratory centrifuge
Lamm equation
Lipofection
Liquid gas
Liquid-liquid
extraction
List of purification
methods in chemistry
Low copy number
Lowry protein assay
Lysis buffer
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
Magnetofection
Mason-Weaver
equation
Microscopy
•
•
•
Real-time
polymerase
chain reaction
Recrystallization
Restriction
landmark
genomic
scanning
Reverse
transcription
polymerase
chain reaction
SDS-PAGE
Salting out
Sedimentation
Sequencing by
hybridization
Serial dilution
Sham operated
group
Size exclusion
chromatography
Solid phase
microextraction
Sonication
Southern blot
Southwestern
blot
Sparging
(chemistry)
Standard
addition
Starch indicator
Sublimation
•
Ethanol precipitation
F
•
Murashige and Skoog
medium
•
N
•
•
•
•
•
FLAG-tag
Filtration
Finisher
Fluorescent in situ
hybridization
Fosmid
•
•
•
•
•
•
•
•
•
Nanopore sequencing
Nanovid microscopy
•
Native PAGE
Nested polymerase
T
chain reaction
Nick translation
Northern blot
•
Nucleic acid
hybridization
•
•
O
•
•
•
Oligonucleotide
synthesis
Organ culture
Overlay assay
•
•
P
•
•
Peptide mass
fingerprinting
(chemistry)
Sublimation
apparatus
Sucrose gradient
centrifugation
Suction
filtration
SuperSAGE
TMB Liquid
substrate for
ELISA
TUNEL assay
Terminal
restriction
fragment length
polymorphism
Tissue culture
Touchdown
polymerase
chain reaction
Twodimensional gel
electrophoresis
V
•
Visualized
Experimental
Biology
•
•
•
Western blot
Wet laboratory
Winogradsky
column
•
•
Zoo blot
Zymography
W
Z
Retrieved from "http://en.wikipedia.org/wiki/Category:Laboratory_techniques"
Categories: Chemistry | Laboratories | Biological techniques and tools
Views
•
•
•
•
Category
Discussion
Edit this page
History
Personal tools
•
Log in / create account
Navigation
•
•
•
•
•
Main Page
Contents
Featured content
Current events
Random article
Interaction
•
•
•
•
•
•
About Wikipedia
Community portal
Recent changes
Contact Wikipedia
Donate to Wikipedia
Help
Search
Toolbox
•
•
•
•
•
•
What links here
Related changes
Upload file
Special pages
Printable version
Permanent link
Languages
•
•
•
•
Català
Français
Bahasa Indonesia
中文
•
•
•
•
•
This page was last modified on 26 December 2007, at 17:35.
All text is available under the terms of the GNU Free Documentation License.
(See Copyrights for details.)
Wikipedia® is a registered trademark of the Wikimedia Foundation, Inc., a
U.S. registered 501(c)(3) tax-deductible nonprofit charity.
Privacy policy
About Wikipedia
Disclaimers
http://www.who.int/mediacentre/factsheets/fs094/en/
WHO > Programmes and projects > Media centre > Fact sheets
Main content
printable version
Fact sheet N°94
May 2007
Malaria
- Malaria is both preventable and curable.
- A child dies of malaria every 30 seconds.
- More than one million people die of malaria every year, mostly infants, young
children and pregnant women and most of them in Africa.
INFECTION AND TRANSMISSION
Malaria is a disease which can be transmitted to people of all ages. It is caused by
parasites of the species Plasmodium that are spread from person to person through the
bites of infected mosquitoes. The common first symptoms – fever, headache, chills, and
vomiting – appear 10 to 15 days after a person is infected. If not treated promptly with
effective medicines, malaria can cause severe illness that is often fatal.
There are four types of human malaria – Plasmodium falciparum, P.vivax, P.malariae, and
P.ovale. P.falciparum and P.vivax are the most common. P.falciparum is by far the most
deadly type of malaria infection.
Malaria transmission differs in intensity and regularity depending on local factors such as
rainfall patterns, proximity of mosquito breeding sites and mosquito species. Some regions
have a fairly constant number of cases throughout the year – these are malaria endemic –
whereas in other areas there are “malaria” seasons, usually coinciding with the rainy
season.
Malaria transmission differs in intensity and regularity depending on local factors such as
rainfall patterns, proximity of mosquito breeding sites and mosquito species. Some regions
have a fairly constant number of cases throughout the year – these are malaria endemic –
whereas in other areas there are “malaria” seasons, usually coinciding with the rainy
season.
Large and devastating epidemics can occur in areas where people have had little contact
with the malaria parasite, and therefore have little or no immunity. These epidemics can be
triggered by weather conditions and further aggravated by complex emergencies or natural
disasters.
GLOBAL AND REGIONAL RISK
Approximately, 40% of the world’s population, mostly
those living in the world’s poorest countries, are at risk of
malaria. Every year, more than 500 million people become
severely ill with malaria. Most cases and deaths are in subSaharan Africa. However, Asia, Latin America, the Middle
East and parts of Europe are also affected. Travellers from
malaria-free regions going to areas where there is malaria
transmission are highly vulnerable – they have little or no
immunity and are often exposed to delayed or wrong
malaria diagnosis when returning to their home country.
Related links
Global Malaria
Programme
::
Roll Back Malaria
Partnership
::
Malaria (Special
Programme for Research
and Training in Tropical
Diseases, TDR)
::
TREATMENT
Early diagnosis and prompt treatment are the basic
elements of malaria control. Early and effective treatment of malaria disease will shorten
its duration and prevent the development of complications and the great majority of deaths
from malaria. Access to disease management should be seen not only as a component of
malaria control but a fundamental right of all populations at risk. Malaria control must be
an essential part of health care development. In contemporary control, treatment is
provided to cure patients rather than to reduce parasite reservoirs.
Antimalarial treatment policies will vary between countries depending on the
epidemiology of the disease, transmission, patterns of drug resistance and political and
economic contexts.
DRUG RESISTANCE
The rapid spread of antimalarial drug resistance over the past few decades has required
more intensive monitoring of drug resistance to ensure proper management of clinical
cases and early detection of changing patterns of resistance so that national malaria
treatment policies can be revised where necessary. Surveillance of therapeutic efficacy
over time is an essential component of malaria control. Recent efforts to scale-up malaria
control in endemic countries throughout the world including increased support for
commodities and health systems, as well as the proposed price subsidy on artemisininbased combination therapies (ACTs) is resulting in greater access to and a vastly increased
use of antimalarial medicines, in particular ACTs. This is leading to a much higher degree
of drug pressure on the parasite which will almost certainly increase the likelihood of
selecting for resistant parasite genotypes. There are currently no effective alternatives to
artemisinins for the treatment of P. falciparum malaria either on the market or towards the
end of the development pipeline.
The parasite's resistance to medicines continues to undermine malaria control efforts.
WHO has therefore called for continuous monitoring of the efficacy of recently
implemented ACTs, and countries are being assisted in strengthening their drug resistance
surveillance systems. In order to preserve the efficacy of artemisinins as an essential
component of life-saving ACTs, WHO has called for a ban on the use of oral artemisinin
monotherapies, at various levels, including manufacturers, international drug suppliers,
national health authorities and international aid and funding agencies involved in the
funding of essential antimalarial medicines.
PREVENTION: VECTOR CONTROL AND INTERMITTENT
PREVENTIVE THERAPY IN PREGNANT WOMEN
The main objective of malaria vector control is to significantly reduce both the number
and rate of parasite infection and clinical malaria by controlling the malaria-bearing
mosquito and thereby reducing and/or interrupting transmission. There are two main
operational interventions for malaria vector control currently available: Indoor Residual
Spraying of long-acting insecticide (IRS) and Long-Lasting Insecticidal Nets (LLINs).
These core interventions can be locally complemented by other methods (e.g. larval
control or environmental management) in the context of Integrated Vector Management
(IVM). Effective and sustained implementation of malaria vector control interventions
(IRS or LLINs) requires clear political commitment and engagement from national
authorities as well as long-term support from funding partners.
Pregnant women are at high risk of malaria. Non-immune pregnant women risk both acute
and severe clinical disease, resulting in up to 60% fetal loss and over 10% maternal deaths,
including 50% mortality for severe disease. Semi-immune pregnant women with malaria
infection risk severe anaemia and impaired fetal growth, even if they show no signs of
acute clinical disease. An estimated 10 000 of these women and 200 000 of their infants
die annually as a result of malaria infection during pregnancy. HIV-infected pregnant
women are at increased risk. WHO recommends that all endemic countries provide a
package of interventions for prevention and management of malaria in pregnancy,
consisting of (1) diagnosis and treatment for all episodes of clinical disease and anaemia
and (2) insecticide-treated nets for night-time prevention of mosquito bites and infection.
In highly endemic falciparum malaria areas, this should be complemented by (3)
intermittent preventive treatment with sulfadoxine–pyrimethamine (IPT/SP) to clear the
placenta periodically of parasites.
INSECTICIDE RESISTANCE
In spite of increased national and international efforts to scale up cost-effective malaria
vector control interventions and maximize the protection of populations at risk, significant
challenges continue to threaten these objectives and the sustainability of achievements.
Challenges include increasing resistance of vector mosquitoes to insecticides, the
behaviour and ecology of local malaria vectors – which often change as a result of vector
control interventions -- and the diminishing number of available insecticides that can be
used against malaria vectors (adulticides).
There are currently no alternatives to DDT and pyrethroids and the development of new
insecticides will be an expensive long-term endeavour. Therefore, immediate sound vector
resistance management practices are required to assure the continued utility of the
currently available insecticides. At present there is only limited evidence of the impact of
various resistance mechanisms on the efficacy of vector control interventions, whether
they are implemented singly or in combination.
Recent evidence from Africa indicates that pyrethroid and DDT resistance is more
widespread than anticipated. It is believed that the same level of resistance will have a
more detrimental impact on the efficacy of IRS than on that of LLINs, but evidence for
this is very limited. Networks for vector resistance monitoring still need greater
strengthening in order to make resistance detection a routine operational feature of
national programmes, particularly in countries in Africa and the Eastern Mediterranean
region. Regional level databases feeding into a global database accessible by governments,
scientists and policy-makers would greatly assist in the rational use and deployment of
vector control interventions.
SOCIOECONOMIC IMPACT
Malaria causes an average loss of 1.3% annual economic growth in countries with intense
transmission. When compounded over the years, this loss has lead to substantial
differences in GDP between countries with and without malaria. Malaria traps families
and communities in a downward spiral of poverty, disproportionately affecting
marginalized populations and poor people who cannot afford treatment or who have
limited access to health care. Malaria’s direct costs include a combination of personal and
public expenditures on both prevention and treatment of disease. In some countries with a
very heavy malaria burden, the disease may account for as much as 40% of public health
expenditure, 30-50% of inpatient admissions and up to 60% of outpatient visits. Malaria
has lifelong effects through increased poverty, impaired learning and decreases attendance
in schools and the workplace.
For more information contact:
WHO Media centre
Telephone: +41 22 791 2222
E-mail: [email protected]
http://pathmicro.med.sc.edu/mycology/mycology-1.htm
MYCOLOGY - CHAPTER ONE
INTRODUCTION TO MYCOLOGY
INTRODUCTION
A. CLASSIFICATION
Figure 1.
Chaetomium globosum
spores. Chaetomium is an
ascomycete, and in most
species the spores are lemonshaped, with a single germ
pore
© Dennis Kunkel Microscopy, Inc. Used
with permission
Fungi are eukaryotic organisms that do not contain chlorophyll, but have cell walls,
filamentous structures, and produce spores. These organisms grow as
saprophytes and decompose dead organic matter. There are between 100,000 to
200,000 species depending on how they are classified. About 300 species are
presently known to be pathogenic for man.
There are five kingdoms of living things. The fungi are in the Kingdom Fungi.
KINGDOM
Figure 2.
Bracket fungus basidiocarp
(fruiting body) lower surface
showing generative hyphae
(gill, spore producing).
Reproductive spores are
dispersed through pores in
the surface of the brackets.
© Dennis Kunkel Microscopy, Inc. Used
with permission
Figure 3.
Mucor spp. fruiting structure
with spores. The fruiting
structure (condiophore) has
matured and its outer
membrane is disintegrating
allowing the spores (conidia)
to be released. Mucor is a
common fungus found in
many environments. It is a
Zygomycetes fungus which
may be allergenic and is often
found as saprobes in soils,
dead plant material (such as
hay), horse dung, and fruits. It
is an opportunistic pathogen
and may cause mucorosis in
immuno-compromised
individuals. The sites of
infections are the lung, nasal
sinus, brain, eye, and skin.
Few species have been
isolated from cases of
zygomycosis, but the term
mucormycosis has often been
used. Zygomycosis includes
mucocutaneous and
rhinocerebral infections, as
well as renal infections,
gastritis, and pulmonary
CHARACTERISTIC
EXAMPLE
Monera
Prokaryocyte
Bacteria
Actinomycetes
Protista
Eukaryocyte
Protozoa
Fungi
Eukaryocyte *
Fungi
Plantae
Eukaryocyte
Plants, Moss
Animalia
Eukaryocyte *
Arthropods
Mammals
Man
*This common characteristic is responsible for the therapeutic dilemma in antimycotic therapy.
The taxonomy of the Kingdom Fungi is evolving and is controversial. Formerly
based on gross and light microscopic morphology, studies of ultra structure,
biochemistry and molecular biology provide new evidence on which to base
taxonomic positions. Medically important fungi are in four phyla:
1. Ascomycota - Sexual reproduction in a sack called an ascus with the production
of ascopspores (figure 1).
2. Basidiomycota -Sexual reproduction in a sack called a basidium with the
production of basidiospores (figure 2).
3. Zygomycota - sexual reproduction by gametes and asexual reproduction with
the formation of zygospores (figure 3).
4. Mitosporic Fungi (Fungi Imperfecti) - no recognizable form of sexual
reproduction. Includes most pathogenic fungi.
B. MORPHOLOGY
Pathogenic fungi can exist as yeasts or as hyphae (figure 4). A mass of hyphae is
called mycelia. Yeasts are unicellular organisms and mycelia are multicellular
filamentous structures, constituted by tubular cells with cell walls. The yeasts
reproduce by budding. The mycelial forms branch and the pattern of branching is
an aid to the morphological identification. If the mycelia do not have SEPTA, they
are called coenocytic (nonseptate). The terms "hypha" and "mycelium" are
frequently used interchangeably. Some fungi occur in both the yeast and mycelial
forms. These are called dimorphic fungi.
Figure 4.
Candida albicans - yeast and
hyphae stages. A yeast-like
fungus commonly occuring on
human skin, in the upper
respiratory, alimentary &
female genital tracts. This
fungus has a dimorphic life
cycle with yeast and hyphal
stages. The yeast produces
hyphae (strands) and
pseudohyphae. The
pseudohyphae can give rise
to yeast cells by apical or
lateral budding. Causes
candidiasis which includes
thrush (an infection of the
mouth & vagina) and vulvovaginitis. © Dennis Kunkel
Microscopy, Inc. Used with permission
Dimorphic fungi
The dimorphic fungi have two forms (figure 5):
1. YEAST - (parasitic or pathogenic form). This is the form usually seen in tissue,
in exudates, or if cultured in an incubator at 37 degrees C.
2. MYCELIUM - (saprophytic form). The form observed in nature or when cultured
at 25 degrees C. Conversion to the yeast form appears to be essential for
pathogenicity. In the dimorphic fungi. Fungi are identified by several morphological
or biochemical characteristics, including the appearance of their fruiting bodies.
The asexual spores may be large (macroconidia, chlamydospores) or small
(microconidia, blastospores, arthroconidia).
There are four types of mycotic diseases:
1. Hypersensitivity - an allergic reaction to molds and spores.
2. Mycotoxicoses - poisoning of man and animals by feeds and food products
contaminated by fungi which produce toxins from the grain substrate.
3. Mycetismus - the ingestion of toxin (mushroom poisoning).
4. Infection
We shall be concerned only with the last type: pathogenic fungi that cause
infections. Most common pathogenic fungi do not produce toxins but they do show
Growth and Division of
physiologic modifications during a parasitic infection (e.g., increased metabolic
Budding Yeast
(Saccharomyces cerevisiae) rate, modified metabolic pathways and modified cell wall structure). The
mechanisms that cause these modifications as well as their significance as a
High Resolution
pathogenic mechanism are just being described. Most pathogenic fungi are also
Low resolution
thermotolerant, and can resist the effects of the active oxygen radicals released
© Philip Meaden
Heriot-Watt University
during the respiratory burst of phagocytes. Thus, fungi are able to withstand many
Edinburgh, Scotland and The
host defenses. Fungi are ubiquitous in nature and most people are exposed to
MicrobeLibrary
them. The establishment of a mycotic infection usually depends on the size of the
inoculum and on the resistance of the host. The severity of the infection seems to
depend mostly on the immunologic status of the host. Thus, the demonstration of
fungi, for example, in blood drawn from an intravenous catheter can correspond to
VIDEO
colonization of the catheter, to transient fungemia (i.e., dissemination of fungi
through the blood stream), or to a true infection. The physician must decide which
is the clinical status of the patient based on clinical parameters, general status of
the patient, laboratory results, etc. The decision is not trivial, since treatment of
systemic fungal infections requires the aggressive use of drugs with considerable
toxicity. Most mycotic agents are soil saprophytes and mycotic diseases are
generally not communicable from person-to-person (occasional exceptions:
Candida and some dermatophytes). Outbreaks of disease may occur, but these
are due to a common environmental exposure, not communicability. Most of the
fungi which cause systemic infections have a peculiar, characteristic ecologic
niche in nature. This habitat is specific for several fungi which will be discussed
later. In this environment, the normally saprophytic organisms proliferate and
develop. This habitat is also the source of fungal elements and/or spores, where
man and animals, incidental hosts, are exposed to the infectious particles. It is
important to be aware of these associations to diagnose mycotic diseases. The
physician must be able to elicit a complete history from the patient including
occupation, avocation and travel history. This information is frequently required to
raise, or confirm, your differential diagnosis. The incidence of mycotic infections is
currently increasing dramatically, due to an increased population of susceptibles.
Examples are patients with AIDS, patients on immunosuppressive therapy, and
the use of more invasive diagnostic and surgical procedures (prosthetic implants).
Fungal diseases are non-contagious and non-reportable diseases in the national
public health statistics. However, in South Carolina most of the important mycotic
(fungal) diseases were notifiable to the public health authorities until 1994.
A
Candida albicans is a
dimorphic fungus in that it
grows as a unicellular yeast
under some environmental
conditions and as a
filamentous fungus under
other conditions.
Budding yeast cells. C.
albicans was grown at 37°C
with aeration for 3 h in yeastpeptone-dextrose (YPD)
medium. In this image,
unstained cells are magnified
x400. The image was taken
with phase- contrast
microscopy.
B
Budding yeast with septum. The septum has formed between the daughter bud and the mother
cell, but separation of the two has not occurred. This image is from a culture of cells grown at 37°
C for 3 h in YPD medium. The unstained cell is magnified x1,000 using phase- contrast
microscopy.
C
Candida albicans mother and daughter cells. Cells were grown under conditions that induced
hypha formation for 30 min. The daughter cell is on the right; the mother cell is on the left. The
daughter cell has not reached a threshold volume and therefore has not yet formed a hypha. The
mother cell has passed the threshold volume and has started forming a germ tube which will
become a hypha. The germ tube seen here is 6 min old. A septum between the germ tube and the
mother cell has not yet formed. The unstained cells are magnified x1,000 using phase-contrast
microscopy.
© Phillip Stafford
Dartmouth Medical School
Hanover, New Hampshire and
The MicrobeLibrary
Figure 5 A-E
D
C. albicans cell at 3 h. Three hours after the appearance of the germ tube, the hypha has septa. A
new germ tube at the distal pole of the
cell is also evident at this time. The unstained cells are magnified x1,000 using phase-contrast
microscopy.
E
C. albicans hyphal cells at 5 h. After 5 h in hypha-inducing medium, many hyphae are evident.
Clumping of the hyphae is
also apparent, and hyphae are beginning to form hypha blastospores, which are new budding
cells.
C. DIAGNOSIS
1. Skin scrapings suspected to contain dermatophytes or pus from a lesion can be
mounted in KOH on a slide and examined directly under the microscope.
2. Skin testing (dermal hypersensitivity) used to be popular as a diagnostic tool,
but this use is now discouraged because the skin test may interfere with
serological studies, by causing false positive results. It may still be used to
evaluate the patient's immunity, as well as a population exposure index in
epidemiological studies.
3. Serology may be helpful when it is applied to a specific fungal disease; there
are no screening antigens for 'fungi' in general. Because fungi are poor antigens,
the efficacy of serology varies with different fungal infections. The serologic tests
will be discussed under each mycosis. The most common serological tests for
fungi are based on latex agglutination, double immunodiffusion, complement
Figure 6 fixation and enzyme immunoassays. While latex agglutination may favor the
A Sabouraud’s dextrose agar detection of IgM antibodies, double immunodiffusion and complement fixation
plate culture growing a
usually detect IgG antibodies. Some EIA tests are being developed to detect both
Mexican isolate of T. rubrum IgG and IgM antibodies. There are some tests which can detect specific fungal
var. rodhaini. Dermatophytic
antigens, but they are just coming into general use.
members of the genus
Trichophyton are some of the
leading causes of hair, skin,
and nail infections in humans,
known as dermatophytoses.
The genus includes
anthropophilic, zoophilic, and
geophilic species
CDC/Dr. Libero Ajello
4. Direct fluorescent microscopy may be used for identification, even on nonviable cultures or on fixed tissue sections. The reagents for this test are difficult to
obtain.
5. Biopsy and histopathology. A biopsy may be very useful for the identification
and as a source of the of tissue-invading fungi. Usually the Gomori methenamine
silver (GMS) stain is used to reveal the organisms which stain black against a
green background. The H&E stain does not always tint the organism, but it will
stain the inflammatory cells.
6. Culture. A definitive diagnosis requires a culture and identification. Pathogenic
fungi are usually grown on Sabouraud dextrose agar (figure 6). It has a slightly
acidic pH (~5.6); cyclohexamide, penicillin, streptomycin or other inhibitory
antibiotics are often added to prevent bacterial contamination and overgrowth.
Two cultures are inoculated and incubated separately at 25 degrees C and 37
degrees C to reveal dimorphism. The cultures are examined macroscopically and
microscopically. They are not considered negative for growth until after 4 weeks of
incubation.
MOLECULAR
STRUCTURE
Amphotericin B
Ketoconazole
Griseofulvin
5-fluorocytosine
D. TREATMENT
Mammalian cells do not contain the enzymes which will degrade the cell wall
polysaccharides of fungi. Therefore, these pathogens are difficult to eradicate by
the animal host defense mechanisms. Because mammals and fungi are both
eukaryotic, the cellular milieu is biochemically similar in both. The cell membranes
of all eukaryotic cells contain sterols; ergosterol in the fungal cell membrane and
cholesterol in the mammalian cell membrane. Thus, most substances which may
impair the invading fungus will usually have serious side effects on the host.
Although one of the first chemotherapeutic agents (oral iodides) was an antimycotic used in 1903, the further development of such agents has been left far
behind the development of anti-bacterial agents. The selective toxicity necessary
to inhibit the invading organism with minimal damage to the host has been difficult
to establish within eukaryotic cells.
The primary antifungal agents are:
Amphotericin B
A polyene antimycotic. It is usually the drug of choice for most systemic fungal
infections. It has a greater affinity for ergosterol in the cell membranes of fungi
than for the cholesterol in the host's cells; once bound to ergosterol, it causes
disruption of the cell membrane and death of the fungal cell. Amphotericin B is
usually administered intravenously (patient usually needs to be hospitalized), often
for 2-3 months. The drug is rather toxic; thrombo-phlebitis, nephrotoxicity, fever,
chills and anemia frequently occur during administration.
Azoles
The azoles (imidazoles and triazoles), including ketoconazole, fluconazole, and
itraconozole, are being used for muco-cutaneous candidiasis, dermatophytosis,
and for some systemic fungal infections. Fluconazole is presently essential for the
maintenance of AIDS patients with cryptococcosis. The general mechanism of
action of the azoles is the inhibition of ergosterol synthesis. Oral administration
and reduced toxicity are distinct advantages.
Griseofulvin
Griseofulvin is a very slow-acting drug which is used for severe skin and nail
infections. Its effect depends on its accumulation in the stratum corneum where it
is incorporated into the tissue and forms a barrier which stops further fungal
penetration and growth. It is administered orally. The exact mechanism of action is
unknown.
5-fluorocytosine
5-fluorocytosine (Flucytosine or 5-FC) inhibits RNA synthesis and has found its
main application in cryptococcosis (to be discussed later). It is administered orally.
E. CLINICAL CLASSIFICATION OF THE MYCOSES
Fungal diseases may be discussed in a variety of ways. The most practical
method for medical students is the clinical taxonomy which divides the fungi into:
a. Superficial mycoses
b. Subcutaneous mycoses
c. Systemic mycoses
MOLECULAR
STRUCTURE
Ergosterol
Figure 7.
Ringworm on the skin of the
neck due to Trichophyton
rubrum.
CDC/Lucille K. Georg
Return to the Mycology Section of Microbiology and Immunology On-line
This page copyright 2007, The Board of Trustees of the University of South Carolina
This page last changed on
Page maintained by Richard Hunt
Please report any problems to [email protected]
http://en.wikipedia.org/wiki/Microbial_metabolism
Microbial metabolism
From Wikipedia, the free encyclopedia
Jump to: navigation, search
Microbial metabolism is the means by which a microbe obtains the energy and
nutrients (e.g. carbon) it needs to live and reproduce. Microbes use many different
types of metabolic strategies and species can often be differentiated from each other
based on metabolic characteristics. The specific metabolic properties of a microbe are
the major factors in determining that microbe’s ecological niche, and often allow for
that microbe to be useful in industrial processes or responsible for biogeochemical
cycles.
Contents
[hide]
•
•
•
•
•
•
•
•
•
•
1 Types of microbial metabolism
2 Heterotrophic microbial metabolism
3 Fermentation
4 Special metabolic properties
o 4.1 Methylotrophy
o 4.2 Syntrophy
5 Anaerobic respiration
o 5.1 Denitrification
o 5.2 Sulfate reduction
o 5.3 Acetogenesis
o 5.4 Inorganic electron acceptors
o 5.5 Organic terminal electron acceptors
6 Chemolithotrophy
o 6.1 Hydrogen oxidation
o 6.2 Sulfur oxidation
2+
o 6.3 Ferrous iron (Fe ) oxidation
o 6.4 Nitrification
o 6.5 Anammox
7 Phototrophy
8 Nitrogen fixation
9 See also
10 References
Types of microbial metabolism
Flow chart to determine the metabolic characteristics of microorganisms
Main article: Primary nutritional groups
All microbial metabolism can be arranged according to three principles:
1. How the organism obtains carbon for synthesising cell mass:
•
•
•
autotrophic – carbon is obtained from carbon dioxide (CO2)
heterotrophic – carbon is obtained from organic compounds
mixotrophic – carbon is obtained from both organic compounds and by fixing
carbon dioxide
2. How the organism obtains reducing equivalents used either in energy conservation
or in biosynthetic reactions:
•
•
lithotrophic – reducing equivalents are obtained from inorganic compounds
organotrophic – reducing equivalents are obtained from organic compounds
3. How the organism obtains energy for living and growing:
•
•
chemotrophic – energy is obtained from external chemical compounds
phototrophic – energy is obtained from light
In practice, these terms are almost freely combined. Typical examples are as follows:
•
•
•
•
•
chemolithoautotrophs obtain energy from the oxidation of inorganic
compounds and carbon from the fixation of carbon dioxide. Examples:
Nitrifying bacteria, Sulfur-oxidising bacteria, Iron-oxidising bacteria,
Knallgas-bacteria
photolithoautotrophs obtain energy from light and carbon from the fixation
of carbon dioxide, using reducing equivalents from inorganic compounds.
Examples: Cyanobacteria (water as reducing equivalent donor),
Chlorobiaceae, Chromaticaceae (hydrogen sulfide as reducing equivalent
donor), Chloroflexus (hydrogen as reducing equivalent donor)
chemolithoheterotrophs obtain energy from the oxidation of inorganic
compounds, but can not fix carbon dioxide. Examples: some Nitrobacter spp.,
Wolinella (with H2 as reducing equivalent donor), some Knallgas-bacteria
chemoorganoheterotrophs obtain energy, carbon and reducing equivalents
for biosynthetic reactions from organic compounds. Examples: most bacteria,
e. g. Escherichia coli, Bacillus spp., Actinobacteria
photoorganoheterotrophs obtain energy from light, carbon and reducing
equivalents for biosynthetic reactions from organic compounds. Some species
are strictly heterotrophic, many others can also fix carbon dioxide and are
mixotrophic. Examples: Rhodobacter, Rhodopseudomonas, Rhodospirillum,
Rhodomicrobium, Rhodocyclus, Heliobacterium, Chloroflexus (alternatively to
photolithoautotrophy with hydrogen)
[edit] Heterotrophic microbial metabolism
Most microbes are heterotrophic (more precisely chemoorganoheterotrophic), using
organic compounds as both carbon and energy sources. Heterotrophic microbes live
off of nutrients that they scavenge from living hosts (as commensals or parasites) or
find in dead organic matter of all kind (saprophages). Microbial metabolism is the
main contribution for the bodily decay of all organisms after death. Many eukaryotic
microorganisms are heterotrophic by predation or parasitism, properties also found in
some bacteria such as Bdellovibrio (an intracellular parasite of other bacteria, causing
death of its victims) and Myxobacteria such as Myxococcus (predators of other
bacteria which are killed and lysed by cooperating swarms of many single cells of
Myxobacteria). Most pathogenic bacteria can be viewed as heterotrophic parasites of
humans or whatever other eukaryotic species they affect. Heterotrophic microbes are
extremely abundant in nature and are responsible for the breakdown of large organic
polymers such as cellulose, chitin or lignin which are generally indigestible to larger
animals. Generally, the breakdown of large polymers to carbon dioxide
(mineralization) requires several different organisms, with one breaking down the
polymer into its constituent monomers, one able to use the monomers and excreting
simpler waste compounds as by-products and one able to use the excreted wastes.
There are many variations on this theme, as different organisms are able to degrade
different polymers and secrete different waste products. Some organisms are even
able to degrade more recalcitrant compounds such as petroleum compounds or
pesticides, making them useful in bioremediation.
Biochemically, prokaryotic heterotrophic metabolism is much more versatile than that
of eukaryotic organisms, although many prokaryotes share the most basic metabolic
models with eukaryotes, e. g. using glycolysis (also called EMP pathway) for sugar
metabolism and the citric acid cycle to degrade acetate, producing energy in the form
of ATP and reducing power in the form of NADH or quinols. These basic pathways
are well conserved because they are also involved in biosynthesis of many conserved
building blocks needed for cell growth (sometimes in reverse direction). However,
many bacteria and archaea utilise alternative metabolic pathways other than glycolysis
and the citric acid cycle. A well studied example is sugar metabolism via the ketodeoxy-phosphogluconate pathway (also called ED pathway) in Pseudomonas] instead
of the glycolytic pathway. Moreover, there is even a third alternative sugar-catabolic
pathway used by some bacteria, the pentose-phosphate pathway. This metabolic
diversity and ability of prokaryotes to use a huge variety of organic compounds arises
from the much deeper evolutionary history and diversity of prokaryotes, as compared
to eukaryotes. It is also noteworthy that the mitochondrion, the small membranebound intracellular organelle that is the site of eukaryotic energy metabolism, arose
from the endosymbiosis of a bacterium related to obligate intracellular Rickettsia, and
also to plant-associated Rhizobium or Agrobacterium. Therefore it is not surprising
that all mitrochondriate eukaryotes share metabolic properties with these
Proteobacteria. Most microbes respire (use an electron transport chain), although
oxygen is not the only terminal electron acceptor that may be used. As discussed
below, the use of terminal electron acceptors other than oxygen has important
biogeochemical consequences.
[edit] Fermentation
Main article: Fermentation (biochemistry)
Fermentation is a specific type of heterotrophic metabolism that uses organic carbon
instead of oxygen as a terminal electron acceptor. This means that these organisms do
not use an electron transport chain to oxidize NADH to NAD+ and therefore must
have an alternative method of using this reducing power and maintaining a supply of
NAD+ for the proper functioning of normal metabolic pathways (e.g. glycolysis). As
oxygen is not required, fermentative organisms are anaerobic. Many organisms can
use fermentation under anaerobic conditions and respiration when oxygen is not
present. These organisms are facultative anaerobes. To avoid the overproduction of
NADH obligately fermentative organisms usually do not have a complete citric acid
cycle. Instead of using an ATPase as in respiration, ATP in fermentative organisms is
produced by substrate-level phosphorylation where a phosphate group is transferred
from a high-energy organic compound to ADP to form ATP. As a result of the need to
produce high energy phosphate-containing organic compounds (generally in the form
of CoA-esters) fermentative organisms use NADH and other cofactors to produce
many different reduced metabolic by-products, often including hydrogen gas (H2).
These reduced organic compounds are generally small organic acids and alcohols
derived from pyruvate, the end product of glycolysis. Examples include ethanol,
acetate, lactate and butyrate. Fermentative organisms are very important industrially
and are used to make many different types of food products. The different metabolic
end products produced by each specific bacterial species are responsible for the
different tastes and properties of each food.
Not all fermentative organisms use substrate-level phosphorylation. Instead, some
organisms are able to couple the oxidation of low-energy organic compounds directly
to the formation of a proton (or sodium) motive force and therefore ATP synthesis.
Examples of these unusual forms of fermentation include succinate fermentation by
Propionigenium modestum and oxalate fermentation by Oxalobacter formigenes.
These reactions are extremely low energy-yielding. Humans and other higher animals
also use fermentation to use excess NADH to produce lactate, although this is not the
major form of metabolism as it is in fermentative microorganisms.
[edit] Special metabolic properties
[edit] Methylotrophy
Methylotrophy refers to the ability of an organism to use C1-compounds as energy
sources. These compounds include methanol, methyl amines, formaldehyde and
formate. Several other, less common substrates may also be used for metabolism, all
of which lack carbon-carbon bonds. Examples of methylotrophs include the bacteria
Methylomonas and Methylobacter. Methanotrophs are a specific type of methylotroph
that are also able to use methane (CH4) as a carbon source by oxidizing it sequentially
to methanol (CH3OH), formaldehyde (CH2O), formate (HCOO-) and finally carbon
dioxide CO2 initially using the important enzyme methane monooxygenase. As
oxygen is required for this process, all (conventional) methanotrophs are obligate
aerobes. Reducing power in the form of quinones and NADH is produced during
these oxidations to produce a proton motive force and therefore ATP generation.
Methylotrophs and methanotrophs are not considered as autotrophic, because they are
able to incorporate some of the oxidized methane (or other metabolites) into cellular
carbon before it is completely oxidised to CO2 (at the level of formaldehyde), using
either the serine pathway (Methylosinus, Methylocystis) or the ribulose
monophosphate pathway (Methylococcus), depending on the species of methylotroph.
In addition to aerobic methylotrophy, methane can also be oxidized anaerobically.
This occurs by a consortium of sulfate-reducing bacteria and relatives of
methanogenic Archaea working syntrophically (see below). Little is currently known
about the biochemistry and ecology of this process.
Methanogenesis is the biological production of methane. It is carried out by
methanogens, strictly anaerobic archaea such as Methanococcus,
Methanocaldococcus, Methanobacterium, Methanothermus, Methanosarcina,
Methanosaeta and Methanopyrus. The biochemistry of methanogenesis is unique in
nature in its use of a number of unusual cofactors to sequentially reduce
methanogenic substrates to methane. These cofactors are responsible (among other
things) for the establishment of a proton gradient across the outer membrane thereby
driving ATP synthesis. Several different types of methanogenesis occurs, which differ
in the starting compounds oxidized. Some methanogens reduce carbon dioxide (CO2)
to methane (CH4) using electrons (most often) from hydrogen gas (H2)
chemolithoautotrophically. These methanogens can often be found in environments
containing fermentative organisms. The tight association of methanogens and
fermentative bacteria can be considered to be syntrophic (see below) because the
methanogens, which rely on the fermentors for hydrogen, relieve feedback inhibition
of the fermentors by the build-up of excess hydrogen that would otherwise inhibit
their growth. This type of syntrophic relationship is specifically known as interspecies
hydrogen transfer. A second group of methanogens use methanol (CH3OH) as a
substrate for methanogenesis. These are chemoorganotrophic, but still autotrophic in
using CO2 as only carbon source. The biochemistry of this process is quite different
from that of the carbon dioxide reducing methanogens. Lastly, a third group of
methanogens produce both methane and carbon dioxide from acetate (CH3COO-) with
the acetate being literally split between the two carbons. These acetate-cleaving
organisms are the only chemoorganoheterotrophic methanogens. All
autotrophicmethanogens use a variation of the acetyl-CoA pathway to fix CO2 and
obtain cellular carbon.
[edit] Syntrophy
Syntrophy, in the context of microbial metabolism, refers to the pairing of multiple
species to achieve a chemical reaction that, on its own, would be energetically
unfavorable. The best studied example of this process is the oxidation of fermentative
end products (such as acetate, ethanol and butyrate) by organisms such as
Syntrophomonas. Alone, the oxidation of butyrate to acetate and hydrogen gas is
energetically unfavorable. However, when a hydrogenotrophic (hydrogen using)
methanogen is present the use of the hydrogen gas will significantly lower the
concentration of hydrogen (down to 10-5 atm) and thereby shift the equilibrium of the
butyrate oxidation reaction under standard conditions (ΔGº’) to non-standard
conditions (ΔG’). Because the concentration of one product is lowered, the reaction is
"pulled" towards the products and shifted towards net energetically favorable
conditions (for butyrate oxidation: ΔGº’= +48.2 kJ/mol, but ΔG' = -8.9 kJ/mol at 10-5
atm hydrogen and even lower if also the initially produced acetate is further
metabolised by methanogens). Conversely, the available free energy from
methanogenesis is lowered from ΔGº’= -131 kJ/mol under standard conditions to ΔG'
= -17 kJ/mol at 10-5 atm hydrogen. This is an example of intraspecies hydrogen
transfer. In this way, low energy-yielding carbon sources can be used by a consortium
of organisms to achieve further degradation and eventual mineralization of these
compounds. These reactions help prevent the excess sequestration of carbon over
geologic time scales, releasing it back to the biosphere in usable forms such as
methane and CO2.
[edit] Anaerobic respiration
In aerobic organisms, oxygen is used as a terminal electron acceptor during
respiration. This is largely because oxygen has a very low reduction potential
allowing for aerobic organisms to utilize their electron transport systems most
efficiently. In anaerobic organisms, terminal electron acceptors other than oxygen are
used. These inorganic compounds have a higher reduction potential compared to
oxygen, meaning that respiration is less efficient in these organisms generally leading
to slower growth rates compared to aerobes. Many facultative anaerobes can use
either oxygen or alternative terminal electron acceptors for respiration depending on
the environmental conditions. Most respiring anaerobes are heterotrophs, although
some do live autotrophically. All of the processes described below are dissimilative,
meaning that they are used during energy production and not to provide nutrients for
the cell (assimilative). Assimilative pathways for many forms of anaerobic respiration
are also known.
[edit] Denitrification
Main article: Denitrification
Denitrification is the utilization of nitrate (NO3-) as a terminal electron acceptor. It is a
widespread process that is used by many members of the Proteobacteria. Many
facultative anaerobes use denitrification because nitrate, like oxygen, has a low
reduction potential. Many denitrifying bacteria can also use ferric iron (Fe3+) and
some organic electron acceptors. Denitrification involves the stepwise reduction of
nitrate to nitrite (NO2-), nitric oxide (NO), nitrous oxide (N2O) and dinitrogen (N2) by
the enzymes nitrate reductase, nitrite reductase, nitric oxide reductase and nitrous
oxide reductase, respectively. Protons are transported across the membrane by the
initial NADH reductase, quinones and nitrous oxide reductase to produce the
electrochemical gradient critical for respiration. Some organisms (e.g. E. coli) only
produce nitrate reductase and therefore can accomplish only the first reduction
leading to the accumulation of nitrite. Others (e.g. Paracoccus denitrificans or
Pseudomonas stutzeri) reduce nitrate completely. Complete denitrification is an
environmentally significant process because some intermediates of denitrification
(nitric oxide and nitrous oxide) are important greenhouse gases that react with
sunlight and ozone to produce nitric acid, a component of acid rain. Denitrification is
also important in biological wastewater treatment where it is used to reduce the
amount of nitrogen released into the environment thereby reducing eutrophication.
[edit] Sulfate reduction
Sulfate reduction is a relatively energetically poor process used by many Gram
negative bacteria found within the δ-Proteobacteria, Gram positive organisms relating
to Desulfotomaculum or the archaeon Archaeoglobus. Hydrogen sulfide (H2S) is
produced as a metabolic end product. Many sulfate reducers are heterotrophic, using
carbon compounds such as lactate and pyruvate (among many others) as electron
donors while others are autotrophic, using hydrogen gas (H2) as an electron donor.
Some unusual autotrophic sulfate reducing-bacteria can use phosphite (HPO3-) as an
electron donor (e.g. Desulfotignum phosphitoxidans) or are capable of sulfur
disproportionation (splitting one compound into two different compounds, in this case
an electron donor and an electron acceptor) using thiosulfate (S2O32- e.g.
Desulfovibrio sulfodismutans). All sulfate reducing-organisms are strict anaerobes.
Because sulfate is energetically stable before it can be metabolized it must first be
activated by adenylation to form APS (adenosine 5’-phosphosulfate) thereby
consuming ATP. The APS is then reduced by the enzyme APS reductase to form
sulfite (SO32- and AMP. In organisms that use carbon compounds as electron donors,
the ATP consumed is accounted for by fermentation of the carbon substrate. The
hydrogen produced during fermentation is actually what drives respiration during
sulfate reduction. Electrons are passed from the hydrogenase enzyme eventually to the
APS reductase, which along with sulfite reductase completes the reduction of sulfate
to hydrogen sulfide. A proton motive force is established due to a fact that the
hydrogenase, which converts H2 to 2H+ is located in the periplasm (or extracellularly
in Gram positive bacteria).
[edit] Acetogenesis
Main article: Acetogenesis
Acetogenesis is a type of microbial metabolism that uses hydrogen (H2) as an electron
donor and carbon dioxide (CO2) as an electron acceptor to produce acetate. This is
similar to methanogenesis (see above) in having the same electron donors and
acceptors. Bacteria that can autotrophically synthesize acetate are called
homoacetogens. Carbon dioxide reduction in all homoacetogens occurs by the acetylCoA pathway. This pathway is also used for carbon fixation by autotrophic sulfatereducing bacteria and hydrogenotrophic methanogens. Often homoacetogens can also
be fermentative, using the hydrogen and carbon dioxide produced as a result of
fermentation to produce acetate, which is secreted as an end product.
[edit] Inorganic electron acceptors
Ferric iron (Fe3+) is a widespread anaerobic terminal electron acceptor both for
autotrophic and heterotrophic organisms. Electron flow in these organisms is similar
to those in electron transport ending in oxygen or nitrate except that in ferric ironreducing organisms the final enzyme in this system is a ferric iron reductase. Model
organisms include Shewanella putrifaciens and Geobacter metallireducens. Since
some ferric iron-reducing bacteria (e.g. G. metallireducens) can use toxic
hydrocarbons such as toluene as a carbon source there is significant interest in using
these organisms as bioremediation agents in ferric iron-rich contaminated aquifers.
Although Ferric iron is the most prevalent inorganic electron acceptor, a number of
organisms (including the iron-reducing bacteria mentioned above) can use other
inorganic ions in anaerobic respiration. While these processes may often be less
significant ecologically, they are of considerable interest for bioremediation,
especially when heavy metals or radionuclides are used as electron acceptors.
Examples include:
•
•
•
•
Manganic ion (Mn4+) reduction to manganous ion (Mn2+)
Selenate (SeO42-) reduction to selenite (SeO32-) and selenite reduction to
inorganic selenium (Se0)
Arsenate (AsO43-) reduction to arsenite (AsO33-)
Uranyl ion ion (UO22+) reduction to uranium dioxide (UO2)
[edit] Organic terminal electron acceptors
An number of organisms, instead of using inorganic compounds as terminal electron
acceptors are able to use organic compounds to accept electrons from respiration.
Examples include:
•
•
•
•
Fumarate reduction to succinate
Trimethylamine N-oxide (TMAO) reduction to trimethylamine (TMA)
Dimethyl sulfoxide (DMSO) reduction to Dimethyl sulfide (DMS)
Reductive dechlorination
TMAO is a chemical commonly produced by fish, and when reduced to TMA
produces a strong odor. DMSO is a common marine and freshwater chemical which is
also odiferous when reduced to DMS. Reductive dechlorination is the process by
which chlorinated organic compounds are reduced to form their non-chlorinated
endproducts. As chlorinated organic compounds are often important (and difficult to
degrade) environmental polutants, reductive dechlorination is an important process in
bioremediation.
[edit] Chemolithotrophy
Chemolithotrophy is a type of metabolism where energy is obtained from the
oxidation of inorganic compounds. Most chemolithotrophic organisms are also
autotrophic. There are two major objectives to chemolithotrophy: the generation of
energy (ATP) and the generation of reducing power (NADH).
[edit] Hydrogen oxidation
Many organisms are capable of using hydrogen (H2) as a source of energy. While
several mechanisms of anaerobic hydrogen oxidation have been mentioned previously
(e.g. sulfate reducing- and acetogenic bacteria) hydrogen can also be used as an
energy source aerobically. In these organisms hydrogen is oxidized by a membranebound hydrogenase causing proton pumping via electron transfer to various quinones
and cytochromes. In many organisms, a second cytoplasmic hydrogenase is used to
generate reducing power in the form of NADH, which is subsequently used to fix
carbon dioxide via the Calvin cycle. Hydrogen oxidizing organisms, such as
Cupriavidus necator (formerly Ralstonia eutropha)--Louegger (talk) 16:11, 3
February 2008 (UTC), often inhabit oxic-anoxic interfaces in nature to take advantage
of the hydrogen produced by anaerobic fermentative organisms while still maintaining
a supply of oxygen.
[edit] Sulfur oxidation
Sulfur oxidation involves the oxidation of reduced sulfur compounds (such as sulfide
(H2S), inorganic sulfur (S0) and thiosulfate (S2O22-) ) to form sulfuric acid (H2SO4). A
classic example of a sulfur oxidizing bacterium is Beggiatoa, a microbe originally
described by Sergei Winogradsky, one of the founders of microbiology. Generally,
the oxidation of sulfide occurs in stages, with inorganic sulfur being stored either
inside or outside of the cell until needed. This two step process occurs because
energetically sulfide is a better electron donor than inorganic sulfur or thiosulfate,
allowing for a greater number of protons to be translocated across the membrane.
Sulfur oxidizing organisms generate reducing power for carbon dioxide fixation via
the Calvin cycle using reverse electron flow, an energy-requiring process that pushes
the electrons against their thermodynamic gradient to produce NADH. Biochemically,
reduced sulfur compounds are converted to sulfite (SO32-) and subsequently converted
to sulfate by the enzyme sulfite oxidase. Some organisms, however, accomplish the
same oxidation using a reversal of the APS reductase system used by sulfate-reducing
bacteria (see above). In all cases the energy liberated is transferred to the electron
transport chain for ATP and NADH production. In addition to aerobic sulfur
oxidation, some organisms (e.g. Thiobacillus denitrificans) use nitrate (NO32-) as a
terminal electron acceptor and therefore grow anaerobically.
[edit] Ferrous iron (Fe2+) oxidation
Ferrous iron is a soluble form of iron that is stable at extremely low pHs or under
anaerobic conditions. Under aerobic, moderate pH conditions ferrous iron is oxidized
spontaneously to the ferric (Fe3+) form and is hydrolyzed abiotically to insoluble
ferric hydroxide (Fe(OH)3). There exists, therefore, three distinct types of ferrous
iron-oxidizing microbes. The first are acidophiles, such as the bacteria
Acidithiobacillus ferooxidans and Leptospirrillum ferrooxidans, as well as the
archaeon Ferroplasma. These microbes oxidize iron in environments that have a very
low pH and are important in acid mine drainage. The second type of microbes oxidize
ferrous iron at neutral pH along oxic-anoxic interfaces. Both these bacteria, such as
Gallionella ferruginea and Sphaerotilus natans, and the acidophilic iron oxidizingbacteria are aerobes. The third type of iron-oxidizing microbes are anaerobic
photosynthetic bacteria such as Chlorobium, which use ferrous iron to produce
NADH for autotrophic carbon dioxide fixation. Biochemically, aerobic iron reduction
is a very energetically poor process which therefore requires large amounts of iron to
be oxidized by the enzyme rusticyanin to facilitate the formation of proton motive
force. Like during sulfur oxidation reverse electron flow must be used to form the
NADH used for carbon dioxide fixation via the Calvin cycle.
[edit] Nitrification
Nitrification is the process by which ammonia (NH3) is converted to nitrate (NO3-).
Nitrification is actually the net result of two distinct processes: oxidation of ammonia
to nitrite (NO2-) by nitrosifying bacteria (e.g. Nitrosomonas) and oxidation of nitrite to
nitrate by the nitrite-oxidizing bacteria (e.g. Nitrobacter). Both of these processes are
extremely poor energetically leading to very slow growth rates for both types of
organisms. Biochemically, ammonia oxidation occurs by the stepwise oxidation of
ammonia to hydroxylamine (NH2OH) by the enzyme ammonia monooxygenase in the
cytoplasm followed by the oxidation of hydroxylamine to nitrite by the enzyme
hydroxylamine oxidoreductase in the periplasm.
Electron and proton cycling are very complex but as a net result only one proton is
translocated across the membrane per molecule of ammonia oxidized. Nitrite
reduction is much simpler, with nitrite being oxidized by the enzyme nitrite
oxidoreductase coupled to proton translocation by a very short electron transport
chain, again leading to very low growth rates for these organisms. In both ammoniaand nitrite-oxidation oxygen is required, meaning that both nitrosifying and nitriteoxidizing bacteria are aerobes. As in sulfur and iron oxidation, NADH for carbon
dioxide fixation using the Calvin cycle is generated by reverse electron flow, thereby
placing a further metabolic burden on an already energy-poor process.
[edit] Anammox
Anammox stands for anaerobic ammonia oxidation and is a relatively recently (late
1990’s) discovered process. It occurs in members of the Planctomycetes (e.g.
Candidatus Brocadia anammoxidans) and involves the coupling of ammonia
oxidation to nitrite reduction. As oxygen is not required for this process these
organisms are strict anaerobes. Amazingly, hydrazine (N2H4-rocket fuel) is produced
as an intermediate during anammox metabolism. To deal with the high toxicity of
hydrazine, anammox bacteria contain an hydrazine-containing intracellular organelle
called the anammoxasome surrounded by highly compact (and unusual) ladderane
lipid membrane. These lipids are unique in nature, as is the use of hydrazine as a
metabolic intermediate. Anammox organisms are autotrophs although the mechanism
for carbon dioxide fixation is unclear. Because of this property, these organisms have
been applied industrially to remove nitrogen in wastewater treatment processes.
Anammox has also been shown have widespread occurrence in anaerobic aquatic
systems and has been speculated to account for approximately 50% of nitrogen gas
production in some marine environments.
[edit] Phototrophy
Many microbes are capable of using light as a source of energy (phototrophy). Of
these, algae are particularly significant because they are oxygenic, using water as an
electron donor for electron transfer during photosynthesis.[citation needed] Phototrophic
bacteria are found in the phyla Cyanobacteria, Chlorobi, Proteobacteria, Chloroflexi
and Firmicutes.[1] Along with plants these microbes are responsible for all biological
generation of diatomic oxygen on Earth. Because chloroplasts were derived from a
lineage of the Cyanobacteria, the general principles of metabolism in these
endosymbionts can also be applied to chloroplasts. In addition to oxygenic
photosynthesis, many bacteria can also photosynthesize anaerobically, typically using
sulfide (H2S) as an electron donor to produce sulfate. Inorganic sulfur (S0), thiosulfate
(S2O32-) and ferrous iron (Fe2+) can also be used by some organisms.
Phylogenetically, all oxygenic photosynthetic bacteria are Cyanobacteria, while
anoxygenic photosynthetic bacteria belong to the purple bacteria (Proteobacteria),
Green sulfur bacteria (e.g. Chlorobium), Green non-sulfur bacteria (e.g. Chloroflexus)
or the heliobacteria (Low %G+C Gram positives). In addition to these organisms,
some microbes (e.g. the archaeon Halobacterium or the bacterium Roseobacter,
among others) can utilize light to produce energy using the enzyme
bacteriorhodopsin, a light-driven proton pump. This type of metabolism is not
considered to be photosynthesis but rather photophosphorylation, since it generates
energy, but does not directly fix carbon.
As befits the large diversity of photosynthetic bacteria, there exist many different
mechanisms by which light is converted into energy for metabolism. All
photosynthetic organisms locate their photosynthetic reaction centers within a
membrane, which may be invaginations of the cytoplasmic membrane (purple
bacteria), thylakoid membranes (Cyanobacteria), specialized antenna structures called
chlorosomes (Green sulfur and non-sulfur bacteria) or the cytoplasmic membrane
itself (heliobacteria). Different photosynthetic bacteria also contain different
photosynthetic pigments such as chlorophylls and carotenoids allowing them to take
advantage of different portions of the electromagnetic spectrum and thereby inhabit
different niches. Some groups of organisms contain more specialized light-harvesting
structures e.g. phycobilisomes in Cyanobacteria and chlorosomes in Green sulfur and
non-sulfur bacteria, allowing for increased light utilization efficiency.
Biochemically, anoxygenic photosynthesis is very different from oxygenic
photosynthesis. Cyanobacteria (and by extension chloroplasts) use the Z scheme of
electron flow in which electrons eventually are used to form NADH. Two different
reaction centers (photosystems) are used and proton motive force is generated both by
using cyclic electron flow and the quinone pool. In anoxygenic photosynthetic
bacteria electron flow is cyclic, with all electrons used in photosynthesis eventually
being transferred back to the single reaction center. A proton motive force is
generated using only the quinone pool. In heliobacteria, Green sulfur and non-sulfur
bacteria NADH is formed using the protein ferredoxin, an energetically favorable
reaction. In purple bacteria NADH is formed by reverse electron flow due to the
lower chemical potential of this reaction centre. In all cases, however, a proton motive
force is generated and used to drive ATP production via an ATPase.
Most photosynthetic microbes are autotrophic, fixing carbon dioxide via the Calvin
cycle. Some photosynthetic bacteria (e.g. Chloroflexus) are photoheterotrophs,
meaning that they use organic carbon compounds as a carbon source for growth.
Some photosynthetic organisms also fix nitrogen (see below).
[edit] Nitrogen fixation
Main article: Nitrogen fixation
Nitrogen is an element required for growth by all biological systems. While extremely
common (80% by volume) in the atmosphere, dinitrogen gas (N2) is generally
biologically inaccessible due to its high activation energy. Throughout all of nature,
only specialized bacteria are capable of nitrogen fixation, converting dinitrogen gas
into ammonia (NH3), which is easily assimilated by all organisms. These bacteria,
therefore are very important ecologically and are often essential for the survival entire
ecosystems. This is especially true in the ocean, where nitrogen-fixing cyanobacteria
are often the only sources or fixed nitrogen and in soils where specialized symbioses
exist between legumes and their nitrogen-fixing partners to provide the nitrogen
needed by these plants for growth.
Nitrogen fixation can be found distributed throughout nearly all bacterial lineages and
physiological classes but is not a universal property. Because the enzyme nitrogenase,
responsible for nitrogen fixation, is very sensitive to oxygen which will inhibit it
irreversibly, all nitrogen-fixing organisms must possess some mechanism to keep the
concentration of oxygen low. Examples include:
•
•
•
•
heterocyst formation (cyanobacteria e.g. Anabaena) where one cell does not
photosynthesize but instead fixed nitrogen for its neighbors which in turn
provide it with energy
root nodule symbioses (e.g. Rhizobium) with plants that supply oxygen to the
bacteria bound to molecules of leghaemoglobin
anaerobic lifestyle (e.g. Clostridium pasteurianum)
very fast metabolism (e.g. Azotobacter vinelandii)
The production and activity of nitrogenases is very highly regulated, both because
nitrogen fixation is an extremely energetically expensive process (16-24 ATP are used
per N2 fixed) and due to the extreme sensitivity of the nitrogenase to oxygen.
[edit] See also
•
lipophilic bacteria, a minority of bacteria with lipid metabolism
[edit] References
1. ^ D.A. Bryant & N.-U. Frigaard (Nov 2006). "Prokaryotic photosynthesis and
phototrophy illuminated". Trends Microbiol. 14 (11): 488.
doi:doi:10.1016/j.tim.2006.09.001.
•
Madigan, M. T., Martinko, J. M. "Brock Biology of Microorganisms, 11th
Ed." (2005) Pearson
http://en.wikipedia.org/wiki/Category:Microbiology_techniques
Category:Microbiology techniques
From Wikipedia, the free encyclopedia
Jump to: navigation, search
Pages in category "Microbiology techniques"
There are 19 pages in this section of this category.
A
C
•
Antibiogram
M cont.
•
Clonogenic assay
•
Microscopy
•
•
•
Aseptic technique
Auraminerhodamine stain
Axenic
•
Bacterial water
analysis
Blood culture
Cryogenic grinding
•
Guthrie test
G
B
•
•
•
Miles and Misra
method
•
Oxidase test
•
Replica plating
•
Streaking
(microbiology)
•
Ziehl-Neelsen
stain
O
I
R
•
•
Indole test
Industrial fermentation
K
S
•
Kirby-Bauer antibiotic
testing
M
Z
•
Microbiological
culture
Retrieved from "http://en.wikipedia.org/wiki/Category:Microbiology_techniques"
Categories: Microbiology | Laboratory techniques
Microscopy mi·cros·co·py (Pronunciation[mahy-kros-kuh-pee, mahy-kruh-skoh-pee])
is the technical field of using microscopes to view samples or objects. There are three
well-known branches of microscopy, optical, electron and scanning probe
microscopy.
Optical and electron microscopy involve the diffraction, reflection, or refraction of
electromagnetic radiation incident upon the subject of study, and the subsequent
collection of this scattered radiation in order to build up an image. This process may
be carried out by wide field irradiation of the sample (for example standard light
microscopy and transmission electron microscopy) or by scanning of a fine beam over
the sample (for example confocal microscopy and scanning electron microscopy).
Scanning probe microscopy involves the interaction of a scanning probe with the
surface or object of interest. The development of microscopy revolutionized biology
and remains an essential tool in that science, along with many others.
Scanning electron microscope image of pollen.
Contents
[hide]
•
•
•
•
1 Optical microscopy
o 1.1 Limitations of optical microscopy
o 1.2 Optical microscopy techniques
1.2.1 Bright field optical microscopy and what it means
1.2.2 Oblique illumination and what it means
1.2.3 Dark field optical microscopy and what it means
1.2.4 Phase contrast optical microscopy
1.2.5 Differential interference contrast microscopy
1.2.6 Fluorescence microscopy
1.2.7 Confocal laser scanning microscopy
1.2.8 Deconvolution microscopy
o 1.3 Sub-diffraction optical microscopy techniques
1.3.1 NSOM
1.3.2 Local enhancement / ANSOM / bowties
1.3.3 STED
1.3.4 Fitting the PSF
1.3.5 PALM & STORM
1.3.6 Structured illumination
o 1.4 Extensions of the optical microscope
o 1.5 Other optical microscope enhancements
o 1.6 X-ray microscopy
o 1.7 Electron Microscopy
o 1.8 Atomic de Broglie microscope
2 Scanning probe microscopy
o 2.1 Ultrasonic force microscopy
3 Infrared microscopy
4 Amateur Microscopy
•
•
•
5 See also
6 References
o 6.1 Further reading
7 External links
o 7.1 Organizations
[edit] Optical microscopy
See also: Optical microscope
Optical or light microscopy involves passing visible light transmitted through or
reflected from the sample through a single or multiple lenses to allow a magnified
view of the sample.[1] The resulting image can be detected directly by the eye, imaged
on a photographic plate or captured digitally. The single lens with its attachments, or
the system of lenses and imaging equipment, along with the appropriate lighting
equipment, sample stage and support, makes up the basic light microscope.
[edit] Limitations of optical microscopy
See also: Microscopy#Super-Resolution Optical Microscopy Techniques
Limitations of standard optical microscopy (bright field microscopy) lie in three
areas;
•
•
•
The technique can only image dark or strongly refracting objects effectively.
Diffraction limits resolution to approximately 0.2 micrometre (see:
microscope).
Out of focus light from points outside the focal plane reduces image clarity.
Live cells in particular generally lack sufficient contrast to be studied successfully,
internal structures of the cell are colourless and transparent. The most common way to
increase contrast is to stain the different structures with selective dyes, but this
involves killing and fixing the sample. Staining may also introduce artifacts, apparent
structural details that are caused by the processing of the specimen and are thus not a
legitimate feature of the specimen.
These limitations have, to some extent, all been overcome by specific microscopy
techniques which can non-invasively increase the contrast of the image. In general,
these techniques make use of differences in the refractive index of cell structures. It is
comparable to looking through a glass window: you (bright field microscopy) don't
see the glass but merely the dirt on the glass. There is however a difference as glass is
a more dense material, and this creates a difference in phase of the light passing
through. The human eye is not sensitive to this difference in phase but clever optical
solutions have been thought out to change this difference in phase into a difference in
amplitude (light intensity).
[edit] Optical microscopy techniques
[edit] Bright field optical microscopy and what it means
Main article: Bright field microscopy
Bright field microscopy is the simplest of all the light microscopy techniques. Sample
illumination is via transmitted white light, i.e. illuminated from below and observed
from above. Limitations include low contrast of most biological samples and low
apparent resolution due to the blur of out of focus material. The simplicity of the
technique and the minimal sample preparation required are significant advantages.
[edit] Oblique illumination and what it means
The use of oblique (from the side) illumination gives the image a 3-dimensional
appearance and can highlight otherwise invisible features. A more recent technique
based on this method is Hoffmann's modulation contrast, a system found on inverted
microscopes for use in cell culture. Oblique illumination suffers from the same
limitations as bright field microscopy (low contrast of many biological samples; low
apparent resolution due to out of focus objects), but may highlight otherwise invisible
structures.
[edit] Dark field optical microscopy and what it means
Main article: Dark field microscopy
Dark field microscopy is a technique for improving the contrast of unstained,
transparent specimens.[2] Darkfield illumination uses a carefully aligned light source
to minimise the quantity of directly-transmitted (un-scattered) light entering the image
plane, collecting only the light scattered by the sample. Darkfield can dramatically
improve image contrast—especially of transparent objects—while requiring little
equipment setup or sample preparation. However, the technique does suffer from low
light intensity in final image of many biological samples, and continues to be affected
by low apparent resolution.
Rheinberg illumination is a special variant of dark field illumination in which
transparent, colored filters are inserted just before the condenser so that light rays at
high aperture are differently colored than those at low aperture (i.e. the background to
the specimen may be blue while the object appears self-luminous yellow). Other color
combinations are possible but their effectiveness is quite variable.[3]
[edit] Phase contrast optical microscopy
Main articles: Phase contrast microscope and Phase contrast microscopy
In electron microscopy: Phase-contrast imaging
More sophisticated techniques will show differences in optical density in proportion.
Phase contrast is a widely used technique that shows differences in refractive index
as difference in contrast. It was developed by the Dutch physicist Frits Zernike in the
1930s (for which he was awarded the Nobel Prize in 1953). The nucleus in a cell for
example will show up darkly against the surrounding cytoplasm. Contrast is excellent;
however it is not for use with thick objects. Frequently, a halo is formed even around
small objects, which obscures detail. The system consists of a circular annulus in the
condenser which produces a cone of light. This cone is superimposed on a similar
sized ring within the phase-objective. Every objective has a different size ring, so for
every objective another condenser setting has to be chosen. The ring in the objective
has special optical properties: it first of all reduces the direct light in intensity, but
more importantly, it creates an artificial phase difference of about a quarter
wavelength. As the physical properties of this direct light have changed, interference
with the diffracted light occurs, resulting in the phase contrast image.
[edit] Differential interference contrast microscopy
Main article: Differential interference contrast microscopy
Superior and much more expensive is the use of interference contrast. Differences in
optical density will show up as differences in relief. A nucleus within a cell will
actually show up as a globule in the most often used differential interference
contrast system according to Georges Nomarski. However, it has to be kept in mind
that this is an optical effect, and the relief does not necessarily resemble the true
shape! Contrast is very good and the condenser aperture can be used fully open,
thereby reducing the depth of field and maximizing resolution.
The system consists of a special prism (Nomarski prism, Wollaston prism) in the
condenser that splits light in an ordinary and an extraordinary beam. The spatial
difference between the two beams is minimal (less than the maximum resolution of
the objective). After passage through the specimen, the beams are reunited by a
similar prism in the objective.
In a homogeneous specimen, there is no difference between the two beams, and no
contrast is being generated. However, near a refractive boundary (say a nucleus within
the cytoplasm), the difference between the ordinary and the extraordinary beam will
generate a relief in the image. Differential interference contrast requires a polarized
light source to function; two polarizing filters have to be fitted in the light path, one
below the condenser (the polarizer), and the other above the objective (the analyzer).
Note: In cases where the optical design of a microscope produces an appreciable
lateral separation of the two beams we have the case of classical interference
microscopy, which does not result in relief images, but can nevertheless be used for
the quantitative determination of mass-thicknesses of microscopic objects.
[edit] Fluorescence microscopy
Main article: Fluorescence microscopy
When certain compounds are illuminated with high energy light, they then emit light
of a different, lower frequency. This effect is known as fluorescence. Often specimens
show their own characteristic autofluorescence image, based on their chemical
makeup.
This method is of critical importance in the modern life sciences, as it can be
extremely sensitive, allowing the detection of single molecules. Many different
fluorescent dyes can be used to stain different structures or chemical compounds. One
particularly powerful method is the combination of antibodies coupled to a
fluorochrome as in immunostaining. Examples of commonly used fluorochromes are
fluorescein or rhodamine. The antibodies can be made tailored specifically for a
chemical compound. For example, one strategy often in use is the artificial production
of proteins, based on the genetic code (DNA). These proteins can then be used to
immunize rabbits, which then form antibodies which bind to the protein. The
antibodies are then coupled chemically to a fluorochrome and then used to trace the
proteins in the cells under study.
Highly-efficient fluorescent proteins such as the green fluorescent protein (GFP) have
been developed using the molecular biology technique of gene fusion, a process
which links the expression of the fluorescent compound to that of the target
protein.Piston DW, Patterson GH, Lippincott-Schwartz J, Claxton NS, Davidson MW
(2007). Nikon MicroscopyU: Introduction to Fluorescent Proteins. Nikon
MicroscopyU. Retrieved on 2007-08-22. This combined fluorescent protein is
generally non-toxic to the organism and rarely interferes with the function of the
protein under study. Genetically modified cells or organisms directly express the
fluorescently-tagged proteins, which enables the study of the function of the original
protein in vivo.
Since fluorescence emission differs in wavelength (color) from the excitation light, a
fluorescent image ideally only shows the structure of interest that was labelled with
the fluorescent dye. This high specificity led to the widespread use of fluorescence
light microscopy in biomedical research. Different fluorescent dyes can be used to
stain different biological structures, which can then be detected simultaneously, while
still being specific due to the individual color of the dye.
To block the excitation light from reaching the observer or the detector, filter sets of
high quality are needed. These typically consist of an excitation filter selecting the
range of excitation wavelengths, a dichroic mirror, and an emission filter blocking the
excitation light. Most fluorescence microscopes are operated in the Epi-illumination
mode (illumination and detection from one side of the sample) to further decrease the
amount of excitation light entering the detector.
See also total internal reflection fluorescence microscope.
[edit] Confocal laser scanning microscopy
Main article: Confocal laser scanning microscopy
Generates the image by a completely different way than the normal visual bright field
microscope. It gives slightly higher resolution, but most importantly it provides
optical sectioning without disturbing out-of-focus light degrading the image.
Therefore it provides sharper images of 3D objects. This is often used in conjunction
with fluorescence microscopy.
[edit] Deconvolution microscopy
Fluorescence microscopy is extremely powerful due to its ability to show specifically
labelled structures within a complex environment but also because of its inherent
ability to provide three dimensional information of biological structures.
Unfortunately this information is blurred by the fact, that upon illumination all
fluorescently labeled structures emit light no matter if they are in focus or not. This
means, that an image of a certain structure is always blurred by the contribution of
light from structures which are out of focus. This phenomenon becomes apparent as a
loss of contrast especially when using objectives with a high resolving power,
typically oil immersion objectives with a high numerical aperture.
Fortunately though, this phenomenon is not caused by random processes such as light
scattering but can be relatively well defined by the optical properties of the image
formation in the microscope imaging system. If one considers a small fluorescent
light source (essentially a bright spot), light coming from this spot spreads out the
further out of focus one is. Under ideal conditions this produces a sort of "hourglass"
shape of this point source in the third (axial) dimension. This shape is called the point
spread function (PSF) of the microscope imaging system. Since any fluorescence
image is made up of a large number of such small fluorescent light sources the image
is said to be "convolved by the point spread function".
Knowing this point spread function means, that it is possible to reverse this process to
a certain extent by computer based methods commonly known as deconvolution
microscopy.[4] There are various algorithms available for 2D or 3D Deconvolution.
They can be roughly classified in non restorative and restorative methods. While the
non restorative methods can improve contrast by removing out of focus light from
focal planes, only the restorative methods can actually reassign light to it proper place
of origin. This can be an advantage over other types of 3D microscopy such as
confocal microscopy, because light is not thrown away but reused. For 3D
deconvolution one typically provides a series of images derived from different focal
planes (called a Z-stack) plus the knowledge of the PSF which can be either derived
experimentally or theoretically from knowing all contributing parameters of the
microscope.
[edit] Sub-diffraction optical microscopy techniques
It is well known that there is a spatial limit to which light can focus: approximately
half of the wavelength of the light you are using. But this is not a true barrier, because
this diffraction limit is only true in the far-field and localization precision can be
increased with many photons and careful analysis (although two objects still cannot
be resolved); and like the sound barrier, the diffraction barrier is breakable. This
section explores some approaches to imaging objects smaller than ~250 nm. Most of
the following information was gathered (with permission) from a chemistry blog's
review of sub-diffraction microscopy techniques Part I and Part II. For a review, see
also reference [5].
[edit] NSOM
Probably the most conceptual way to break the diffraction barrier is to use a light
source and/or a detector that is itself nanometer in scale. Diffraction as we know it is
truly a far-field effect: the light from an aperture is the Fourier transform of the
aperture in the far-field.[6] But in the near-field, all of this is not necessarily the case.
Near-field scanning optical microscopy (NSOM) forces light through the tiny tip of a
pulled fiber—and the aperture can be on the order of tens of nanometers.[7] When the
tip is brought to nanometers away from a molecule, the resolution is not limited by
diffraction but by the size of the tip aperture (because only that one molecule will see
the light coming out of the tip). An image can be built by a raster scan of the tip over
the surface to create an image.
The main down-side to NSOM is the limited number of photons you can force out a
tiny tip, and the minuscule collection efficiency (if you are trying to collect
fluorescence in the near-field). Other techniques such as ANSOM (see below) try to
avoid this drawback.
[edit] Local enhancement / ANSOM / bowties
Instead of forcing photons down a tiny tip, some techniques create a local bright spot
in an otherwise diffraction-limited spot. ANSOM is apertureless NSOM: it uses a tip
very close to a fluorophore to enhance the local electric field the fluorophore sees.[8]
Basically, the ANSOM tip is like a lightning rod which creates a hot spot of light.
Bowtie nanoantennas have been used to greatly and reproducibly enhance the electric
field in the nanometer gap between the tips two gold triangles. Again, the point is to
enhance a very small region of a diffraction-limited spot, thus improving the
mismatch between light and nanoscale objects—and breaking the diffraction barrier.[9]
[edit] STED
A recent favorite is STED—stimulated emission depletion. Stefan Hell at the Max
Planck Institute developed this method, which uses two laser pulses. The first pulse is
a diffraction-limited spot that is tuned to the absorption wavelength, so excites any
fluorophores in that region; an immediate second pulse is red-shifted to the emission
wavelength and stimulates emission back to the ground state before, thus depleting
the excited state of any fluorophores in this depletion pulse. The trick is that the
depletion pulse goes through a phase modulator that makes the pulse illuminate the
sample in the shape of a donut, so the outer part of the diffraction limited spot is
depleted and the small center can still fluoresce. By saturating the depletion pulse, the
center of the donut gets smaller and smaller until they can get resolution of tens of
nanometers.[10]
This technique also requires a raster scan like NSOM and standard confocal laser
scanning microscopy.
[edit] Fitting the PSF
The methods above (and below) use experimental techniques to circumvent the
diffraction barrier, but one can also use crafty analysis to increase the ability to know
where a nanoscale object is located. The image of a point source on a charge-coupled
device camera is called a point-spread function (PSF), which is limited by diffraction
to be no less than approximately half the wavelength of the light. But it is possible to
simply fit that PSF with a Gaussian to locate the center of the PSF—and thus the
location of the fluorophore. The precision by which this technique can locate the
center depends on the number of photons collected (as well as the CCD pixel size and
other factors).[11] Regardless, groups like the Selvin lab and many others have
employed this analysis to localize single fluorophores to a few nanometers. This, of
course, requires careful measurements and collecting many photons.
[edit] PALM & STORM
What fitting a PSF is to localization, photo-activated localization microscopy (PALM)
is to "resolution"—this term is here used loosely to mean measuring the distance
between objects, not true optical resolution. Eric Betzig and colleagues developed
PALM;[12] Xiaowei Zhuang at Harvard used a similar techniques and calls it STORM:
stochastic optical reconstruction microscopy.[13] The basic premise of both techniques
is to fill the imaging area with many dark fluorophores that can be photoactivated into
a fluorescing state by a flash of light. Because photoactivation is stochastic, only a
few, well separated molecules "turn on." Then Gaussians are fit to their PSFs to high
precision (see section above). After the few bright dots photobleach, another flash of
the photoactivating light activates random fluorophores again and the PSFs are fit of
these different well spaced objects. This process is repeated many times, building up
an image molecule-by-molecule; and because the molecules were localized at
different times, the "resolution" of the final image can be much higher than that
limited by diffraction.
The major problem with these techniques is that to get these beautiful pictures, it
takes on the order of hours to collect the data. This is certainly not the technique to
study dynamics (fitting the PSF is better for that).
[edit] Structured illumination
There is also the wide-field structured-illumination (SI) approach to breaking the
diffraction limit of light.[14][15] SI—or patterned illumination—relies on both specific
microscopy protocols and extensive software analysis post-exposure. But, because SI
is a wide-field technique, it is usually able to capture images at a higher rate than
confocal-based schemes like STED. (This is only a generalization, because SI isn't
actually super fast. I'm sure someone could make STED fast and SI slow!) The main
concept of SI is to illuminate a sample with patterned light and increase the resolution
by measuring the fringes in the Moiré pattern (from the interference of the
illumination pattern and the sample). "Otherwise-unobservable sample information
can be deduced from the fringes and computationally restored."[16]
SI enhances spatial resolution by collecting information from frequency space outside
the observable region. This process is done in reciprocal space: the Fourier transform
(FT) of an SI image contains superimposed additional information from different
areas of reciprocal space; with several frames with the illumination shifted by some
phase, it is possible to computationally separate and reconstruct the FT image, which
has much more resolution information. The reverse FT returns the reconstructed
image to a super-resolution image.
But this only enhances the resolution by a factor of 2 (because the SI pattern cannot
be focused to anything smaller than half the wavelength of the excitation light). To
further increase the resolution, you can introduce nonlinearities, which show up as
higher-order harmonics in the FT. In reference [16], Gustafsson uses saturation of the
fluorescent sample as the nonlinear effect. A sinusoidal saturating excitation beam
produces the distorted fluorescence intensity pattern in the emission. This
nonpolynomial nonlinearity yields a series of higher-order harmonics in the FT.
Each higher-order harmonic in the FT allows another set of images that can be used to
reconstruct a larger area in reciprocal space, and thus a higher resolution. In this case,
Gustafsson achieves less than 50-nm resolving power, more than five times that of the
microscope in its normal configuration.
The main problems with SI are that, in this incarnation, saturating excitation powers
cause more photodamage and lower fluorophore photostability, and sample drift must
be kept to below the resolving distance. The former limitation might be solved by
using a different nonlinearity (such as stimulated emission depletion or reversible
photoactivation, both of which are used in other sub-diffraction imaging schemes); the
latter limits live-cell imaging and may require faster frame rates or the use of some
fiducial markers for drift subtraction. Nevertheless, SI is certainly a strong contender
for further application in the field of super-resolution microscopy.
[edit] Extensions of the optical microscope
Most modern instruments provide simple solutions for micro-photography and image
recording electronically. However such capabilities are not always present and the
more experienced microscopist will, in many cases, still prefer a hand drawn image
rather than a photograph. This is because a microscopist with knowledge of the
subject can accurately convert a three dimensional image into a precise two
dimensional drawing . In a photograph or other image capture system however, only
one thin plane is ever in good focus.
The creation of careful and accurate micrographs requires a microscopical technique
using a monocular eyepiece. It is essential that both eyes are open and that the eye
that is not observing down the microscope is instead concentrated on a sheet of paper
on the bench besides the microscope. With practice, and without moving the head or
eyes, it is possible to accurately record the observed details by tracing round the
observed shapes by simultaneously "seeing" the pencil point in the microscopical
image.
Practising this technique also establishes good general microscopical technique. It is
always less tiring to observe with the microscope focussed so that the image is seen at
infinity and with both eyes open at all times.
[edit] Other optical microscope enhancements
stereomicroscope
[edit] X-ray microscopy
Main article: X-ray microscopy
As resolution depends on the wavelength of the light. Electron microscopy has been
developed since the 1930s that use electron beams instead of light. Because of the
much lower wavelength of the electron beam, resolution is far higher.
Though less common, X-ray microscopy has also been developed since the late
1940s. The resolution of X-ray microscopy lies between that of light microscopy and
the electron microscopy.
[edit] Electron Microscopy
For light microscopy the wavelength of the light limits the resolution to around 0.2
micrometers. In order to gain higher resolution, the use of an electron beam with a far
smaller wavelength is used in electron microscopes.
•
•
Transmission electron microscopy (TEM) is principally quite similar to the
compound light microscope, by sending an electron beam through a very thin
slice of the specimen. The resolution limit nowadays (2005) is around 0.05
nanometer.
Scanning electron microscopy (SEM) visualizes details on the surfaces of cells
and particles and gives a very nice 3D view. It gives results much like the
stereo light microscope and akin to that its most useful magnification is in the
lower range than that of the transmission electron microscope.
[edit] Atomic de Broglie microscope
Main article: Atomic de Broglie microscope
The atomic de Broglie microscope is an imaging system which is expected to provide
resolution at the nanometer scale using neutral He atoms as probe particles. [17][18].
Such a device could provide the resolution at nanometer scale and be absolutely nondestructive, but it is not developed so well as optical microscope or an electron
microscope.
[edit] Scanning probe microscopy
This is a sub-diffraction technique. Examples of scanning probe microscopes are the
atomic force microscope (AFM), the Scanning tunneling microscope and the photonic
force microscope. All such methods imply a solid probe tip in the vicinity (near field)
of an object, which is supposed to be almost flat. For more detail, see Scanning probe
microscopy.
[edit] Ultrasonic force microscopy
Ultrasonic Force Microscopy (UFM) has been developed in order to improve the
details and image contrast on "flat" areas of interest where the AFM images are
limited in contrast. The combination of AFM-UFM allows a near field acoustic
microscopic image to be generated. The AFM tip is used to detect the ultrasonic
waves and overcomes the limitation of wavelength that occurs in acoustic
microscopy. By using the elastic changes under the AFM tip, an image of much
greater detail than the AFM topography can be generated.
Ultrasonic force microscopy allows the local mapping of elasticity in atomic force
microscopy by the application of ultrasonic vibration to the cantilever or sample. In an
attempt to analyse the results of ultrasonic force microscopy in a quantitative fashion,
a force-distance curve measurement is done with ultrasonic vibration applied to the
cantilever base, and the results are compared with a model of the cantilever dynamics
and tip-sample interaction based on the finite-difference technique.
[edit] Infrared microscopy
The term infrared microscope covers two main types of diffraction-limited
microscopy. The first provides optical visualisation plus IR spectroscopic data
collection. The second (more recent and more advanced) technique employs focal
plane array detection for infrared chemical imaging, where the image contrast is
determined by the response of individual sample regions to particular IR wavelengths
selected by the user.
IR versions of sub-diffraction microscopy (see above) exist also. These include IR
NSOM [19] and photothermal microspectroscopy.
[edit] Amateur Microscopy
Amateur Microscopy is the investigation and observation of biological and nonbiological specimens for recreational purposes using an optical microscope (light
microscopes). Collectors of minerals, insects, seashells and plants may use
microscopes as tools to uncover features that help them classify their collected items.
Other amateurs may be interested in observing the life found in pond water and of
other samples. Microscopes may also prove useful for the water quality assessment
for people that keep a home aquarium. Photographic documentation and drawing of
the microscopic images are additional tasks that augment the spectrum of tasks of the
amateur. There are even competitions for photomicrograph art. Participants of this
pastime may either use commercially prepared microscopic slides or may engage in
the task of specimen preparation.
While microscopy is a central tool in the documentation of biological specimens, it is
rarely sufficient to justify the discovery of a new species based on microscopic
investigations alone. Often genetic and biochemical tests are necessary to confirm the
discovery of a new species. A fully equipped laboratory may be necessary, something
often not available to amateurs. For this reason it may be unlikely that amateur
microscopists are capable of substantiating their find to the extent to yield a scientific
publication.
In the late 1800's amateur microscopy became a popular hobby in the United States
and Europe. Professor John Phin published "Practical Hints on the Selection and Use
of the Microscope (Second Edition, 1878)," and was also the editor of the “American
Journal of Microscopy.”
[edit] See also
•
•
Köhler illumination
Two-photon excitation microscopy
[edit] References
1. ^ Abramowitz M, Davidson MW (2007). Introduction to Microscopy. Molecular
Expressions. Retrieved on 2007-08-22.
2. ^ Abramowitz M, Davidson MW (2007). Darkfield Illumination. Retrieved on 200708-22.
3. ^ Abramowitz M, Davidson MW (2007). Rheinberg Illumination. Retrieved on 200708-22.
4. ^ Wallace W, Schaefer LH, Swedlow JR (2001). "A workingperson's guide to
deconvolution in light microscopy". BioTechniques 31 (5): 1076-8, 1080, 1082
passim. PMID 11730015.
5. ^ WEM News and Views
6. ^ Fresnel Diffraction Applet (Java applet). Retrieved on 2007-08-22.
7. ^ Cummings JR, Fellers TJ, Davidson MW (2007). Specialized Microscopy
Techniques - Near-Field Scanning Optical Microscopy. Olympus Microscopy
Resource Center. Retrieved on 2007-08-22.
8. ^ Sánchez EJ, Novotny L, Xie XS (1999). "Near-Field Fluorescence Microscopy
Based on Two-Photon Excitation with Metal Tips". Phys Rev Lett 82: 4014-7.
doi:10.1103/PhysRevLett.82.4014.
9. ^ Schuck PJ, Fromm DP, Sundaramurthy A, Kino GS, Moerner WE (2005).
"Improving the Mismatch between Light and Nanoscale Objects with Gold Bowtie
Nanoantennas". Phys Rev Lett 94: 017402. doi:10.1103/PhysRevLett.94.017402.
10. ^ STED
11. ^ Webb paper
12. ^ PALM
13. ^ STORM
14. ^ Bailey, B.; Farkas, D. L.; Taylor, D. L.; Lanni, F. Enhancement of axial resolution
in fluorescence microscopy by standing-wave excitation. Nature 1993, 366, 44–48.
15. ^ Gustafsson, M. G. L. Surpassing the lateral resolution limit by a factor of two using
structured illumination microscopy. J. of Microsc. 2000, 198(2), 82–87.
16. ^ a b Gustafsson, M. G. L. http://dx.doi.org/10.1073/pnas.0406877102 Nonlinear
structured-illumination microscopy: Wide-field fluorescence imaging with
theoretically unlimited resolution. PNAS 2005, 102(37), 13081–13086.
17. ^ D.Kouznetsov; H. Oberst, K. Shimizu, A. Neumann, Y. Kuznetsova, J.-F. Bisson,
K. Ueda, S. R. J. Brueck (2006). "Ridged atomic mirrors and atomic nanoscope".
JOPB 39: 1605-1623.
18. ^ Atom Optics and Helium Atom Microscopy. Cambridge University, http://wwwsp.phy.cam.ac.uk/research/mirror.php3
19. ^ H M Pollock and D A Smith, The use of near-field probes for vibrational
spectroscopy and photothermal imaging, in Handbook of vibrational spectroscopy,
J.M. Chalmers and P.R. Griffiths (eds), John Wiley & Sons Ltd, Vol. 2, pp. 1472 1492 (2002)
[edit] Further reading
•
•
•
•
Advanced Light Microscopy vol. 1 Principles and Basic Properties by Maksymilian
Pluta, Elsevier (1988)
Advanced Light Microscopy vol. 2 Specialised Methods by Maksymilian Pluta,
Elsevier (1989)
Introduction to Light Microscopy by S. Bradbury, B. Bracegirdle, BIOS Scientific
Publishers (1998)
Video Microscopy by Shinya Inoue, Plenum Press (1986)
•
A review of sub-diffraction microscopy techniques Part I and Part II - a blog post
with helpful information, some of which appears in this article
[edit] External links
•
•
•
•
•
•
•
•
Microscopy Techniques Various Techniques Used In Microscopy
Carl Zeiss "Microscopy from the very beginning", a step by step tutorial into
the basics of microscopy.
Interactive Fluorescence Dye and Filter Database Carl Zeiss Interactive
Fluorescence Dye and Filter Database.
Nikon MicroscopyU - The source for microscopy education
Olympus Microscopy Resource Center
Microscopy in Detail - A resource with many illustrations elaborating the most
common microscopy techniques
Images formed by simple microscopes - examples of observations with singlelens microscopes.
Portraits of life, one molecule at a time, a feature article on sub-diffraction
microscopy from the March 1, 2007 issue of Analytical Chemistry
[edit] Organizations
•
•
•
Royal Microscopical Society (RMS)
Microscopy Society of America (MSA)
European Microscopy Society (EMS)
[hide]
v•d•e
Analytical chemistry
Atomic absorption spectrometer · Flame emmission spectrometer ·
Gas chromatograph · High performance liquid chromatograph ·
Instrumentation
Infrared Spectrometer · Mass spectrometer · Melting point apparatus ·
Microscope · Spectrometer · Spectrophotometer
Techniques
Calorimetry · Chemometrics · Chromatography · Electrochemistry ·
Gravimetric analysis
Coning and quartering · Dilution · Dissolution · Filtration · Masking ·
Sampling Pulverization · Sample preparation · Separation process · Subsampling
Prominent
Analytical chemistry
publications
http://mycology.cornell.edu/fteach.html
Fungi Perfecti (Olympia, Washington, USA) supplies a plethora of mushroomgrowing equipment, spawn and kits, books, and dried edible and medicinal
mushrooms. Their online catalog and information about Paul Stamets' mushroom
cultivation seminars and consultation services can be found here. This elegant web
site includes many impressive images of mushrooms and other products, including
scanning electron micrographs of mushroom ultrastructure.
George Barron's website
This website includes some lovely images of fungi, including Entomophthora,
Spinellus, and some nematode parasites. It also includes information on Barron's book
"Mushrooms of Northeast North America" (in Canada entitled "Mushrooms of
Ontario and Eastern Canada").
Glossary of Technical Terms in Plant Pathology
This useful Glossary of technical terms in Plant Pathology was created by Phil
Arneson of Cornell University. It includes definitions, illustrations, and sound files by
Richard Korf to aid pronunciation.
Irish Potato Famine
A compilation of information on the Irish Potato Famine of the 1840s, during which
time over 3 million Irish died, and many others (including some of my own ancestors)
emigrated to other parts of the world. The Famine resulted from an outbreak of late
blight, caused by Phytophthora infestans.
John C. Tacoma Mushroom Slide Collection
Many, many scanned images of mushrooms and allies, from photographs taken by
John C. Tacoma, 1968-1978. Maintained by the Library of Indiana University-Purdue
University Indianapolis.
LichenLand
Lichenland provides a fine introduction to lichens for both professionals and
amateurs. Synoptic keys to taxa and to terms lead to many fine images of lichens, a
compilation of their characteristics, and pertinent literature.
Meredith Blackwell's Lab
Meredith Blackwell's lab at Louisiana State University provides information on
current research on insect-fungus associations, history of mycology, the genealogy of
American mycologists, teaching resources, the LSU herbarium, and other tidbits.
Microfungal home page
Color images of many microfungi taken under the microscope. Over 100 genera of
molds are represented.
Moulds: their isolation, cultivation and identification
An online version of David Malloch's excellent guide to moulds (University of
Toronto Press, 1981), complete with keys, media recipes, and illustrations of common
genera. This book makes a great introduction to hyphomycetes for those with access
to a microscope.
Mushroom Toxins
This discussion of mushroom toxins and the symptoms they produce forms a chapter
of the "Bad Bug Book" by the US Food and Drug Administration. Other mycotoxins
(aflatoxin and ilk) are discussed in a subsequent chapter.
Mushrooms and Magic
The Mycotheology Home Page provides an interesting discussion of the role of fungi
in magic, folklore, and religion.
Mushrooms of North Carolina
Mycology students at Duke University (NC, USA) have prepared this site
documenting the mushrooms of North Carolina. Their excellent photographs are
available here.
Mycologue Publications
Mycologue is a publishing company founded by W. Bryce Kendrick. It provides
books, teaching materials, and computerized keys to fungi (Canada). The site also
includes information and many illustrations of fungi that complement Dr. Kendrick's
textbook, The Fifth Kingdom (q.v.).
Mycology class at Arizona State University
Home page of the General Mycology class at Arizona State University, USA.
Mycology class at Oregon State University
Home page of the mycology class at Oregon State University, USA.
Mycology class at Towson University
The home page of the Mycology class at Towson University, in Maryland, USA.
Mycology classes at Humboldt University
Home page of Mycology classes at Humboldt University, California, USA.
Mycology Course at the University of Illinois at Urbana/Champaign
This web site for Dr. Carol Shearer's Mycology class includes a syllabus, lab
exercises, and many excellent lecture illustrations.
Mycology Online
Mycology Online is a guide to fungal pathogens of humans, the diseases they cause,
and selected case studies. This Australian site is searchable, nicely illustrated (not for
the squeamish!), and replete with information.
Mycorrhiza information exchange
The Mycorrhiza Information Exchange covers everything you need: literature
databases, job ads, teaching tips, images, inoculum sources, links, etc. Participation is
invited.
Mycorrhizae and Plant Phylogeny
A website devoted to mycorrhizae and plant systematics, and the evolution of
mycorrhizal symbiosis.
Mycorrhizas webpage
This guide to mycorrhizal associations (adapted and excerpted from a larger book) is
provided by Mark Brundett at CSIRO (Australia). It details the structure and
development of mycorrhizae, with handsome images and good textual explanation. It
makes a wonderful teaching tool.
Mycorrhizospheres of boreal forest trees
This site from the Biocenter at the University of Helsinki (Finland) includes scientific
publications documenting the diversity, interactions and functions of forest tree
mycorrhizae.
Mycotoxin homepage
A unit of the US Dept. of Agriculture, Agricultural Research Service that focuses on
mycotoxin research. 3-dimensional molecular structures of a few mycotoxins
produced by molds are available here.
MyxoWeb
This web site devoted to myxomycetes provides information on the plasmodial slime
molds, including some impressively gooey images.
Natural Perspective's introduction to fungi
Natural Perspective's nicely illustrated introduction to the fungal kingdom.
North American Lichen Project
The North American Lichen Project includes essays on lichen biology and the uses of
lichens by people and animals, as well as excerpts and lovely photographs from the
forthcoming book Lichens of North America, by I.M. Brodo, S.D. Sharnoff, and S.
Sharnoff (Yale University Press).
North American Mycological Association
NAMA is a great group for amateur mycologists. It provides a national mushroom
poisoning registry, sponsors an annual foray, and publishes a fine annual journal,
McIlvainea, and a bimonthly newsletter, The Mycophile. Also available through
NAMA are suggestions for teaching K-12 students about fungi, and other tidbits.
Penn State Mushroom Spawn Lab
Pennsylvania State University's strong program in mushroom cultivation presents fact
sheets and other information about commercial mushroom production on these pages.
PSU's mushroom growers' information pages are part of this site.
Plant Pathogenic Fungi
The University of Kentucky's course in plant pathogenic fungi has web pages that
include the syllabus and other information.
Plant Pathology
The Plant Pathology courses at the University of Nebraska-Lincoln (USA). Most
materials are for registered students only; a distance learning course is offered.
Plant Pathology Internet Guidebook
The Plant Pathology Internet Guidebook is a comprehensive source for Plant
Pathology resources online. It is available through the Institute of Plant Diseases and
Plant Protection in Hannover, Germany.
Plant Pathology Simulations
Computer simulations for teaching aspects of plant pathology and epidemiology.
Plasmodiophorid Home Page
These are pages devoted to the Plasmodiophorales that include information about life
histories, cytology, and biology of this interesting group of fungus-like protists. The
site is no longer being updated.
Pythium insidiosum
Pythiosis is a disease of humans and animals that can be caused by the subject of this
web page, Pythium insidiosum. The site includes graphic images and information on
biology, epidemiology, diagnosis, and treatment.
Spongospora Homepage
Spongospora subterranea is a plasmodiophorid pathogen of potatoes (and other plants)
and an emerging pathogen in some regions. This workshop site introduces the biology
and control of S. subterranea and related species, and includes images and a
discussion board.
The Fifth Kingdom
W.B. Kendrick's delightful introductory mycology textbook, The Fifth Kingdom, is
partly available online. This site includes over 800 lavish, colorful illustrations as a
supplement to the text, which is available from Mycologue Publications (q.v.). The
text of sample chapters is available, too. Dr. Kendrick's website also includes other
publications for sale.
The Rhynie Chert and its Flora
The Rhynie Chert is a fossilized Devonian lake shore in Scotland that includes some
of the oldest fossils of plants and their associated fungi. This nice site introduces the
botanical and mycological finds of the Rhynie Chert, and provides photos of the
oldest known lichen and early arbuscular mycorrhizal fungi.
This is Not Just Plant Pathogenic Fungi!
Students at Texas A M University have prepared a guide to plant pathogenic (and
other) fungi.
Tom Volk's web pages
One stop shopping for mycology. These pages feature a "fungus of the month"
column, with entertaining text and nice photos, in addition to a plethora of other
information about fungi. Tom is a professor at the University of Wisconsin-La Crosse,
USA.
Tree of Life
This phylogenetic navigator provides a tree that shows the evolutionary relationships
of living organisms, including fungi. It also supplies descriptive pages on selected
terminal taxa. Like biological systematics itself, it's a work in progress.
UC Berkeley's Introduction to Fungi
The Museum of Paleontology at the University of California, Berkeley provides a
well-prepared introduction to the kingdom Fungi, and also to two groups that have
historically been studied by mycologists, the Oomycota and slime molds. Similar
introductions are available for all other taxa. This link makes a valuable addition to
any teaching program.
University of Tennessee Mycology Labs
Drs. Ron Petersen and Karen Hughes maintain a nice set of web pages that include a
primer on Botanical Nomenclature, a synopsis of molecular phylogenetic techniques.
These pages also provide an important resources on color standards used by
mycologists: a synopsis of Fries' color terminology, and a concordance of colors in
the Ridgway and Methuen color handbooks. Lots of information is also provided on
the projects of staff and students.
Views of the Famine
An illustrated history of news coverage of the Irish Potato Famine that occurred in the
1840s due to Phytophthora infestans, causal agent of late blight of potato.
Wayne's Word on the fungal kingdom
A delightful introduction to selected members of the kingdom Fungi from the e-zine,
Wayne's Word.
Western Montana Mycological Association (USA): Fungal Jungal
The Western Montana Mycological Association maintains this nice site. It includes
photos of Montana mushrooms, recipes, an oyster mushroom cultivation project, a
mushroom "trunk" for teachers, a morel information site, and information on the
WMMA's current activities.
World-Wide Web Virtual Library
You're there now! This is a distributed library of resources maintained at many
different sites all over the world. Unlike some of the big search engines, VL site
maintainers personally select and evaluate the links they recommend, with the result
that VL sites generally have a high signal to noise ratio. The WWW VL is a good
place to start when looking for electronic information on all kinds of different topics.
HOME -- ABOUT -- COLLECTIONS -- DIRECTORIES -- DISCUSSIONS -- GENERAL -- GENETICS -- GUIDES -- MUSHROOMS
-- SUPPLIES -- TAXONOMY -- TEACHING -- INDEX
Robert Koch
From Wikipedia, the free encyclopedia
Jump to: navigation, search
Robert Koch
Robert Koch
Born
December 11, 1843
Clausthal, Hanover
Died
May 27, 1910 (aged 66)
Baden-Baden, Germany
Field
Microbiology
Institutions
Imperial Health Office, Berlin, University of Berlin
Alma mater
University of Göttingen
Academic advisor
Known for
Notable awards
Friedrich Gustav Jakob Henle
Co-founder of bacteriology,
Koch's postulates of germ theory,
Isolator of anthrax, tuberculosis and cholera
Nobel Prize in Medicine, 1905
For the American lobbyist, see Bobby Koch.
Robert Koch (December 11, 1843 – May 27, 1910) was a German physician. He
became famous for isolating Bacillus anthracis (1877), the tuberculosis bacillus
(1882) and the cholera vibrio (1883) and for his development of Koch's postulates.
He was awarded the Nobel Prize in Physiology or Medicine for his tuberculosis
findings in 1905. He is considered one of the founders of microbiology - he inspired
such major figures as Paul Ehrlich and Gerhard Domagk.
Contents
[hide]
•
•
•
•
•
1 Biography
2 References
3 Consult
4 See also
5 External links
[edit] Biography
Robert Koch was born in Clausthal, Germany as the son of a mining official. He
studied medicine under Friedrich Gustav Jakob Henle at the University of Göttingen
and graduated in 1866. He then served in the Franco-Prussian War and later became
district medical officer in Wollstein (now Wolsztyn, Poland). Working with very
limited resources, he became one of the founders of bacteriology, the other major
figure being Louis Pasteur.
After Casimir Davaine showed the direct transmission of the anthrax bacillus between
cows, Koch studied anthrax more closely. He invented methods to purify the bacillus
from blood samples and grow pure cultures. He found that, while it could not survive
outside a host for long, anthrax built persisting endospores that could last a long time.
These endospores, embedded in soil, were the cause of unexplained "spontaneous"
outbreaks of anthrax. Koch published his findings in 1876, and was rewarded with a
job at the Imperial Health Office in Berlin in 1880. In 1881, he urged the sterilization
of surgical instruments using heat.
In Berlin, he improved the methods he used in Wollstein, including staining and
purification techniques, and bacterial growth media, including agar plates (thanks to
the advice of Angelina and Walther Hesse) and the Petri dish, named after its
inventor, his assistant Julius Richard Petri. These devices are still used today. With
these techniques, he was able to discover the bacterium causing tuberculosis
(Mycobacterium tuberculosis) in 1882 (he announced the discovery on March 24).
Tuberculosis was the cause of one in seven deaths in the mid-19th century.
In 1883, Koch worked with a French research team in Alexandria, Egypt, studying
cholera. Koch identified the vibrio bacterium that caused cholera, though he never
managed to prove it in experiments. The bacterium had been previously isolated by
Italian anatomist Filippo Pacini in 1854, but his work had been ignored due to the
predominance of the miasma theory of disease. Koch was unaware of Pacini's work
and made an independent discovery, and his greater preeminence allowed the
discovery to be widely spread for the benefit of others. In 1965, however, the
bacterium was formally renamed Vibrio cholera Pacini 1854.
In 1885, he became professor of hygiene at the University of Berlin, and later, in
1891, director of the newly formed Institute of Infectious Diseases, a position which
he resigned from in 1904. He started traveling around the world, studying diseases in
South Africa, India, and Java.
Probably as important as his work on tuberculosis, for which he was awarded a Nobel
Prize (1905), are Koch's postulates, which say that to establish that an organism is the
cause of a disease, it must be:
•
•
•
•
found in all cases of the disease examined
prepared and maintained in a pure culture
capable of producing the original infection, even after several generations in
culture
be retrievable from an inoculated animal and cultured again.
After Koch's success the quality of his own research declined (especially with the
fiasco over his ineffective TB cure "tuberculin"), although his pupils found the
organisms responsible for diphtheria, typhoid, pneumonia, gonorrhoea, cerebrospinal
meningitis, leprosy, bubonic plague, tetanus, and syphilis, among others, by using his
methods.
He died on 27 May 1910 of a heart-attack in Baden-Baden, aged 66.[1]
Koch crater on the Moon was named after him. The Robert Koch Prize and Medal
were created to honour Microbiologists who make groundbreaking discoveries or who
contribute to global health in a unique way. The first non-German to be awarded the
medal was Professor Bill Hutchison of Strathclyde University in Glasgow.[2]
[edit] References
1. ^ Robert Koch Institute
2. ^ Parasitology in Scotland
[edit] Consult
•
Thomas Brock, Robert Koch: A Life in Medicine and Bacteriology,
Washington D.C. (1999)
[edit] See also
•
•
•
History of medicine
Microbiology
Timeline of medicine and medical technology
[edit] External links
•
•
Biography at the Nobel Foundation website
Biography and bibliography in the Virtual Laboratory of the Max Planck
Institute for the History of Science
[hide]
v•d•e
Nobel Laureates in Physiology or Medicine
Emil Behring (1901) · Ronald Ross (1902) · Niels Finsen (1903) · Ivan Pavlov (1904) ·
Robert Koch (1905) · Camillo Golgi / Santiago Ramón y Cajal (1906) · Alphonse
Laveran (1907) · Ilya Mechnikov / Paul Ehrlich (1908) · Emil Kocher (1909) · Albrecht
Kossel (1910) · Allvar Gullstrand (1911) · Alexis Carrel (1912) · Charles Robert Richet
(1913) · Robert Bárány (1914) · Jules Bordet (1919) · August Krogh (1920) · Archibald
Hill / Otto Meyerhof (1922) · Frederick Banting / John Macleod (1923) · Willem
Einthoven (1924)
Complete roster · 1901–1925 · 1926–1950 · 1951–1975 · 1976–2000 · 2001–present
Retrieved from "http://en.wikipedia.org/wiki/Robert_Koch"
Categories: 1843 births | 1910 deaths | German biologists | German physicians |
German inventors | German microbiologists | Tuberculosis | Nobel laureates in
Physiology or Medicine | German Nobel laureates | German military personnel of the
Franco-Prussian War | University of Göttingen alumni | Deaths by myocardial
infarction | People from the Kingdom of Hanover
Views
•
•
•
•
Article
Discussion
Edit this page
History
Personal tools
•
Log in / create account
Navigation
•
•
•
•
•
Main Page
Contents
Featured content
Current events
Random article
Interaction
•
•
•
•
•
•
About Wikipedia
Community portal
Recent changes
Contact Wikipedia
Donate to Wikipedia
Help
Search
Toolbox
•
•
•
•
•
•
•
What links here
Related changes
Upload file
Special pages
Printable version
Permanent link
Cite this page
Languages
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
Afrikaans
ﺍﻝﻉﺭﺏﻱﺓ
Bosanski
Brezhoneg
Български
Català
Česky
Dansk
Deutsch
Eesti
Español
Esperanto
Euskara
ﻑﺍﺭﺱﯼ
Français
한국어
Hrvatski
Bahasa Indonesia
Italiano
עברית
Kiswahili
Kurdî ﻙﻭﺭﺩﯼ/
Latina
Lëtzebuergesch
Македонски
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
Nederlands
日本語
(Norsk (nynorsk)(
(Norsk (bokmål)(
Polski
Português
Română
Runa Simi
Русский
Slovenčina
Slovenščina
Српски / Srpski
Suomi
Svenska
Tiếng Việt
Türkçe
Українська
中文
•
•
This page was last modified on 13 March 2008, at 18:06.
All text is available under the terms of the GNU Free Documentation License.
(See Copyrights for details.)
Wikipedia® is a registered trademark of the Wikimedia Foundation, Inc., a
U.S. registered 501(c)(3) tax-deductible nonprofit charity.
Privacy policy
About Wikipedia
Disclaimers
•
•
•
http://www.virology.net/Big_Virology/BVHomePage.html
Ways to find the
virus picture you're
The Big Picture Book of Viruses is intended to
interested in:
by Name:
List of Virus
Families
viral families with
example viruses
List of Individual
Viruses
all named viruses,
listed with their
assigned family
serve as both a catalog of virus pictures on the
Internet and as an educational resource to those
seeking more information about viruses. To this
end, it is intimately linked to All the Virology on
the WWW, and our collection of Virology Courses
and Tutorials.
There are several ways to access the information
in the Big Picture Book of Viruses. All viruses are
listed according to the family to which they have
been assigned by the International Committee on
Taxonomy of Viruses (ICTV). The images and
other data can be obtained by the routes listed at
the left.
What you can find here:
by
Structure/Genome:
Virus Families by
genome type
RNA, DNA, positive,
negative, etc.
Virus Families
Grouped by the
Baltimore Method
Listed with example
viruses and hosts
by Host:
Virus Families by
Host
includes broad host
categories (algae,
fungi, etc.) with
example viruses that
infect them
On this page, several types of information about
viruses can be found. First and foremost, we
show you what they look like, either by electron
microscopy or by computerassisted imaging. The
viral images are listed by their taxonomic groups.
Images listed have been gathered from several
well known sources on the web. Each image is
presented in a miniature format on this page, with
the original fullsized image being available by
simply clicking on the image of interest. (Note: if
the original image has moved, please contact me
for assistance) Also listed on the page are links to
detailed information (Tutorials and On-Line
Courses) about the viruses, and links to other
WWW sites (All the Virology on the WWW) with
additional information.
Other Virology Resources:
•
The full table of contents of this site's
parent with links to all the virology web
sites.
•
by Disease:
Virus Families by
All the Virology on the WWW
On-line Virology Courses
Some of the best note sets, diagrams,
tutorials and video available online.
Infectious Disease
includes a list of
infectious diseases in
humans, and links to
pictures of the viruses
that cause them
Guides to Virus
Structure:
Principles of Virus
Architecture
contains descriptions
and diagrams of virus
structure (and a little
history).
Virus Structure from
ATV's own on-line
virology courses.
An Introduction to Viral Taxonomy:
(from the National Library of Medicine)
•
•
•
•
The Universal System of Virus Taxonomy
Virus Nomenclature
Replicative Properties of Viruses Used in
Taxonomy
References
How to Add your Favorite Image to this Site:
If you know of a virus picture that is not listed
here, or would like to update the listing of a site,
please use our virology site submission form or
email me with the address. We are counting on a
continuation of community support to keep this
site up to date.
Are you interested in more information, or
assistance with your organization's Web site?
New!
ICTV Net is an email
network of ICTV
members that provides
the virology
community with the
opportunity to interact
with ICTV, ask
questions, and even
make proposals...
Check it out!
If you are interested in developing a WWW site
for your lab or organization, please feel free to
contact me for any needed advice and/or
assistance.
David M. Sander, Ph.D. ([email protected]
)
Don't forget to sign our Guestbook!
ATV Home | Table of Contents | Submit a Site | Search
Tulane University | Garry Lab Contact Info | FAQ | Garry Lab Home | Tulane Medical
Center
© 1995-2007. D. Sander
Established 5/95.
Dedication to Hans Zinsser
http://www.textbookofbacteriology.net/
Welcome to Todar's Online Textbook of Bacteriology textbookofbacteriology.net.
This textbook has evolved from online and live-in-person lectures presented in my
bacteriology courses at the University of Wisconsin-Madison. Its contents are suitable
for reading or presentation in courses or course modules concerning general
microbiology and medical bacteriology at the college and advanced high school levels
of education. As an electronic text, new material is constantly being added, and
current material is constantly being revised and updated. This is an inherent advantage
of the web-based text over the tree-burner.
The textbook will never be complete, as the rate of production of new information in
microbiology far outruns the author's ability to acquire and properly present it. If you
have suggestions, comments or criticisms regarding the textbook or its contents, or the
idea of this type of textbook, please send email to me at the address below.
Kenneth Todar
University of Wisconsin
Department of Bacteriology
Madison, Wisconsin 53706
[email protected]
To search the entire book, enter a term or phrase in the form below
Search WWW
General Bacteriology
Overview of Bacteriology
Search Textbook of Bacteriology
The Impact of Microbes on the Environment and Human Activities
Structure and Function of Procaryotes
Nutrition and Growth of Bacteria
Growth of Bacterial Populations
Control of Microbial Growth
The Diversity of Procaryotic Metabolism
Regulation and Control of Metabolic Activities
Bacteriophage
Procaryotes in the Environment
Important Groups of Procaryotes
Bacterial Relationships with Animals
The Nature of Host-Parasite Interactions
The Bacterial Flora of Humans
Mechanisms of Bacterial Pathogenicity
Bacteria of Medical Importance
Immune Defense against Microbial Pathogens: Innate Immunity
Immune Defense against Microbial Pathogens: Adaptive Immunity
Principles of Bacterial Pathogenesis
Bacterial Structure in Relationship to Pathogenicity
Colonization and Invasion by Bacterial Pathogens
Bacterial Defense against Phagocytosis
Bacterial Defense against Immune Responses
Bacterial Protein Toxins
Bacterial Endotoxin
Antimicrobial Agents Used in the Treatment of Infectious Disease
Bacterial Resistance to Antimicrobial Agents
Bacterial Pathogens and Diseases of Humans
Staphylococcus and Staphylococcal Disease
Streptococcus and Streptococcal Disease
Streptococcus pneumoniae
Listeria monocytogenes and Listeriosis
Neisseria: Gonorrhea and Meningitis
Haemophilus influenzae including Hib Meningitis
Opportunistic Infections Caused by Pseudomonas aeruginosa
Whooping Cough (Pertussis)
E. coli: Gastroenteritis, Urinary Tract Infections and Neonatal Meningitis
Cholera
Salmonella and Salmonellosis
Shigella and Shigellosis
Pathogenic Clostridia, including Tetanus and Botulism
Bacillus cereus Food Poisoning
Bacillus anthracis and Anthrax
Diphtheria
Tuberculosis
Rickettsial Diseases, including Rocky Mountain Spotted Fever
Emerging Pathogens
Borrelia burgdorferi
Vibrio vulnificus
Important Groups of Procaryotes In progress: Enteric bacteria; Lactic acid bacteria;
Plant pathogenic bacteria
Bacillus and Related Endospore-forming Bacteria
Kenneth Todar has taught microbiology to undergraduate students at The University of Texas,
University of Alaska and University of Wisconsin since 1969. He received a PhD in Microbiology
in 1972 from The University of Texas-Austin. His main teaching interests are in general
microbiology, bacterial diversity, microbial ecology and pathogenic bacteriology. Currently, he is
an emeritus lecturer at the University of Wisconsin-Madison, where he teaches Microbiology 100,
"The Microbial World". He resides in Madison, Wisconsin and Silvergate, Montana.
WEB TEXT REVIEW (SCIENCE Magazine Vol 304: 1421)
"The Good, the Bad, and the Deadly"
The pearly droplets in this photo are colonies of Bacillus
anthracis, the bacterium that causes anthrax. The bugs exude a
goopy coating that repels immune system assaults and allows
them to establish a foothold in the body. Learn more about the
tricks bacteria use to prosper almost everywhere on Earth in
this Web text from microbiologist Kenneth Todar of the
University of Wisconsin, Madison. High school and college
students can absorb the basics of bacterial structure, physiology,
classification, and ecology.The book emphasizes medical
microbiology, exploring how bacteria hitch a ride from host to
host, how the body tries to corral invading microbes, and how
the bugs elude these defenses. For example, the cholera
bacterium releases a toxin that induces intestinal cells to spill
ions and water, producing potentially lethal diarrhea.
textbookofbacteriology.net
OTHER CITATIONS, REVIEWS, ADAPTATIONS
© 2008 Kenneth Todar University of Wisconsin-Madison Department of
Bacteriology.
Written and edited by Kenneth Todar University of Wisconsin-Madison
Department of Bacteriology. All rights reserved.