* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Electromagnetic Spectrum Wavelength Wavenumber Frequency
Astronomical spectroscopy wikipedia , lookup
Stability constants of complexes wikipedia , lookup
Reflection high-energy electron diffraction wikipedia , lookup
Homoaromaticity wikipedia , lookup
Magnetic circular dichroism wikipedia , lookup
Ultrafast laser spectroscopy wikipedia , lookup
Molecular orbital wikipedia , lookup
Electrochemistry wikipedia , lookup
Degenerate matter wikipedia , lookup
Mössbauer spectroscopy wikipedia , lookup
Auger electron spectroscopy wikipedia , lookup
Heat transfer physics wikipedia , lookup
Electron scattering wikipedia , lookup
Metastable inner-shell molecular state wikipedia , lookup
Ultraviolet–visible spectroscopy wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Photoelectric effect wikipedia , lookup
Atomic orbital wikipedia , lookup
Chemical bond wikipedia , lookup
X-ray fluorescence wikipedia , lookup
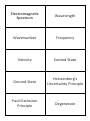
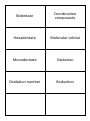
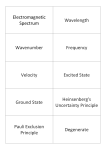
Electromagnetic Spectrum Wavelength Wavenumber Frequency Velocity Excited State Ground State Heinsenberg's Uncertainty Principle Pauli Exclusion Principle Degenerate This is the range of frequencies or wavelengths of electromagnetic radiation The distance between adjacent crests or troughs of a wave. The reciprocal of wavelength and has the units of cm -1 (number of cycles per cm.) The number of wavelengths that pass a fixed point in one second. The units are Hertz (Hz). Speed of light = 3.00 x 10⁸ ms-1 When an electron absorbs energy, it goes to a higher energy level. This is the lowest possible electronic configuration the electrons in an atom can adopt. When an electron moves down to its ground state, energy is given out. This states that it is impossible to state precisely the position and the momentum of an electron at the same instant. This states that an orbital holds a maximum of two electrons. Of equal energy. Aufbau Principle Hund's Rule Spectroscopic Notation Atomic Emission Spectroscopy Atomic Absorption Spectroscopy Complex Ligand Spectrochemical Series Dative Covalent Bond Coordination number This states that orbitals are filled in order of increasing energy. When degenerate orbitals are available, electrons fill each singly, keeping their spins parallel before pairing starts. 1s² 2s² 2p⁶ 3s² 3p⁶ 4s²3d¹⁰ A way of performing chemical analysis of elements contained within a sample. The sample is converted into a gas and then excited using a flame/electricity. The excited gas atoms emit light energy. The intensity of this light can be use to determine exactly which elements are in the sample. A technique for determining the concentration of a particular metal element in a sample. The electrons are promoted to higher energy levels by absorbing energy, and the wavelength of the absorbed energy can be used to determine which element is present. The intensity of the absorbed light can be used to determine the concentration of the elements. A central metal ion surrounded by ligands. Negative ions or uncharged molecules with one or more lone pairs of electrons. When one atom provides both of the electrons that form the bond A list of ligands in order of the size of the crystal field splitting caused in the d-orbitals. CN⁻ > NH₃ > H₂O > OH⁻ > F⁻ >Cl⁻ > Br⁻ > I⁻ This is the number of nearest neighbours by which an atom or ion is surrounded in a structure. Bidentate Coordination compounds Hexadentate Molecular orbital Monodentate Oxidation Oxidation number Reduction A ligand that contains two atoms with lone pairs of electrons capable of bondingto a metal ion. Compounds in which a central metal ion is attached to a group of surrounding molecules or ions by dative covalent bonds (also known as coordinate bonds). A ligand that bonds to a metal ion using electron pairs on six donor atoms. A molecular orbital is a region in space between the nuclei where there is a high probability of finding electrons. It is formed by the overlap of atomic orbitals. A ligand that bonds to a metal ion using the electron pair of a single donor atom. This is the loss of electrons from a substance. It can also be described as an increase in oxidation number. The formal charge assigned to each atom in a compound according to certain rules. This is the gain of electrons by a substance. It can also be described as a decrease in oxidation number.